New Approaches Based on Non-Invasive Brain Stimulation and Mental Representation Techniques Targeting Pain in Parkinson’s Disease Patients: Two Study Protocols for Two Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
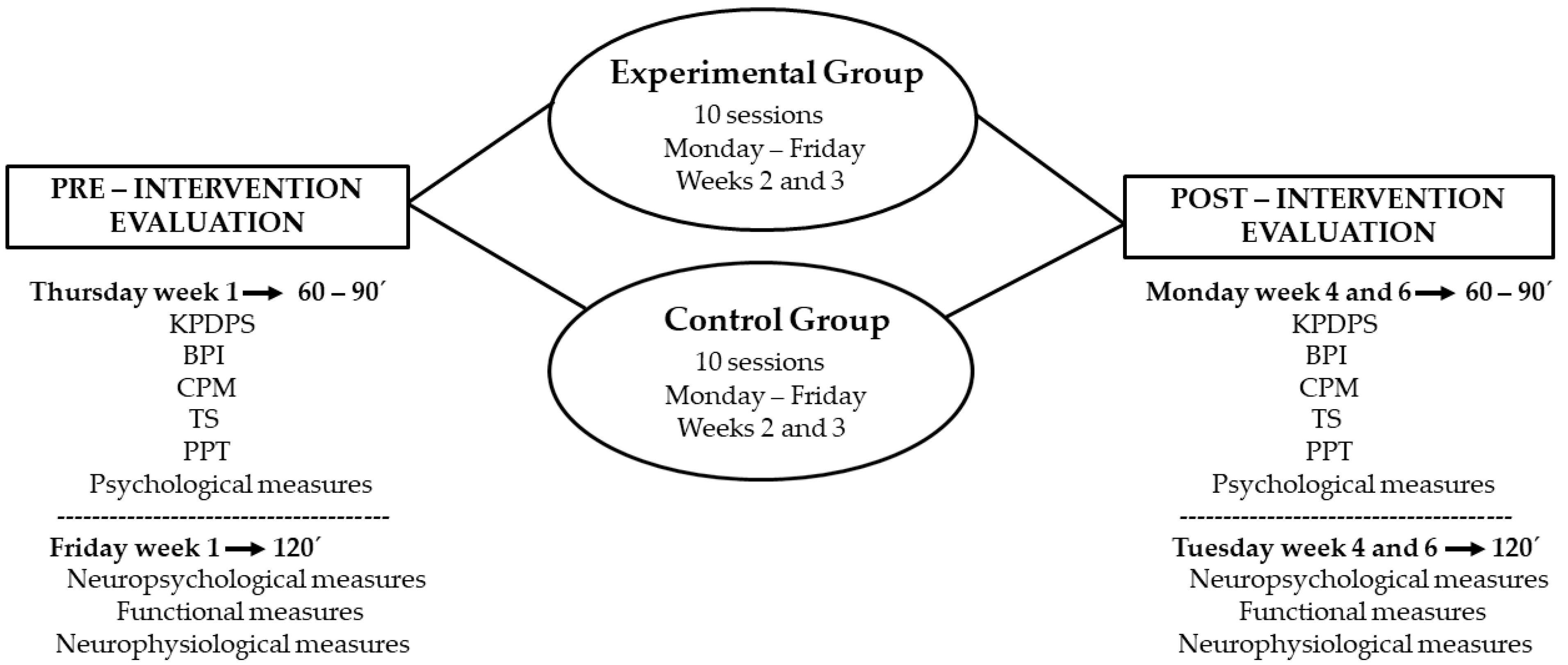
2.1. Study Design and Participants
2.1.1. tDCS-Based Study
2.1.2. AO+MI-Based Study
2.2. Intervention Protocols
2.2.1. tDCS-Based Study
2.2.2. AO+MI-Based Study
2.3. Outcomes Measurement
2.3.1. Main Outcomes
King’s Parkinson’s Disease Pain Scale (KPDPS)
Brief Pain Inventory (BPI)
Pain Pressure Threshold (PPT)
Temporal Summation (TS)
Conditioned Pain Modulation (CPM)
2.3.2. Secondary Outcomes
Psychological and Functional Outcomes
Neuropsychological Outcome
Neurophysiological Outcomes
2.4. Sample Size Calculation
2.4.1. tDCS-Based Study
2.4.2. AO+MI-Based Study
2.5. Data Analyses
2.6. Further Analysis
2.7. Dissemination Plans
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Karri, M.; Ramasamy, B.; Kalidoss, R. Prevalence of Non-motor Symptoms in Parkinson’s Disease and Its Impact on Quality of Life in Tertiary Care Center in India. Ann. Indian Acad. Neurol. 2020, 23, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Silverdale, M.A.; Kobylecki, C.; Kass-Iliyya, L.; Martinez-Martin, P.; Lawton, M.; Cotterill, S.; Chaudhuri, K.R.; Morris, H.; Baig, F.; Williams, N.; et al. A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 56, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Kurtis, M.M.; Chaudhuri, K.R.; NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov. Disord. 2011, 26, 399–406. [Google Scholar] [CrossRef]
- Gerdelat-Mas, A.; Simonetta-Moreau, M.; Thalamas, C.; Ory-Magne, F.; Slaoui, T.; Rascol, O.; Brefel-Courbon, C. Levodopa raises objective pain threshold in Parkinson’s disease: A RIII reflex study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mao, C.-J.; Li, S.-J.; Wang, F.; Chen, J.; Zhang, H.-J.; Li, L.; Guo, S.-S.; Yang, Y.-P.; Liu, C.-F. Quantitative and fiber-selective evaluation of pain and sensory dysfunction in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Granovsky, Y.; Schlesinger, I.; Fadel, S.; Erikh, I.; Sprecher, E.; Yarnitsky, D. Asymmetric pain processing in Parkinson’s disease. Eur. J. Neurol. 2013, 20, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Karnik, V.; Farcy, N.; Zamorano, C.; Bruno, V. Current Status of Pain Management in Parkinson’s Disease. Can. J. Neurol. Sci. 2020, 47, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, K.; Leta, V.; Sportelli, C.; Buhidma, Y.; Duty, S.; Malcangio, M.; Ray Chaudhuri, K. Pain in Parkinson’s disease: New concepts in pathogenesis and treatment. Curr. Opin. Neurol. 2019, 32, 579–588. [Google Scholar] [CrossRef]
- Gandolfi, M.; Geroin, C.; Antonini, A.; Smania, N.; Tinazzi, M. Understanding and Treating Pain Syndromes in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 827–858. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Tinazzi, M.; Abbruzzese, G.; Berardelli, A.; Chaudhuri, K.R.; Defazio, G.; Ferreira, J.; Martinez-Martin, P.; Trenkwalder, C.; Rascol, O. Pain in Parkinson’s disease: Facts and uncertainties. Eur. J. Neurol. 2018, 25, 917-e69. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, A.; Sandrini, G.; Serrao, M.; Buscone, S.; Tassorelli, C.; Tinazzi, M.; Zangaglia, R.; Pacchetti, C.; Bartolo, M.; Pierelli, F.; et al. Facilitated temporal summation of pain at spinal level in Parkinson’s disease: Temporal Summation of Pain in Parkinson’s Disease. Mov. Disord. 2011, 26, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Chipchase, L.S.; Schabrun, S.M. Primary sensory and motor cortex function in response to acute muscle pain: A systematic review and meta-analysis. Eur. J. Pain 2016, 20, 1203–1213. [Google Scholar] [CrossRef]
- Lefaucheur, J.P. The use of repetitive transcranial magnetic stimulation (rTMS) in chronic neuropathic pain. Neurophysiol. Clin. 2006, 36, 117–124. [Google Scholar] [CrossRef]
- Dasilva, A.F.; Mendonca, M.E.; Zaghi, S.; Lopes, M.; Dossantos, M.F.; Spierings, E.L.; Bajwa, Z.; Datta, A.; Bikson, M.; Fregni, F. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache 2012, 52, 1283–1295. [Google Scholar] [CrossRef]
- Chen, K.-H.S.; Chen, R. Invasive and Noninvasive Brain Stimulation in Parkinson’s Disease: Clinical Effects and Future Perspectives. Clin. Pharm. 2019, 106, 763–775. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson’s disease: Influence of antiparkinsonian treatment and cortical stimulation. Clin. Neurophysiol. 2005, 116, 244–253. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Ahn, H.; Suchting, R.; Woods, A.J.; Miao, H.; Green, C.; Cho, R.Y.; Choi, E.; Fillingim, R.B. Bayesian analysis of the effect of transcranial direct current stimulation on experimental pain sensitivity in older adults with knee osteoarthritis: Randomized sham-controlled pilot clinical study. J. Pain Res. 2018, 11, 2071–2082. [Google Scholar] [CrossRef]
- Przeklasa-Muszyńska, A.; Kocot-Kępska, M.; Dobrogowski, J.; Wiatr, M.; Mika, J. Transcranial direct current stimulation (tDCS) and its influence on analgesics effectiveness in patients suffering from migraine headache. Pharm. Rep. 2017, 69, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Kim, Y.K.; Kim, H.-R.; Kim, S.E.; Lee, Y.; Shin, H.I. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: A mechanistic PET study. Neurorehabil. Neural Repair. 2014, 28, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Meeker, T.J.; Keaser, M.L.; Khan, S.A.; Gullapalli, R.P.; Seminowicz, D.A.; Greenspan, J.D. Non-invasive Motor Cortex Neuromodulation Reduces Secondary Hyperalgesia and Enhances Activation of the Descending Pain Modulatory Network. Front. Neurosci. 2019, 13, 467. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.A.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov. Disord. 2006, 21, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Hatem, S.; Nineb, A.; Ménard-Lefaucheur, I.; Wendling, S.; Keravel, Y.; Nguyen, J.P. Somatotopic organization of the analgesic effects of motor cortex rTMS in neuropathic pain. Neurology 2006, 67, 1998–2004. [Google Scholar] [CrossRef]
- Lang, N.; Siebner, H.R.; Ward, N.S.; Lee, L.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N.; Frackowiak, R.S. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci. 2005, 22, 495–504. [Google Scholar] [CrossRef]
- Wright, D.J.; Williams, J.; Holmes, P.S. Combined action observation and imagery facilitates corticospinal excitability. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Caspers, S.; Zilles, K.; Laird, A.R.; Eickhoff, S.B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 2010, 50, 1148–1167. [Google Scholar] [CrossRef]
- Tremblay, F.; Léonard, G.; Tremblay, L. Corticomotor facilitation associated with observation and imagery of hand actions is impaired in Parkinson’s disease. Exp. Brain Res. 2008, 185, 249–257. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef]
- Reuter, I.; Mehnert, S.; Leone, P.; Kaps, M.; Oechsner, M.; Engelhardt, M. Effects of a Flexibility and Relaxation Programme, Walking, and Nordic Walking on Parkinson’s Disease. J. Aging Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.-W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef] [PubMed]
- Mylius, V.; Lloret, S.P.; Cury, R.G.; Teixeira, M.J.; Barbosa, V.R.; Barbosa, E.R.; Moreira, L.I.; Listik, C.; Fernandes, A.M.; de Lacera Veiga, D.; et al. The Parkinson’s disease pain classification system (PDPCS): Results from an international mechanism-based classification approach. Pain 2020. [Google Scholar] [CrossRef] [PubMed]
- Chesterton, L.S.; Barlas, P.; Foster, N.E.; Baxter, G.D.; Wright, C.C. Gender differences in pressure pain threshold in healthy humans. Pain 2003, 101, 259–266. [Google Scholar] [CrossRef]
- Sung, S.; Vijiaratnam, N.; Chan, D.W.C.; Farrell, M.; Evans, A.H. Parkinson disease: A systemic review of pain sensitivities and its association with clinical pain and response to dopaminergic stimulation. J. Neurol. Sci. 2018, 395, 172–206. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.D.; Fietsam, A.C.; Rudroff, T. Tolerability and Blinding of Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Critical Review. Brain Sci. 2020, 10, 467. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Drouot, X.; Menard-Lefaucheur, I.; Zerah, F.; Bendib, B.; Cesaro, P.; Keravel, Y.; Nguyen, J.-P. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J. Neurol. Neurosurg. Psychiatry 2004, 75, 612–616. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Lima, M.C.; Ferreira, M.J.L.; Wagner, T.; Rigonatti, S.P.; Castro, A.W.; Souza, D.R.; Riberto, M.; Freedman, S.D.; et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006, 122, 197–209. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Feichtner, K.B.; Hasan, A.; Gauglitz, G.; Langguth, B.; Nitsche, M.A.; Keeser, D.; Padberg, F. The role of contact media at the skin-electrode interface during transcranial direct current stimulation (tDCS). Brain Stimul. 2014, 7, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Renard, Y.; Lotte, F.; Gibert, G.; Congedo, M.; Maby, E.; Delannoy, V.; Bertrand, O.; Lécuyer, A. OpenViBE: An Open-Source Software Platform to Design, Test and Use Brain-Computer Interfaces in Real and Virtual Environments. Presence Teleoperators Virtual Environ. 2010, 19. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Faria, A.L.; Cameirão, M.S.; Badia, S.B. i RehabNet: A distributed architecture for motor and cognitive neuro-rehabilitation. In Proceedings of the 2013 IEEE 15th International Conference on e-Health Networking, Applications and Services (Healthcom 2013), Lisbon, Portugal, 9–12 October 2013; pp. 454–459. [Google Scholar]
- Pfurtscheller, G.; Neuper, C.; Müller, G.R.; Obermaier, B.; Krausz, G.; Schlögl, A.; Scherer, R.; Graimann, B.; Keinrath, C.; Skliris, D.; et al. Graz-BCI: State of the art and clinical applications. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Ferreira, A.; Badia, S.B. i NeuRow: An Immersive VR Environment for Motor-Imagery Training with the Use of Brain-Computer Interfaces and Vibrotactile Feedback. In Proceedings of the 3rd International Conference on Physiological Computing Systems, Lisbon, Portugal, 27–28 July 2016; SCITEPRESS-Science and Technology Publications: Lisbon, Portugal; pp. 43–53. [Google Scholar]
- Morales Tejera, D.; Fernandez-Carnero, J.; Suso-Martí, L.; Cano-de-la-Cuerda, R.; Lerín-Calvo, A.; Remón-Ramiro, L.; La Touche, R. Comparative study of observed actions, motor imagery and control therapeutic exercise on the conditioned pain modulation in the cervical spine: A randomized controlled trial. Somat. Mot. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Jorge, C.; Abreu, R.; Figueiredo, P.; Fernandes, J.-C.; Bermúdez i Badia, S. Efficacy and Brain Imaging Correlates of an Immersive Motor Imagery BCI-Driven VR System for Upper Limb Motor Rehabilitation: A Clinical Case Report. Front. Hum. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Soler, M.D.; Kumru, H.; Pelayo, R.; Vidal, J.; Tormos, J.M.; Fregni, F.; Navarro, X.; Pascual-Leone, A. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 2010, 133, 2565–2577. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Rizos, A.; Trenkwalder, C.; Rascol, O.; Pal, S.; Martino, D.; Carroll, C.; Paviour, D.; Falup-Pecurariu, C.; Kessel, B.; et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov. Disord. 2015, 30, 1623–1631. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Ciampi de Andrade, D.; Lyons, K.E.; Rodríguez-Blázquez, C.; Chaudhuri, K.R.; Deuschl, G.; Cruccu, G.; Sampaio, C.; Goetz, C.G.; Schrag, A.; et al. Rating Scales for Pain in Parkinson’s Disease: Critique and Recommendations. Mov. Disord. Clin. Pr. 2016, 3, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Bann, C.M.; Dodd, S.L.; Schein, J.; Mendoza, T.R.; Cleeland, C.S. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin. J. Pain 2004, 20, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Bisset, L.M.; Evans, K.; Tuttle, N. Reliability of 2 Protocols for Assessing Pressure Pain Threshold in Healthy Young Adults. J. Manip. Physiol. Ther. 2015, 38, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Peña, R.; Muñoz-García, D.; Calvo-Lobo, C.; Fernández-Carnero, J. Pain Expansion and Severity Reflect Central Sensitization in Primary Care Patients with Greater Trochanteric Pain Syndrome. Pain Med. 2019, 20, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Arendt-Nielsen, L.; Andersen, H.; Graven-Nielsen, T. Temporal summation of pain evoked by mechanical stimulation in deep and superficial tissue. J. Pain 2005, 6, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kliger, M.; Stahl, S.; Haddad, M.; Suzan, E.; Adler, R.; Eisenberg, E. Measuring the Intensity of Chronic Pain: Are the Visual Analogue Scale and the Verbal Rating Scale Interchangeable? Pain Pr. 2015, 15, 538–547. [Google Scholar] [CrossRef]
- Olesen, S.S.; van Goor, H.; Bouwense, S.A.W.; Wilder-Smith, O.H.G.; Drewes, A.M. Reliability of static and dynamic quantitative sensory testing in patients with painful chronic pancreatitis. Reg. Anesth. Pain Med. 2012, 37, 530–536. [Google Scholar] [CrossRef]
- Imai, Y.; Petersen, K.K.; Mørch, C.D.; Arendt Nielsen, L. Comparing test-retest reliability and magnitude of conditioned pain modulation using different combinations of test and conditioning stimuli. Somat. Mot. Res. 2016, 33, 169–177. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. Relationships between specific and general tests of cerebral functioning. Clin. Neuropsychol. 1996, 10, 37–42. [Google Scholar] [CrossRef]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2006; ISBN 978-0-19-515957-8. [Google Scholar]
- Bronte-Stewart, H.M.; Ding, L.; Alexander, C.; Zhou, Y.; Moore, G.P. Quantitative digitography (QDG): A sensitive measure of digital motor control in idiopathic Parkinson’s disease. Mov. Disord. 2000, 15, 36–47. [Google Scholar] [CrossRef]
- Jensen, A.R. Clocking the Mind: Mental Chronometry and Individual Differences; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 978-0-08-046372-8. [Google Scholar]
- Reicker, L.I.; Tombaugh, T.N.; Walker, L.; Freedman, M.S. Reaction time: An alternative method for assessing the effects of multiple sclerosis on information processing speed. Arch. Clin. Neuropsychol. 2007, 22, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, L.; Uygur Kucukseymen, E.; Duarte, D.; El-Hagrassy, M.M.; Bonin Pinto, C.; Gunduz, M.E.; Cardenas-Rojas, A.; Pacheco-Barrios, K.; Yang, Y.; Gonzalez-Mego, P.; et al. Optimised transcranial direct current stimulation (tDCS) for fibromyalgia-targeting the endogenous pain control system: A randomised, double-blind, factorial clinical trial protocol. BMJ Open 2019, 9, e032710. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, D.; Oreiro, M.; Pérez, P.; Fanjul, G.; Paz González, J.M.; Feal Painceiras, M.J.; Cores Bartolomé, C.; Valdés Aymerich, L.; García Sancho, C.; Castellanos Rodrigo, M.D.M. Impact of Coronavirus Disease 2019 Pandemic on Parkinson’s Disease: A Cross-Sectional Survey of 568 Spanish Patients. Mov. Disord. 2020, 35, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Older than 18. Neuroimaging study without previous pathologies. Score > 5 in transfers (bed to chair and back) item in Barthel Index. Score = or > 24 in Mini-Mental State Examination. Tolerability for the application of electrotherapy. Able to provide informed consent to participate in the study. | History of neurologic disease different from PD. Presence of non-related to PD pain. Dermatologic problems, wounds, or ulcers in the electrode’s application area. Presence of implants or metal pieces in the head. Presence of cardiac pacemaker, vagal, brain or transcutaneous stimulators, medication pumps, ventriculoperitoneal shunts or aneurysm clips. Significative difficulties in language. History of alcohol or drugs abuse. Non-controlled medical problems. Pregnancy. Epilepsy. |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Older than 18. Neuroimaging study without previous pathologies. Score > 5 in transfers (bed to chair and back) item in Barthel Index. Score = or > 24 in Mini-Mental State Examination. Able to provide informed consent to participate in the study. | History of neurologic disease different from PD. Presence of non-related to PD pain. Dermatologic problems, wounds, or ulcers in the electrode’s application area. Significative difficulties in language. History of alcohol or drugs abuse. Non-controlled medical problems. Pregnancy. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Zamorano, Y.; Fernández-Carnero, J.; Sánchez-Cuesta, F.J.; Arroyo-Ferrer, A.; Vourvopoulos, A.; Figueiredo, P.; Serrano, J.I.; Romero, J.P. New Approaches Based on Non-Invasive Brain Stimulation and Mental Representation Techniques Targeting Pain in Parkinson’s Disease Patients: Two Study Protocols for Two Randomized Controlled Trials. Brain Sci. 2021, 11, 65. https://doi.org/10.3390/brainsci11010065
González-Zamorano Y, Fernández-Carnero J, Sánchez-Cuesta FJ, Arroyo-Ferrer A, Vourvopoulos A, Figueiredo P, Serrano JI, Romero JP. New Approaches Based on Non-Invasive Brain Stimulation and Mental Representation Techniques Targeting Pain in Parkinson’s Disease Patients: Two Study Protocols for Two Randomized Controlled Trials. Brain Sciences. 2021; 11(1):65. https://doi.org/10.3390/brainsci11010065
Chicago/Turabian StyleGonzález-Zamorano, Yeray, Josué Fernández-Carnero, Francisco José Sánchez-Cuesta, Aida Arroyo-Ferrer, Athanasios Vourvopoulos, Patricia Figueiredo, José Ignacio Serrano, and Juan Pablo Romero. 2021. "New Approaches Based on Non-Invasive Brain Stimulation and Mental Representation Techniques Targeting Pain in Parkinson’s Disease Patients: Two Study Protocols for Two Randomized Controlled Trials" Brain Sciences 11, no. 1: 65. https://doi.org/10.3390/brainsci11010065
APA StyleGonzález-Zamorano, Y., Fernández-Carnero, J., Sánchez-Cuesta, F. J., Arroyo-Ferrer, A., Vourvopoulos, A., Figueiredo, P., Serrano, J. I., & Romero, J. P. (2021). New Approaches Based on Non-Invasive Brain Stimulation and Mental Representation Techniques Targeting Pain in Parkinson’s Disease Patients: Two Study Protocols for Two Randomized Controlled Trials. Brain Sciences, 11(1), 65. https://doi.org/10.3390/brainsci11010065










