Epidemiology of Anthropometric Factors in Glioblastoma Multiforme—Literature Review
Abstract
1. Introduction
2. Characteristics of Glioblastoma Multiforme
3. The Influence of Gender on the Incidence of Glioblastoma Multiforme
4. Effect of Age on the Incidence of Glioblastoma Multiforme
5. Glioblastoma Multiforme Incidence Depending on Tumor Location in the Central Nervous System
6. Influence of Overweight and Obesity on the Incidence of Glioblastoma Multiforme
7. Height as a Risk Factor for GBM Incidence
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DeAngelis, L.M.; Mellinghoff, I.K. Virchow 2011 or How to ID(H) Human Glioblastoma. J. Clin. Oncol. 2011, 29, 4473–4474. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classi-fication of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Chowdhury, R.; Yeoh, K.K.; Tian, Y.M.; Hillringhaus, L.; Bagg, E.A.; Rose, N.R.; Leung, I.K.H.; Li, X.S.; Woon, E.C.Y.; Yang, M.; et al. The Oncometabolite 2-Hydroxyglutarate Inhibits Histone Lysine Demethylases. EMBO Rep. 2011, 12, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sanson, M.; Marie, Y.; Paris, S.; Idbaih, A.; Laffaire, J.; Ducray, F.; El Hallani, S.; Boisselier, B.; Mokhtari, K.; Hoang-Xuan, K.; et al. Isocitrate Dehydrogenase 1 Codon 132 Mutation Is an Important Prognostic Biomarker in Gliomas. J. Clin. Oncol. 2009, 27, 4150–4154. [Google Scholar] [CrossRef] [PubMed]
- Houillier, C.; Wang, X.; Kaloshi, G.; Mokhtari, K.; Guillevin, R.; Laffaire, J.; Paris, S.; Boisselier, B.; Idbaih, A.; Laigle-Donadey, F.; et al. IDH1 or IDH2 Mutations Predict Longer Survival and Response to Temozolomide in Low-Grade Gliomas. Neurology 2010, 75, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Liu, W.L.; Cao, H.; Wen, C.; Chen, L.; Jiang, G. O6-Methylguanine DNA Methyltransferase as a Promising Target for the Treatment of Temozolomide-Resistant Gliomas. Cell Death Dis. 2013, 4, e876. [Google Scholar] [CrossRef]
- Binabaj, M.M.; Bahrami, A.; ShahidSales, S.; Joodi, M.; Joudi Mashhad, M.; Hassanian, S.M.; Anvari, K.; Avan, A. The Prognostic Value of MGMT Promoter Methylation in Glioblastoma: A Meta-Analysis of Clinical Trials. J. Cell. Physiol. 2018, 233, 378–386. [Google Scholar] [CrossRef]
- Gladitz, J.; Klink, B.; Seifert, M. Network-Based Analysis of Oligodendrogliomas Predicts Novel Cancer Gene Candidates within the Region of the 1p/19q Co-Deletion. Acta Neuropathol. Commun. 2018, 6, 49. [Google Scholar] [CrossRef]
- Qiu, J.; Shi, Z.; Jiang, J. Cyclooxygenase-2 in Glioblastoma Multiforme. Drug Discov. Today 2017, 22, 148–156. [Google Scholar] [CrossRef]
- Schwartzbaum, J.A.; Fisher, J.L.; Aldape, K.D.; Wrensch, M. Epidemiology and Molecular Pathology of Glioma. Nat. Clin. Pract. Neurol. 2006, 2, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Linos, E.; Raine, T.; Alonso, A.; Michaud, D. Atopy and Risk of Brain Tumors: A Meta-Analysis. J. Natl. Cancer Inst. 2007, 99, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.L.; Schwartzbaum, J.A.; Wrensch, M.; Wiemels, J.L. Epidemiology of Brain Tumors. Neurol. Clin. 2007, 25, 867–890. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. Genetic Alterations and Signaling Pathways in the Evolution of Gliomas. Cancer Sci. 2009, 100, 2235–2241. [Google Scholar] [CrossRef]
- Kim, S.E.; Paik, H.Y.; Yoon, H.; Lee, J.E.; Kim, N.; Sung, M.K. Sex- and Gender-Specific Disparities in Colorectal Cancer Risk. World J. Gastroenterol. 2015, 21, 5167–5175. [Google Scholar] [CrossRef]
- DeCosse, J.J.; Ngoi, S.S.; Jacobson, J.S.; Cennerazzo, W.J. Gender and Colorectal Cancer. Eur. J. Cancer Prev. 1993, 2, 105–115. [Google Scholar] [CrossRef]
- Lucca, I.; Klatte, T.; Fajkovic, H.; De Martino, M.; Shariat, S.F. Gender Differences in Incidence and Outcomes of Urothelial and Kidney Cancer. Nat. Rev. Urol. 2015, 12, 585–592. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the Ageing Microenvironment Influences Tumour Progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Tricoli, J.V.; Bleyer, A. Adolescent and Young Adult Cancer Biology. Cancer J. 2018, 24, 267–274. [Google Scholar] [CrossRef]
- Sandiford, O.A.; Moore, C.A.; Du, J.; Boulad, M.; Gergues, M.; Eltouky, H.; Rameshwar, P. Human Aging and Cancer: Role of MiRNA in Tumor Microenvironment. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1056, pp. 137–152. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, J.; Sun, K.; Zhao, L.; Zhao, F.; Wang, L.; Jiao, Y. Obesity and Incidence of Lung Cancer: A Meta-Analysis. Int. J. Cancer. 2013, 132, 1162–1169. [Google Scholar] [CrossRef]
- Porter, M.P.; Stanford, J.L. Obesity and the Risk of Prostate Cancer. Prostate 2005, 62, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gong, X.; Zhang, T.; Jiang, W. Height and Risk of Colorectal Cancer. Eur. J. Cancer Prev. 2018, 27, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Lv, G.; Chen, W.; Jiang, J.; Wang, J. Height and Kidney Cancer Risk: A Meta-Analysis of Prospective Studies. J. Cancer Res. Clin. Oncol. 2015, 141, 1799–1807. [Google Scholar] [CrossRef]
- Aune, D.; Vieira, A.R.; Chan, D.S.M.; Rosenblatt, D.A.N.; Vieira, R.; Greenwood, D.C.; Cade, J.E.; Burley, V.J.; Norat, T. Height and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies. Cancer Causes Control 2012, 23, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Lotan, E.; Jain, R.; Razavian, N.; Fatterpekar, G.M.; Lui, Y.W. State of the Art: Machine Learning Applications in Glioma Imaging. Am. J. Roentgenol. 2019, 212, 26–37. [Google Scholar] [CrossRef]
- Pope, W.B.; Brandal, G. Conventional and Advanced Magnetic Resonance Imaging in Patients with High-Grade Glioma. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 239–253. [Google Scholar] [CrossRef]
- Ahmed, R.; Oborski, M.J.; Hwang, M.; Lieberman, F.S.; Mountz, J.M. Malignant Gliomas: Current Perspectives in Diagnosis, Treatment, and Early Response Assessment Using Advanced Quantitative Imaging Methods. Cancer Manag. Res. 2014, 6, 149–170. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2010–2014. Neuro Oncol. 2017, 19 (Suppl. 5), v1–v88. [Google Scholar] [CrossRef]
- Walker, E.V.; Davis, F.G. CBTR founding affiliates. Malignant Primary Brain and Other Central Nervous System Tumors Diagnosed in Canada from 2009 to 2013. Neuro Oncol. 2019, 21, 360–369. [Google Scholar] [CrossRef]
- Fabbro-Peray, P.; Zouaoui, S.; Darlix, A.; Fabbro, M.; Pallud, J.; Rigau, V.; Mathieu-Daude, H.; Bessaoud, F.; Bauchet, F.; Riondel, A.; et al. Association of Patterns of Care, Prognostic Factors, and Use of Radiotherapy–Temozolomide Therapy with Survival in Patients with Newly Diagnosed Glioblastoma: A French National Population-Based Study. J. Neurooncol. 2019, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gittleman, H.; Boscia, A.; Ostrom, Q.T.; Truitt, G.; Fritz, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Survivorship in Adults with Malignant Brain and Other Central Nervous System Tumor from 2000–2014. Neuro Oncol. 2018, 20, VII6–VII16. [Google Scholar] [CrossRef] [PubMed]
- Brodbelt, A.; Greenberg, D.; Winters, T.; Williams, M.; Vernon, S.; Collins, V.P. Glioblastoma in England: 2007–2011. Eur. J. Cancer 2015, 51, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Bohn, A.; Braley, A.; De La Vega, P.R.; Carlos Zevallos, J.; Barengo, N.C. The Association between Race and Survival in Glioblastoma Patients in the US: A Retrospective Cohort Study. PLoS ONE 2018, 13, e0198581. [Google Scholar] [CrossRef]
- Bruhn, H.; Strandéus, M.; Milos, P.; Hallbeck, M.; Vrethem, M.; Lind, J. Improved Survival of Swedish Glioblastoma Patients Treated According to Stupp. Acta Neurol. Scand. 2018, 138, 332–337. [Google Scholar] [CrossRef]
- Hansen, S.; Rasmussen, B.K.; Laursen, R.J.; Kosteljanetz, M.; Schultz, H.; Nørgård, B.M.; Guldberg, R.; Gradel, K.O. Treatment and Survival of Glioblastoma Patients in Denmark: The Danish Neuro-Oncology Registry 2009–2014. J. Neurooncol. 2018, 139, 479–489. [Google Scholar] [CrossRef]
- Liu, M.; Thakkar, J.P.; Garcia, C.R.; Dolecek, T.A.; Wagner, L.M.; Dressler, E.V.M.; Villano, J.L. National Cancer Database Analysis of Outcomes in Pediatric Glioblastoma. Cancer Med. 2018, 7, 1151–1159. [Google Scholar] [CrossRef]
- Furgason, J.M.; Koncar, R.F.; Michelhaugh, S.K.; Sarkar, F.H.; Mittal, S.; Sloan, A.E.; Barnholtz-Sloan, J.S.; Bahassi, E.M. Whole Genome Sequence Analysis Links Chromothripsis to EGFR, MDM2, MDM4, and CDK4 Amplification in Glioblastoma. Oncoscience 2015, 2, 618–628. [Google Scholar] [CrossRef]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef]
- Bulut, O.; Kilic, G.; Netea, M.G.; Netea, M.G. The CIMPACT-NOW Updates and Their Significance to Current Neuro-Oncology Practice L. Neuro Oncol. Pract. 2020, 30, 1–14. [Google Scholar]
- Mellai, M.; Piazzi, A.; Caldera, V.; Monzeglio, O.; Cassoni, P.; Valente, G.; Schiffer, D. IDH1 and IDH2 Mutations, Immunohistochemistry and Associations in a Series of Brain Tumors. J. Neurooncol. 2011, 105, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; Codon Publications: Singapore, 2017; pp. 143–153. [Google Scholar] [CrossRef]
- Tataranu, L.G.; Ciubotaru, V.; Cazac, T.L.; Alexandru, O.; Purcaru, O.S.; Tache, D.E.; Artene, S.A.; Dricu, A. Current Trends in Glioblastoma Treatment. In Brain Tumors—An Update; InTech: Londou, UK, 2018. [Google Scholar] [CrossRef]
- Sun, T.; Warrington, N.M.; Luo, J.; Brooks, M.D.; Dahiya, S.; Snyder, S.C.; Sengupta, R.; Rubin, J.B. Sexually Dimorphic RB Inactivation Underlies Mesenchymal Glioblastoma Prevalence in Males. J. Clin. Investig. 2014, 124, 4123–4133. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.; Ugiliweneza, B.; Woo, S.; Skirboll, S.; Boaky, M. A Surveillance, Epidemiology and End Results-Medicare Data Analysis of Elderly Patients with Glioblastoma Multiforme: Treatment Patterns, Outcomes and Cost. Mol. Clin. Oncol. 2015, 3, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lu, D.; Guo, Y.; Wang, C.; Liu, X.; Liu, Y.; Liu, D. Trends and Patterns of Incidence of Diffuse Glioma in Adults in the United States, 1973–2014. Cancer Med. 2018, 7, 5281–5290. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Xu, H.; Qin, Z. Geographic Variations in the Incidence of Glioblastoma and Prognostic Factors Predictive of Overall Survival in US Adults from 2004–2013. Front. Aging Neurosci. 2017, 9, 352. [Google Scholar] [CrossRef]
- Bin Abdulrahman, A.K.; Bin Abdulrahman, K.A.; Bukhari, Y.R.; Faqihi, A.M.; Ruiz, J.G. Association between Giant Cell Glioblastoma and Glioblastoma Multiforme in the United States: A Retrospective Cohort Study. Brain Behav. 2019, 9, e01402. [Google Scholar] [CrossRef]
- Xie, J.C.; Yang, S.; Liu, X.Y.; Zhao, Y.X. Effect of Marital Status on Survival in Glioblastoma Multiforme by Demographics, Education, Economic Factors, and Insurance Status. Cancer Med. 2018, 7, 3722–3742. [Google Scholar] [CrossRef]
- Shabihkhani, M.; Telesca, D.; Movassaghi, M.; Naeini, Y.B.; Naeini, K.M.; Hojat, S.A.; Gupta, D.; Lucey, G.M.; Ontiveros, M.; Wang, M.W.; et al. Incidence, Survival, Pathology, and Genetics of Adult Latino Americans with Glioblastoma. J. Neurooncol. 2017, 132, 351–358. [Google Scholar] [CrossRef]
- Cheo, S.T.T.; Lim, G.H.; Lim, K.H.C. Glioblastoma Multiforme Outcomes of 107 Patients Treated in Two Singapore Institutions. Singap. Med. J. 2017, 58, 41–45. [Google Scholar] [CrossRef]
- Tian, M.; Ma, W.; Chen, Y.; Yu, Y.; Zhu, D.; Shi, J.; Zhang, Y. Impact of Gender on the Survival of Patients with Glioblastoma. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- De Witt Hamer, P.C.; Ho, V.K.Y.; Zwinderman, A.H.; Ackermans, L.; Ardon, H.; Boomstra, S.; Bouwknegt, W.; van den Brink, W.A.; Dirven, C.M.; van der Gaag, N.A.; et al. Between-Hospital Variation in Mortality and Survival after Glioblastoma Surgery in the Dutch Quality Registry for Neuro Surgery. J. Neurooncol. 2019, 144, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.T.M.; Hsieh, S.Y.P.; Lau, C.K.Y.; Kam, M.K.M.; Loong, H.H.F.; Tsang, W.K.; Poon, D.M.C.; Poon, W.S. Ten-Year Review of Survival and Management of Malignant Glioma in Hong Kong. Hong Kong Med. J. 2017, 23, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Shubham, S.; Mandal, K.; Trivedi, V.; Chauhan, R.; Naseera, S. Survival and Prognostic Factors for Glioblastoma Multiforme: Retrospective Single-Institutional Study. Indian J. Cancer 2017, 54, 362–367. [Google Scholar] [CrossRef]
- Fuentes-Raspall, R.; Solans, M.; Roca-Barceló, A.; Vilardell, L.; Puigdemont, M.; del Barco, S.; Comas, R.; García-Velasco, A.; Astudillo, A.; Carmona-Garcia, M.C.; et al. Descriptive Epidemiology of Primary Malignant and Non-Malignant Central Nervous Tumors in Spain: Results from the Girona Cancer Registry (1994–2013). Cancer Epidemiol. 2017, 50, 1–8. [Google Scholar] [CrossRef]
- Salehpour, F.; Mirzaei, F.; Meshkini, A.; Parsay, S.; Salehi, S.; Asl, M.M.B. Trends in Primary Brain Tumors: A 5-Year Retrospective Histologically Confirmed Study in Tabriz, Iran, 2011–2016. Asian J. Neurosurg. 2019, 14, 427–431. [Google Scholar] [CrossRef]
- Maxwell, R.; Luksik, A.S.; Garzon-Muvdi, T.; Yang, W.; Huang, J.; Bettegowda, C.; Jallo, G.I.; Terezakis, S.A.; Groves, M.L. Population-Based Study Determining Predictors of Cancer-Specific Mortality and Survival in Pediatric High-Grade Brainstem Glioma. World Neurosurg. 2018, 119, e1006–e1015. [Google Scholar] [CrossRef]
- Lam, S.; Lin, Y.; Zinn, P.; Su, J.; Pan, I.W. Patient and Treatment Factors Associated with Survival among Pediatric Glioblastoma Patients: A Surveillance, Epidemiology, and End Results Study. J. Clin. Neurosci. 2018, 47, 285–293. [Google Scholar] [CrossRef]
- Shanmugavadivel, D.; Liu, J.F.; Murphy, L.; Wilne, S.; Walker, D. Accelerating Diagnosis for Childhood Brain Tumours: An Analysis of the HeadSmart UK Population Data. Arch. Dis. Child. 2019, 105, 355–362. [Google Scholar] [CrossRef]
- Nobusawa, S.; Watanabe, T.; Kleihues, P.; Ohgaki, H. IDH1 Mutations as Molecular Signature and Predictive Factor of Secondary Glioblastomas. Clin. Cancer Res. 2009, 15, 6002–6007. [Google Scholar] [CrossRef]
- Bleeker, F.E.; Atai, N.A.; Lamba, S.; Jonker, A.; Rijkeboer, D.; Bosch, K.S.; Tigchelaar, W.; Troost, D.; Van Dertop, W.P.; Bardelli, A.; et al. The Prognostic IDH1R132 Mutation Is Associated with Reduced NADP+-Dependent IDH Activity in Glioblastoma. Acta Neuropathol. 2010, 119, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.E.; Woo, S.R.; Nam, H.; Nam, D.H.; Lee, J.H.; Joo, K.M. Preclinical and Clinical Implications of TERT Promoter Mutation in Glioblastoma Multiforme. Oncol. Lett. 2017, 14, 8213–8219. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Hosen, I.; Gousias, K.; Rachakonda, S.; Heidenreich, B.; Gessi, M.; Schramm, J.; Hemminki, K.; Waha, A.; Kumar, R. TERT Promoter Mutations: A Novel Independent Prognostic Factor in Primary Glioblastomas. Neuro Oncol. 2015, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Lie, A.; Li, T.; Chowdhury, R.; Liu, F.; Ozer, B.; Wei, B.; Green, R.M.; Ellingson, B.M.; Wang, H.J.; et al. Human TERT Promoter Mutation Enables Survival Advantage from MGMT Promoter Methylation in IDH1 Wild-Type Primary Glioblastoma Treated by Standard Chemoradiotherapy. Neuro Oncol. 2017, 19, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Z.; Shibahara, I.; Yamaki, T.; Yoshioka, E.; Shofuda, T.; Ohe, R.; Matsuda, K.; Saito, R.; Kanamori, M.; Kanemura, Y.; et al. TERT Promoter Mutation Associated with Multifocal Phenotype and Poor Prognosis in Patients with IDH Wild-Type Glioblastoma. Neuro Oncol. Adv. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Kang, K.B.; Wang, T.T.; Woon, C.T.; Cheah, E.S.; Moore, X.L.; Zhu, C.; Wong, M.C. Enhancement of Glioblastoma Radioresponse by a Selective COX-2 Inhibitor Celecoxib: Inhibition of Tumor Angiogenesis with Extensive Tumor Necrosis. Int. J. Radiat. Oncol. 2007, 67, 888–896. [Google Scholar] [CrossRef]
- Ferguson, S.D.; Xiu, J.; Weathers, S.P.; Zhou, S.; Kesari, S.; Weiss, S.E.; Verhaak, R.G.; Hohl, R.J.; Barger, G.R.; Reddy, S.K.; et al. GBM-Associated Mutations and Altered Protein Expression Are More Common in Young Patients. Oncotarget 2016, 7, 69466–69478. [Google Scholar] [CrossRef]
- Shieh, L.T.; Guo, H.R.; Chang, Y.K.; Lu, N.M.; Ho, S.Y. Clinical Implications of Multiple Glioblastomas: An Analysis of Prognostic Factors and Survival to Distinguish from Their Single Counterparts. J. Formos. Med. Assoc. 2020, 119, 728–734. [Google Scholar] [CrossRef]
- Li, H.Y.; Sun, C.R.; He, M.; Yin, L.C.; Du, H.G.; Zhang, J.M. Correlation Between Tumor Location and Clinical Properties of Glioblastomas in Frontal and Temporal Lobes. World Neurosurg. 2018, 112, e407–e414. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Fang, X.; Wei, J.; He, X.; Lian, J.; Han, D.; An, P.; Zhou, T.; Liu, S.; Wang, F.; Min, J. Quantitative Association between Body Mass Index and the Risk of Cancer: A Global Meta-Analysis of Prospective Cohort Studies. Int. J. Cancer 2018, 143, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Helseth, A.; Tretli, S. Pre-Morbid Height and Weight as Risk Factors for Development of Central Nervous System Neoplasms. Neuroepidemiology 1989, 8, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Ali-Osman, F.; Lipp, E.; Marcello, J.E.; McCarthy, B.; McCoy, L.; Rice, T.; Wrensch, M.; Il’Yasova, D. Association between Body Mass Index and Mortality in Patients with Glioblastoma Mutliforme. Cancer Causes Control 2010, 21, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.K.H.; Brunborg, C.; Di Ieva, A.; Lindemann, K.; Johannesen, T.B.; Vatten, L.; Helseth, E.; Zwart, J.A. The Impact of Body Mass Index and Height on the Risk for Glioblastoma and Other Glioma Subgroups: A Large Prospective Cohort Study. Neuro Oncol. 2017, 19, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Rajaraman, P.; Dubrow, R.; Darefsky, A.S.; Koebnick, C.; Hollenbeck, A.; Schatzkin, A.; Leitzmann, M.F. Height, Body Mass Index, and Physical Activity in Relation to Glioma Risk. Cancer Res. 2009, 69, 8349–8355. [Google Scholar] [CrossRef]
- Little, R.B.; Madden, M.H.; Thompson, R.C.; Olson, J.J.; LaRocca, R.V.; Pan, E.; Browning, J.E.; Egan, K.M.; Nabors, L.B. Anthropometric Factors in Relation to Risk of Glioma. Cancer Causes Control 2013, 24, 1025–1031. [Google Scholar] [CrossRef]
- Potharaju, M.; Mangaleswaran, B.; Mathavan, A.; John, R.; Thamburaj, V.; Ghosh, S.; Ganesh, S.; Kalvakonda, C.; Loganathan, M.; Bapu, S.; et al. Body Mass Index as a Prognostic Marker in Glioblastoma Multiforme: A Clinical Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 204–209. [Google Scholar] [CrossRef]
- Sequoia, J.S.P.; Wright, M.E.; McCarron, P.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. A Prospective Investigation of Height and Prostate Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2174–2178. [Google Scholar] [CrossRef][Green Version]
- Tripaldi, R.; Stuppia, L.; Alberti, S. Human Height Genes and Cancer. Biochim. Biophys. Acta Rev. Cancer 2013, 1836, 27–41. [Google Scholar] [CrossRef]
- Perry, J.K.; Liu, D.X.; Wu, Z.S.; Zhu, T.; Lobie, P.E. Growth Hormone and Cancer: An Update on Progress. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Watts, E.L.; Perez-Cornago, A.; Appleby, P.N.; Albanes, D.; Ardanaz, E.; Black, A.; Bueno-de-Mesquita, H.B.; Chan, J.M.; Chen, C.; Chubb, S.A.P.; et al. The Associations of Anthropometric, Behavioural and Sociodemographic Factors with Circulating Concentrations of IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3 in a Pooled Analysis of 16,024 Men from 22 Studies. Int. J. Cancer 2019, 145, 3244–3256. [Google Scholar] [CrossRef] [PubMed]
- Boguszewski, C.L.; Boguszewski, M.C. da S. Growth Hormone’s Links to Cancer. Endocr. Rev. 2019, 40, 558–574. [Google Scholar] [CrossRef]
- Cote, D.J.; Downer, M.K.; Smith, T.R.; Smith-Warner, S.A.; Egan, K.M.; Stampfer, M.J. Height, Waist Circumference, Body Mass Index, and Body Somatotype across the Life Course and Risk of Glioma. Cancer Causes Control 2018, 29, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Wang, S.S.; Melin, B.S.; Wang, Z.; Braganza, M.; Inskip, P.D.; Albanes, D.; Andersson, U.; Beane Freeman, L.E.; Buring, J.E.; et al. Association between Adult Height, Genetic Susceptibility and Risk of Glioma. Int. J. Epidemiol. 2012, 41, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
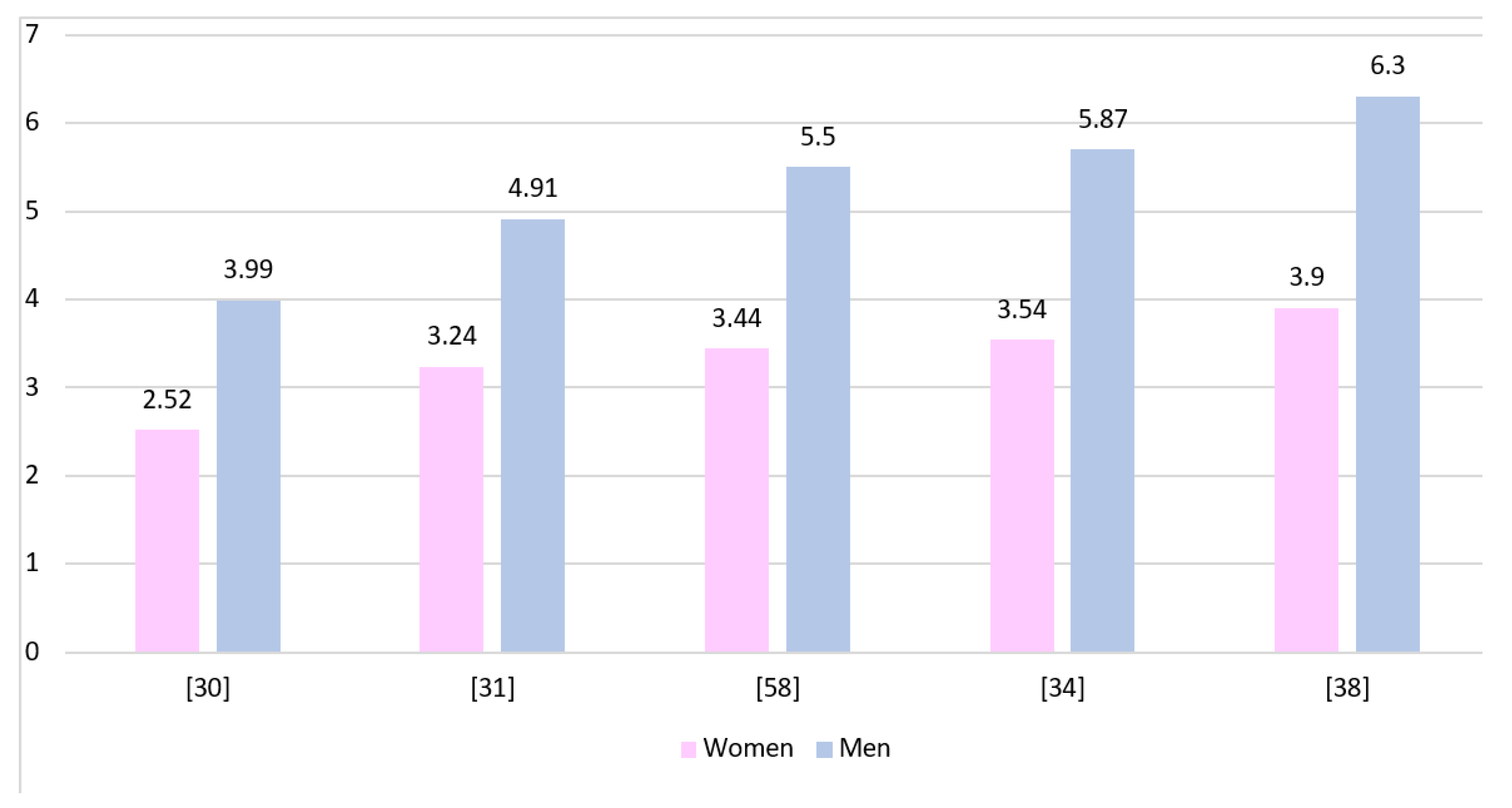
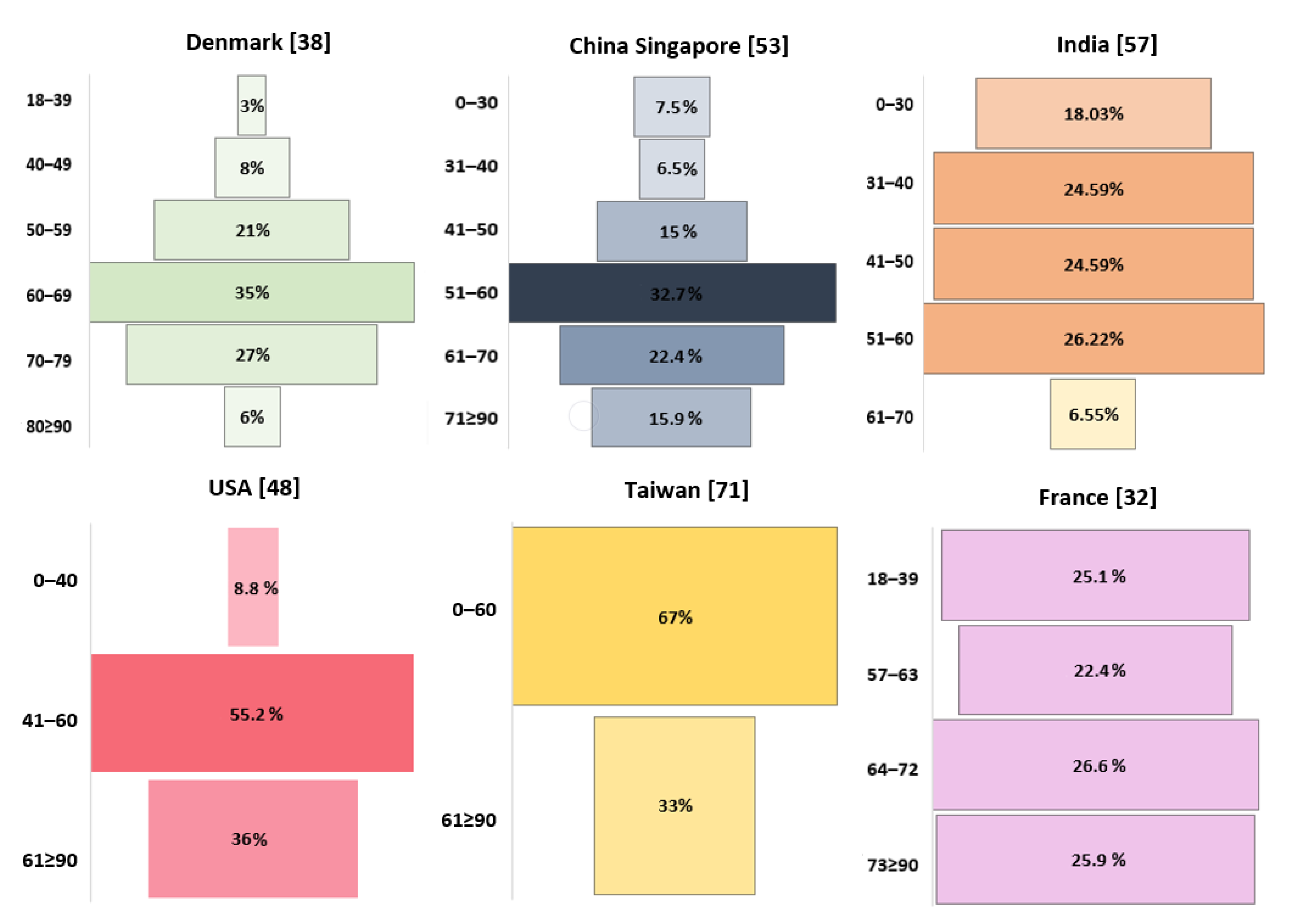
| Country | Dominant Sex | Gender Ratio | Years Range | Population Size | Patient Age | Literature |
|---|---|---|---|---|---|---|
| Adults | ||||||
| USA | Men | 1.148 | 1997–2010 | 3759 | >66 | Burton et al., 2015 [47] |
| USA | Men | 1.347 | 1973–2014 | 49,124 | >20 | K. Li et al., 2018 [48] |
| USA | Men | 1.35 | 2004–2013 | 24,262 | All | Xu et al., 2017 [49] |
| USA | Men | 1.364 | 1985–2014 | 25,117 | 18–60+ | Bin Abdulrahman et al., 2019 [50] |
| USA | Men | 1.375 | 2000–2014 | 6,430,706 | >20 | Gittleman et al., 2018 [33] |
| USA | Men | 1.38 | 2004–2015 | 30,767 | 18–70+ | Xie et al., 2018 [51] |
| USA | Men | 1.395 | 2010–2014 | 33,473 | >18 | Bohn et al., 2018 [36] |
| USA | Men | 1.45 | 2001–2011 | 1826 | >19 | Shabihkhani et al., 2017 [52] |
| Denmark | Men | 1.545 | 2009–2014 | 1364 | >18 | Hansen et al., 2018 [38] |
| China Singapore | Men | 1.547 | 2002–2011 | 107 | 13–85 | Cheo et al., 2017 [53] |
| USA | Men | 1.596 | 2000–2008 | 6586 | 18–70 | Tian et al., 2018 [54] |
| Sweden | Men | 1.6 | 2001–2012 | 143 | 18–99 | Bruhn et al., 2018 [37] |
| Germany | Men | 1.629 | 2011–2014 | 2382 | >18 | De Witt Hamer et al., 2019 [55] |
| China Hong Kong | Men | 1.833 | 2003–2005 2010–2012 | 68 | >18 | Chan et al., 2017 [56] |
| India | Men | 2.588 | 2012–2014 | 61 | 15–68 | Ghosh et al., 2017 [57] |
| Adults and children | ||||||
| Spain | Men | 1.338 | 1993–2014 | 463 | 0–74+ | Fuentes-Raspall et al., 2017 [58] |
| USA | Men | 1.359 | 2010–2014 | 56,421 | All | Ostrom et al., 2017 [30] |
| Canada | Men | 1.459 | 2009–2013 | 5830 | All | Walker et al., 2019 [31] |
| France | Men | 1.5 | 2008–2015 | 2053 | All | Fabbro-Peray et al., 2019 [32] |
| England | Men | 1.503 | 2007–2011 | 10,743 | 0–85+ | Brodbelt et al., 2015 [34] |
| Iran | Men | 2.476 | 2011–2016 | 73 | All | Salehpour et al., 2019 [59] |
| Children | ||||||
| USA | Men | 1.102 | 1973–2013 | 154 | 0–18 | Maxwell et al., 2018 [60] |
| USA | Women | 1.049 | 1973–2013 | 252 | 0–5 | Liu et al., 2018 [39] |
| Men | 1.278 | 1973–2013 | 287 | 6–10 | ||
| Men | 1.438 | 1973–2013 | 634 | 11–19 | ||
| USA | Men | 1.455 | 2000–2010 | 302 | <20 | Lam et al., 2018[61] |
| Country | Frontal Lobe | Temporal Lobe | Parietal Lobe | Occipital Lobe | Cerebellum | Central | Brainstem | Brain | NOS Chamber | NOS Brain | Overlapping Brain Damage | Other | Years Range | Population Size | Literature |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA | 38 | 35 | 21 | 6 | - | - | - | - | - | - | - | - | 2010–2014 | 33,473 | Bohn et al., 2018 [36] |
| England | 24.9 | 21.8 | 16.7 | 4.8 | 0.5 | - | 0.4 | - | - | - | - | 30.9 | 2007–2011 | 10,743 | Brodbelt et al., 2015 [34] |
| USA | 25.25 | 23.6 | 16.7 | 4.2 | 0.5 | - | 0.5 | 3.6 | 0.4 | 0.4 | 17.6 | - | 2000–2008 | 6586 | Tian et al., 2018 [54] |
| Denmark | 30 | 32 | 19 | 11 | - | 8 | - | - | - | - | - | - | 2009–2014 | 1364 | Hansen et al., 2018 [38] |
| USA | 22.8 | 22.8 | 54.3 | - | - | - | - | - | - | - | - | - | 2000–2010 | 302 | Lam et al., 2018 [61] |
| India | 42.62 | 9.83 | 9.83 | 4.91 | - | - | - | - | - | - | - | 31.78 | 2012–2014 | 61 | Ghosh et al., 2017 [57] |
| Taiwan | 31 | 13 | 6 | 9 | 25 | - | - | - | - | - | - | 17 | 2005–2016 | 44 | Shieh et al., 2020 [71] |
| Country | Tested Rate | Observations | Population Size | Literature |
|---|---|---|---|---|
| Norway | Risk factor | No significant interactions | 11,144 | Helseth et al., 1989 [76] |
| Norway | Risk factor | No significant interactions | 3102 | Wiedmann et al., 2017 [78] |
| USA | Risk factor | BMI at 18 correlates with the incidence rate at a later age | 480 | Moore et al., 2009 [79] |
| USA | Risk factor | BMI at 21 correlates with the incidence rate at a later age | 1111 glioma (group with 694 GBM cases) | Little et al., 2013 [80] |
| USA | Overall survival | No significant interactions | 1259 | Jones et al., 2010 [77] |
| India | Prognostic Marker/overall survival | Obesity/overweight improves prognosis | 392 | Potharaju et al., 2018 [81] |
| Country | Tested Rate | OR | Observations | Population Size | Literature |
|---|---|---|---|---|---|
| Norway | Risk factor | 1.36 (+15 cm) | Positive correlation in men | 6391 (men) | Helseth et al., 1989 [76] |
| Norway | Risk factor | 1.18 (+15 cm) | No statistically significant correlation | 4752 (women) | Helseth et al., 1989 [76] |
| USA, Finland, Australia, Sweden | Risk factor | 1.08 (+5 cm) | Positive correlation in men | 405 (men) | Kitahara et al., 2012 [88] |
| USA, Finland, Australia, Sweden | Risk factor | 1.04 (+5 cm) | Positive correlation in women | 308 (women) | Kitahara et al., 2012 [88] |
| Norway | Risk factor | 1.24 (+10 cm) | Positive corelation | 3102 | Wiedmann et al., 2017 [78] |
| USA | Risk factor | 1.07 (+1 inch) | Positive correlation | 321 | Cote et al., 2018 [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simińska, D.; Korbecki, J.; Kojder, K.; Kapczuk, P.; Fabiańska, M.; Gutowska, I.; Machoy-Mokrzyńska, A.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Anthropometric Factors in Glioblastoma Multiforme—Literature Review. Brain Sci. 2021, 11, 116. https://doi.org/10.3390/brainsci11010116
Simińska D, Korbecki J, Kojder K, Kapczuk P, Fabiańska M, Gutowska I, Machoy-Mokrzyńska A, Chlubek D, Baranowska-Bosiacka I. Epidemiology of Anthropometric Factors in Glioblastoma Multiforme—Literature Review. Brain Sciences. 2021; 11(1):116. https://doi.org/10.3390/brainsci11010116
Chicago/Turabian StyleSimińska, Donata, Jan Korbecki, Klaudyna Kojder, Patrycja Kapczuk, Marta Fabiańska, Izabela Gutowska, Anna Machoy-Mokrzyńska, Dariusz Chlubek, and Irena Baranowska-Bosiacka. 2021. "Epidemiology of Anthropometric Factors in Glioblastoma Multiforme—Literature Review" Brain Sciences 11, no. 1: 116. https://doi.org/10.3390/brainsci11010116
APA StyleSimińska, D., Korbecki, J., Kojder, K., Kapczuk, P., Fabiańska, M., Gutowska, I., Machoy-Mokrzyńska, A., Chlubek, D., & Baranowska-Bosiacka, I. (2021). Epidemiology of Anthropometric Factors in Glioblastoma Multiforme—Literature Review. Brain Sciences, 11(1), 116. https://doi.org/10.3390/brainsci11010116