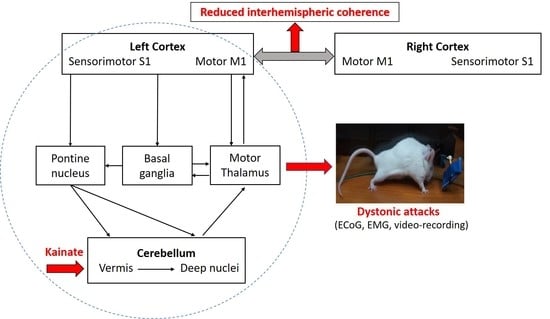
Reduced Interhemispheric Coherence after Cerebellar Vermis Output Perturbation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
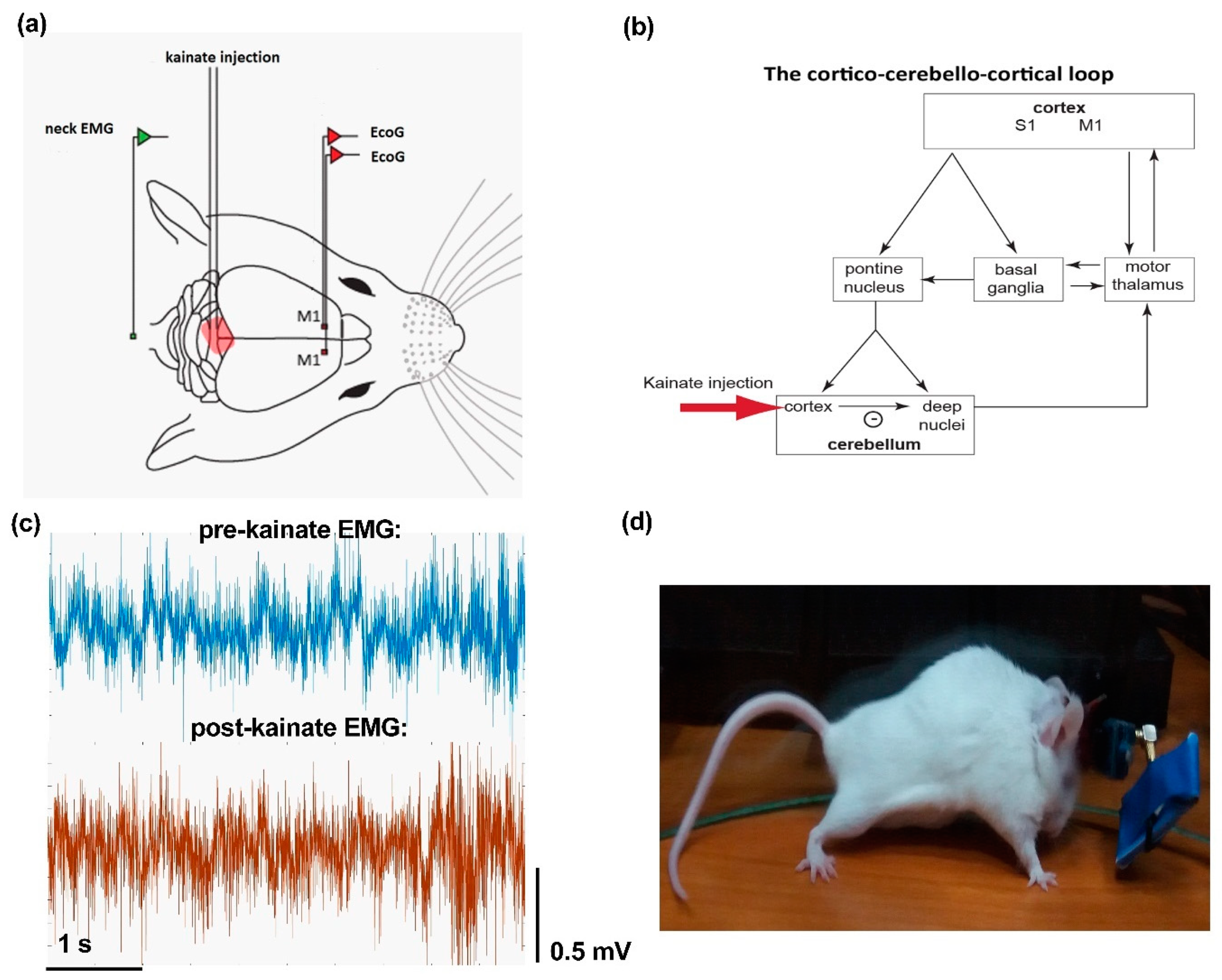
2.2. Surgery
2.3. Kainate Microinjection and ECoG and EMG Recordings
2.4. Data Analysis
3. Results
3.1. Electromyography and Behavior Parameters Reveal Increased Muscular Activity after Cerebellar Kainate Injections
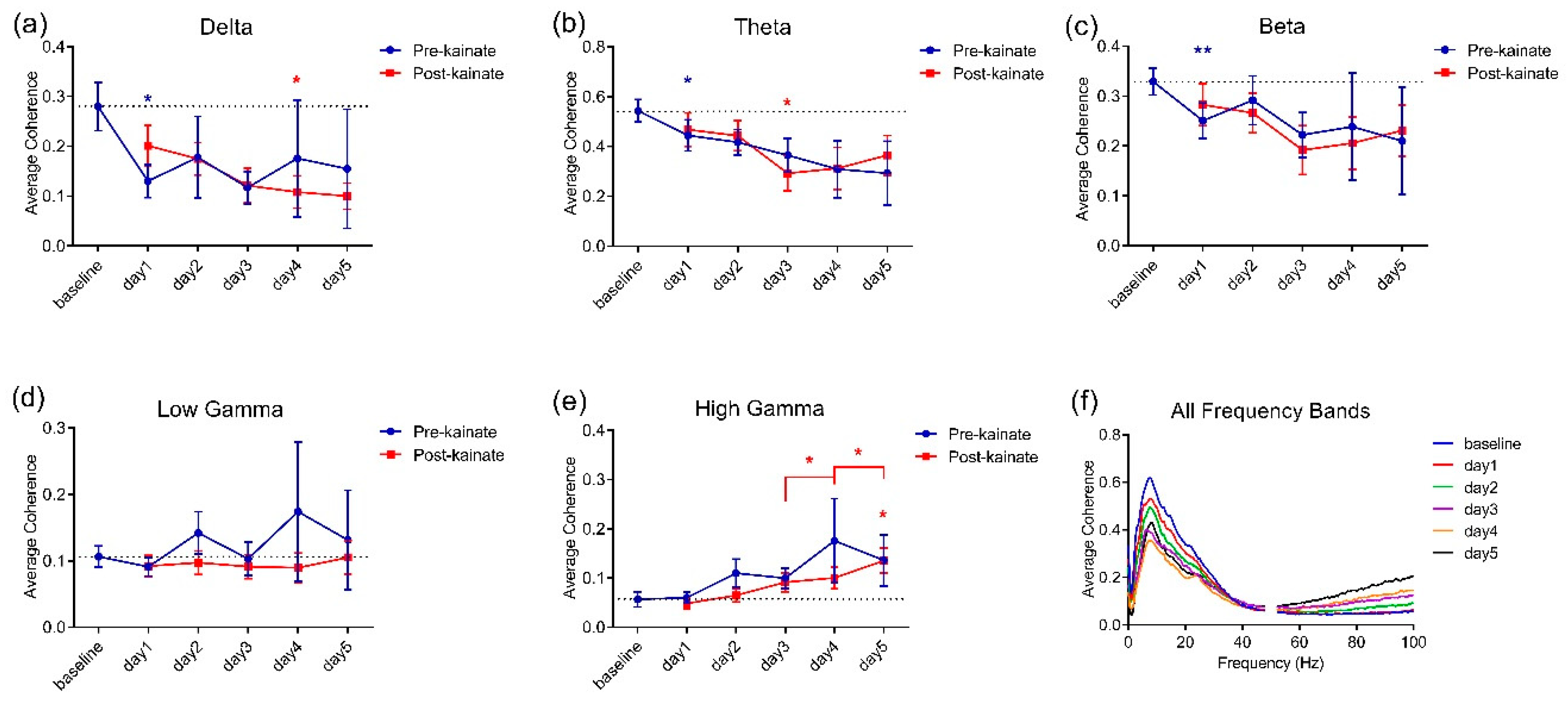
3.2. Interhemispheric Coherence between Primary Motor Cortices before and after Repeated Cerebellar Vermis Kainate Application Was Reduced
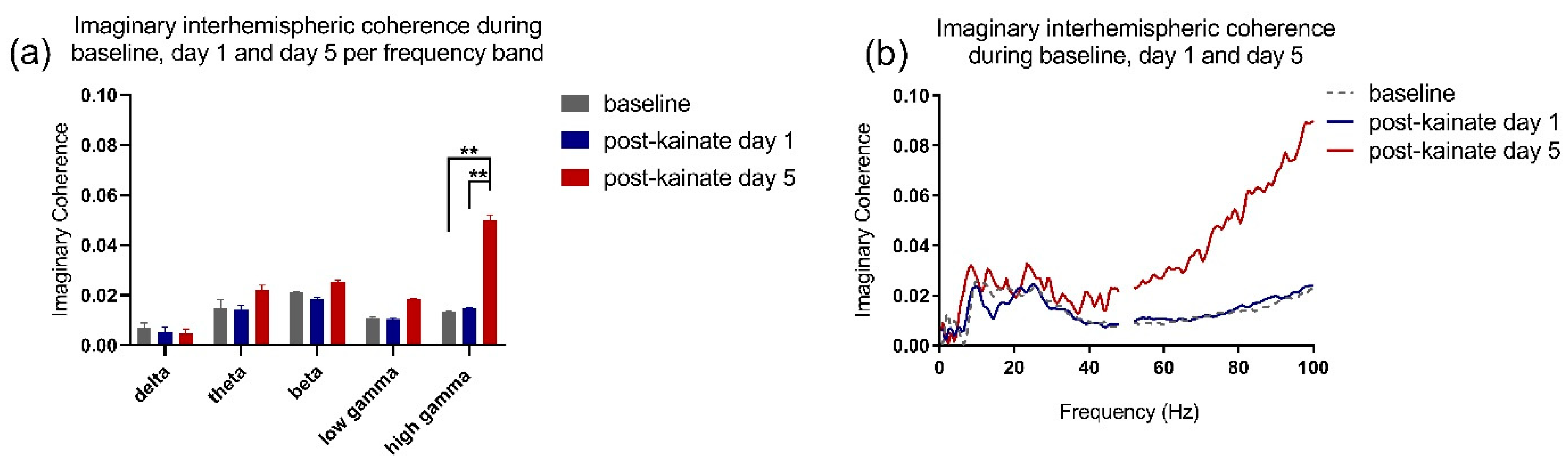
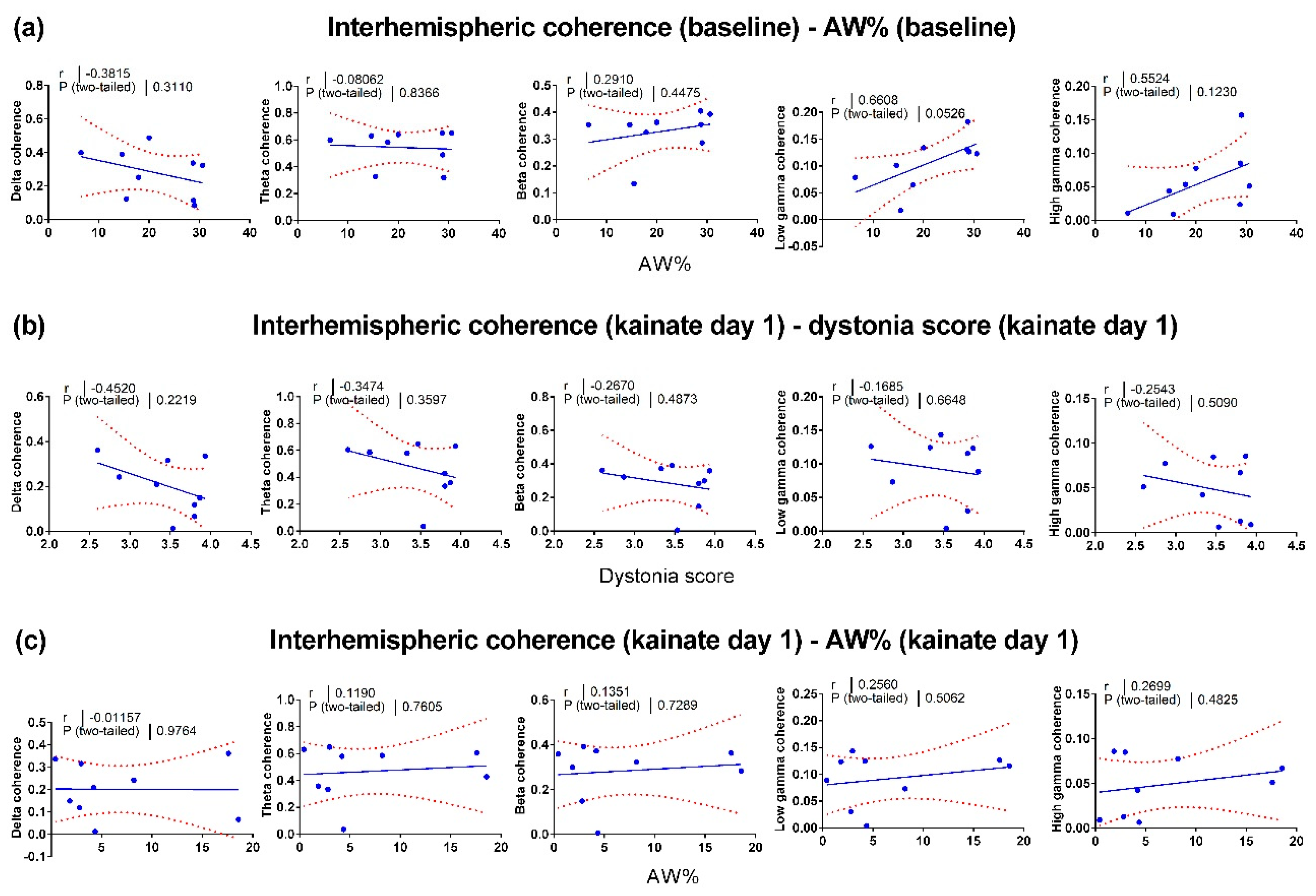
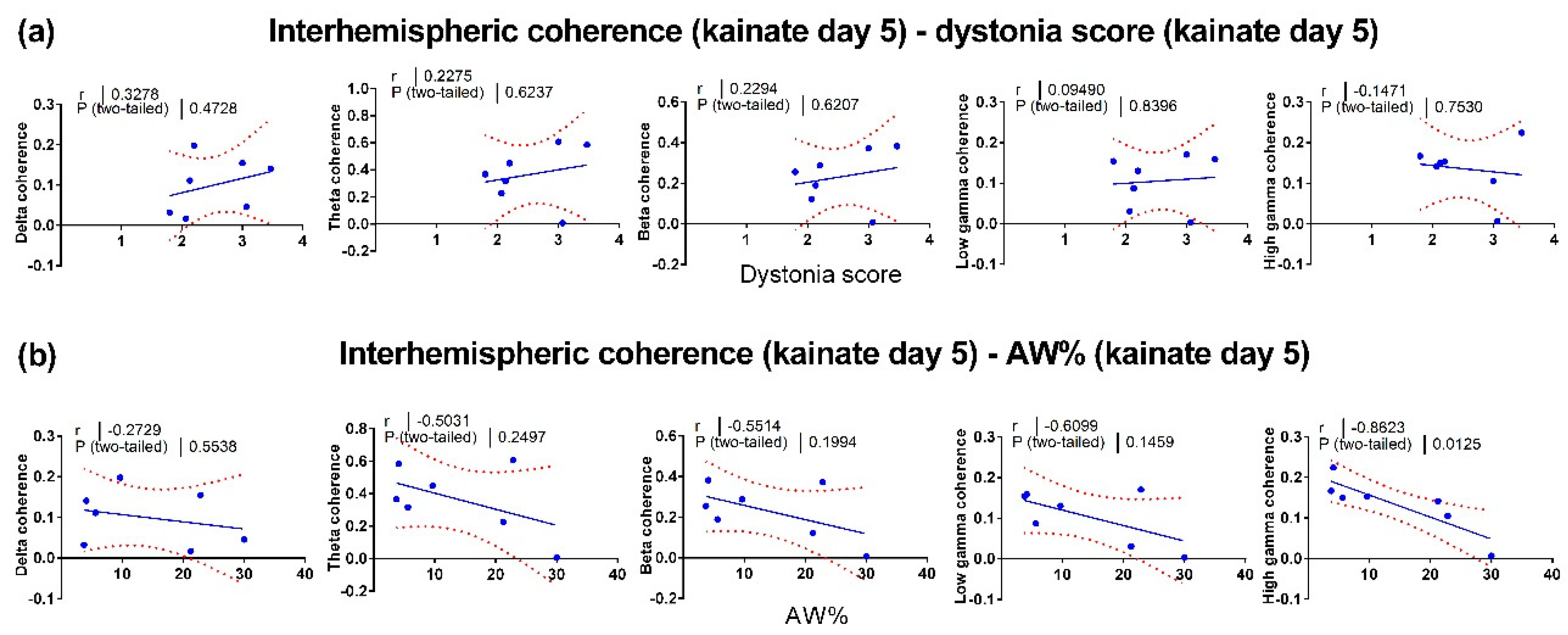
3.3. Correlations between Motor Behavior and Interhemispheric Coherence between Primary Motor Cortices
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006; ISBN 9780195301069. [Google Scholar]
- Melillo, R.; Leisman, G. Autistic Spectrum Disorders as Functional Disconnection Syndrome. Rev. Neurosci. 2009, 20, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Pinto, A.D.; Lang, A.E.; Chen, R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann. Neurol. 2010, 68, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, R.; Murugappan, M.; Ibrahim, N.M.; Kenneth, S.; Iqbal, O.M.; Mohamad, K.; Palaniappan, R.; Satiyan, M. Inter-hemispheric EEG coherence analysis in Parkinson’s disease: Assessing brain activity during emotion processing. J. Neural Transm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Juri, C.; García-Cabezas, M.Á.; Adánez, R. Inter-hemispheric asymmetry of nigrostriatal dopaminergic lesion: A possible compensatory mechanism in Parkinson’s disease. Front. Syst. Neurosci. 2011, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Cash, R.; Dunlop, K.; Vesia, M.; Kucyi, A.; Ghahremani, A.; Downar, J.; Chen, J.; Chen, R. Modulation of cognitive cerebello-cerebral functional connectivity by lateral cerebellar continuous theta burst stimulation. Neuroimage 2017, 158, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Proville, R.D.; Spolidoro, M.; Guyon, N.; Dugué, G.P.; Selimi, F.; Isope, P.; Popa, D.; Léna, C. Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat. Neurosci. 2014, 17, 1233–1239. [Google Scholar] [CrossRef]
- Steriade, M. Two channels in the cerebellothalamocortical system. J. Comp. Neurol. 1995, 354, 57–70. [Google Scholar] [CrossRef]
- D’Angelo, E.; Mazzarello, P.; Prestori, F.; Mapelli, J.; Solinas, S.; Lombardo, P.; Cesana, E.; Gandolfi, D.; Congi, L. The cerebellar network: From structure to function and dynamics. Brain Res. Rev. 2011, 66, 5–15. [Google Scholar] [CrossRef]
- Mann-Metzer, P.; Yarom, Y. Electrotonic coupling synchronizes interneuron activity in the cerebellar cortex. Prog. Brain Res. 2000, 124, 115–122. [Google Scholar] [CrossRef]
- Dugué, G.P.; Brunel, N.; Hakim, V.; Schwartz, E.; Chat, M.; Lévesque, M.; Courtemanche, R.; Léna, C.; Dieudonné, S. Electrical Coupling Mediates Tunable Low-Frequency Oscillations and Resonance in the Cerebellar Golgi Cell Network. Neuron 2009, 61, 126–139. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Hoebeek, F.E.; Schonewille, M. Causes and Consequences of Oscillations in the Cerebellar Cortex. Neuron 2008, 58, 655–658. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E. The critical role of Golgi cells in regulating spatio-temporal integration and plasticity at the cerebellum input stage. Front. Neurosci. 2009, 2, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Forti, L.; Cesana, E.; Mapelli, J.; D’Angelo, E. Ionic mechanisms of autorhythmic firing in rat cerebellar Golgi cells. J. Physiol. 2006, 574, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; DeLong, M.R.; Fahn, S.; Fung, V.S.C.; Hallett, M.; Jankovic, J.; Jinnah, A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A. How many dystonias? Clinical evidence. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef]
- Shakkottai, V.G.; Batla, A.; Bhatia, K.; Dauer, W.T.; Dresel, C.; Niethammer, M.; Eidelberg, D.; Raike, R.S.; Smith, Y.; Jinnah, H.A.; et al. Current Opinions and Areas of Consensus on the Role of the Cerebellum in Dystonia. Cerebellum 2017, 16, 577–594. [Google Scholar] [CrossRef]
- Neychev, V.K.; Fan, X.; Mitev, V.I.; Hess, E.J.; Jinnah, H.A. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain 2008, 131, 2499–2509. [Google Scholar] [CrossRef]
- Fremont, R.; Tewari, A.; Angueyra, C.; Khodakhah, K. A role for cerebellum in the hereditary dystonia DYT1. Elife 2017. [Google Scholar] [CrossRef]
- Tewari, A.; Fremont, R.; Khodakhah, K. It’s not just the basal ganglia: Cerebellum as a target for dystonia therapeutics. Mov. Disord. 2017, 32, 1537–1545. [Google Scholar] [CrossRef]
- Pizoli, C.; Jinnah, H.A.; Billingsley, M.L.; Hess, E.J. Abnormal cerebellar signaling induces dystonia in mice. J. Neurosci. 2002, 22, 7825–7833. [Google Scholar] [CrossRef]
- Brighina, F.; Fierro, B.; Romano, M.; Puma, A.; Giglia, F.; Saia, V.; Giglia, G. Effects of cerebellar TMS on motor cortex of patients with focal dystonia: A preliminary report. Exp. Brain Res. 2009, 192, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Porcacchia, P.; Alvarez de Toledo, A.; Rodrıguez-Baena, A.; Martın-Rodrıguez, J.F.; Palomar, F.J.; Vargas-Gonzalez, L.; Jesus, S.; Koch, G.; Mir, P. Abnormal cerebellar connectivity and plasticity in isolated cervical dystonia. PLoS ONE 2019, 14, e0211367. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, E.L.; Georgescu, I.A.; Zahiu, D.C.; Morozan, V.P.; Pana, A.; Zagrean, A.; Popa, D. Oscillatory cortical activity in an animal model of dystonia caused by cerebellar dysfunction. Front. Cell. Neurosci. 2018, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ilie, A.; Spulber, S.; Avramescu, S.; Nita, D.A.; Zagrean, A.-M.; Zagrean, L.; Moldovan, M. Delayed ischemic electrocortical suppression during rapid repeated cerebral ischemia and kainate-induced seizures in rat. Eur. J. Neurosci. 2006, 23, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Raike, R.S.; Pizoli, C.E.; Weisz, C.; van den Maagdenberg, A.M.J.M.; Jinnah, H.A.; Hess, E.J. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiol. Dis. 2013, 49, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Wenk, G.L. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Rev. 2003, 9, 275–308. [Google Scholar] [CrossRef]
- Jin, S.-H.H.; Lin, P.; Auh, S.; Hallett, M. Abnormal functional connectivity in focal hand dystonia: Mutual information analysis in EEG. Mov. Disord. 2011, 26, 1274–1281. [Google Scholar] [CrossRef]
- Wisden, W.; Seeburg, P.H. A Complex Mosaic of High-Affinity Kainate Receptors in Rat Brain. J. Neurosci. 1993, 13, 3582–3598. [Google Scholar] [CrossRef]
- Nolte, G.; Bai, O.; Wheaton, L.; Mari, Z.; Vorbach, S.; Hallett, M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 2004, 115, 2292–2307. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N. Anatomic Organization of the Basilar Pontine Projections from Prefrontal Cortices in Rhesus Monkey. J. Neurosci. 1997, 17, 438–458. [Google Scholar] [CrossRef]
- Middleton, F.A.; Strick, P.L. Basal-ganglia “projections” to the prefrontal cortex of the primate. J. Neurosci. 2001, 21, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.A.; Corsi-Cabrera, M. EEG coherence or EEG correlation? Int. J. Psychophysiol. 1996, 23, 145–153. [Google Scholar] [CrossRef]
- Lowet, E.; Roberts, M.J.; Bonizzi, P.; Karel, J.; De Weerd, P. Quantifying neural oscillatory synchronization: A comparison between spectral coherence and phase-locking value approaches. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, S.M. Coherence a measure of the brain networks: Past and present. Neuropsychiatr. Electrophysiol. 2016, 2, 1. [Google Scholar] [CrossRef]
- Person, A.L.; Raman, I.M. Synchrony and neural coding in cerebellar circuits. Front. Neural Circuits 2012, 6, 1–32. [Google Scholar] [CrossRef]
- Daskalakis, Z.J.; Paradiso, G.O.; Christensen, B.K.; Fitzgerald, P.B.; Gunraj, C.; Chen, R. Exploring the connectivity between the cerebellum and motor cortex in humans. J. Physiol. 2004, 557, 689–700. [Google Scholar] [CrossRef]
- Gross, J.; Pollok, B.; Dirks, M.; Timmermann, L.; Butz, M.; Schnitzler, A. Task-dependent oscillations during unimanual and bimanual movements in the human primary motor cortex and SMA studied with magnetoencephalography. Neuroimage 2005, 26, 91–98. [Google Scholar] [CrossRef]
- Long, J.; Tazoe, T.; Soteropoulos, D.S.; Perez, M.A. Interhemispheric connectivity during bimanual isometric force generation. J. Neurophysiol. 2016, 1196–1207. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D.; Delong, M.R. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal”; and “limbic”; functions. Prog. Brain Res. 1990, 85, 119–149. [Google Scholar]
- de Carvalho Aguiar, P.M.; Ozelius, L.J. Classification and genetics of dystonia. Neurology 2002, 1, 316–325. [Google Scholar] [CrossRef]
- Dias, F.M.V.; Kummer, A.; Doyle, F.C.P.; Harsányi, E.; Cardoso, F.; Fontenelle, L.F.; Teixeira, A.L. Psychiatric disorders in primary focal dystonia and in Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2011, 7, 111–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lencer, R.; Steinlechner, S.; Stahlberg, J.; Rehling, H.; Orth, M.; Baeumer, T.; Rumpf, H.; Meyer, C.; Klein, C.; Muenchau, A.; et al. Primary focal dystonia: Evidence for distinct neuropsychiatric and personality profiles. J. Neurol. Neurosurg. Psychiatry 2009, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Leisman, G.; Ashkenazi, M. Aetiological factors in dyslexia: Iv. Cerebral hemispheres are functionally equivalent. Neuroscience 1980, 11, 157–164. [Google Scholar] [CrossRef]
- Previc, F.H. Dopamine and the origins of human intelligence. Brain Cogn. 1999, 41, 299–350. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.F.; Braši, J.R.; Singer, H.S.; Schretlen, D.J.; Zhou, Y.; Nandi, A.; Maris, M.A.; Alexander, M.; Ye, W.; Rousset, O.; et al. Mechanisms of Dopaminergic and Serotonergic Neurotransmission in Tourette Syndrome: Clues from an in vivo Neurochemistry Study with PET. Neuropsychopharmacology 2008, 33, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar] [CrossRef]
- Mink, J.W. Basal ganglia dysfunction in Tourette’s syndrome: A new hypothesis. Pediatr. Neurol. 2001, 25, 190–198. [Google Scholar] [CrossRef]
- Ashok, A.H.; Marques, T.R.; Jauhar, S.; Nour, M.M.; Goodwin, G.M.; Young, A.H.; Howes, O.D. The dopamine hypothesis of bipolar affective disorder: The state of the art and implications for treatment. Mol. Psychiatry 2017, 22, 666–679. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Lenz, D.; Junge, S.; Busch, N.A.; Maess, B. Memory matches evoke human gamma-responses. BMC Neurosci. 2004, 5, 1–8. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Jung, J.; Mainy, N.; Dreher, J.C.; Bertrand, O.; Baciu, M.; Minotti, L.; Hoffmann, D.; Kahane, P. Silence is golden: Transient neural deactivation in the prefrontal cortex during attentive reading. Cereb. Cortex 2008, 18, 443–450. [Google Scholar] [CrossRef][Green Version]
- Engel, A.K.; Fries, P.; Singer, W. Dynamic predictions: Oscillations and syncronny in top-down processing. Nat. Rev. Neurosci. 2001, 2, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Riehle, A.; Requin, J. The predictive value for performance speed of preparatory changes in neuronal activity of the monkey motor and premotor cortex. Behav. Brain Res. 1993, 53, 35–49. [Google Scholar] [CrossRef]
- Crammond, D.J.; Kalaska, J.F. Prior information in motor and premotor cortex: Activity during the delay period and effect on pre-movement activity. J. Neurophysiol. 2000, 84, 986–1005. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrate theory of PFC function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Riehle, A.; Grammont, F.; Diesmann, M.; Grün, S. Dynamical changes and temporal precision of synchronized spiking activity in monkey motor cortex during movement preparation. J. Physiol. 2000, 94, 569–582. [Google Scholar] [CrossRef]
- Andreasen, N.C.; O’Leary, D.S.; Cizadlo, T.; Arndt, S.; Rezai, K.; Boles Ponto, L.L.; Watkins, G.L.; Hichwa, R.D. Schizophrenia and cognitive dysmetria: A positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc. Natl. Acad. Sci. USA 1996, 93, 9985–9990. [Google Scholar] [CrossRef]
| Interhemispheric Coherence between Primary Motor Cortices | ||
|---|---|---|
| Frequency Band | Test | p-Value |
| Delta pre-kainate | Mixed-effects model (REML) F (1.953, 15.23) = 0.6940 | 0.5114 |
| day 0 vs. day 1 | Dunnett’s multiple comparisons test (1) | 0.0125 (*) |
| day 0 vs. day 2 | 1 | 0.8189 |
| day 0 vs. day 3 | 1 | 0.0874 |
| day 0 vs. day 4 | 1 | 0.8051 |
| day 0 vs. day 5 | 1 | 0.6919 |
| Delta post-kainate | Mixed-effects model (REML) F (1.662, 12.30) = 4.097 | 0.0492 (*) |
| day 0 vs. day 1 | 1 | 0.0752 |
| day 0 vs. day 2 | 1 | 0.3924 |
| day 0 vs. day 3 | 1 | 0.1531 |
| day 0 vs. day 4 | 1 | 0.0499 (*) |
| day 0 vs. day 5 | 1 | 0.0800 |
| Theta pre-kainate | Mixed-effects model (REML) F (1.553, 12.11) = 1.768 | 0.2129 |
| day 0 vs. day 1 | 1 | 0.0135 (*) |
| day 0 vs. day 2 | 1 | 0.0807 |
| day 0 vs. day 3 | 1 | 0.0833 |
| day 0 vs. day 4 | 1 | 0.1715 |
| day 0 vs. day 5 | 1 | 0.1715 |
| Theta post-kainate | Mixed-effects model (REML) F (2.134, 15.37) = 5.300 | 0.0164 |
| day 0 vs. day 1 | 1 | 0.0985 |
| day 0 vs. day 2 | 1 | 0.1417 |
| day 0 vs. day 3 | 1 | 0.0262 |
| day 0 vs. day 4 | 1 | 0.0868 |
| day 0 vs. day 5 | 1 | 0.1221 |
| Beta pre-kainate | Mixed-effects model (REML) F (1.538, 12.00) = 0.5601 | 0.5414 |
| day 0 vs. day 1 | 1 | 0.0029 (**) |
| day 0 vs. day 2 | 1 | 0.9615 |
| day 0 vs. day 3 | 1 | 0.0986 |
| day 0 vs. day 4 | 1 | 0.8681 |
| day 0 vs. day 5 | 1 | 0.7087 |
| Beta post-kainate | Mixed-effects model (REML) F (2.265, 16.76) = 2.576 | 0.1006 |
| day 0 vs. day 1 | 1 | 0.1348 |
| day 0 vs. day 2 | 1 | 0.1190 |
| day 0 vs. day 3 | 1 | 0.1300 |
| day 0 vs. day 4 | 1 | 0.1999 |
| day 0 vs. day 5 | 1 | 0.1267 |
| Low gamma pre-kainate | Mixed-effects model (REML) F (0.9834, 7.670) = 0.7102 | 0.4225 |
| day 0 vs. day 1 | 1 | 0.2172 |
| day 0 vs. day 2 | 1 | 0.7971 |
| day 0 vs. day 3 | 1 | 0.9998 |
| day 0 vs. day 4 | 1 | 0.9465 |
| day 0 vs. day 5 | 1 | 0.9955 |
| Low gamma post-kainate | Mixed-effects model (REML) F (1.458, 10.79) = 0.6411 | 0.4982 |
| day 0 vs. day 1 | 1 | 0.5557 |
| day 0 vs. day 2 | 1 | 0.7443 |
| day 0 vs. day 3 | 1 | 0.7968 |
| day 0 vs. day 4 | 1 | 0.6618 |
| day 0 vs. day 5 | 1 | 0.9999 |
| High gamma pre-kainate | Mixed-effects model (REML) F (1.253, 9.772) = 1.800 | 0.2140 |
| day 0 vs. day 1 | 1 | 0.9370 |
| day 0 vs. day 2 | 1 | 0.4573 |
| day 0 vs. day 3 | 1 | 0.1390 |
| day 0 vs. day 4 | 1 | 0.5823 |
| day 0 vs. day 5 | 1 | 0.4789 |
| High gamma post-kainate | Mixed-effects model (REML) F (2.549, 18.86) = 5.603 | 0.0084 (**) |
| day 0 vs. day 1 | 1 | 0.8771 |
| day 0 vs. day 2 | 1 | 0.9507 |
| day 0 vs. day 3 | 1 | 0.5170 |
| day 0 vs. day 4 | 1 | 0.1885 |
| day 0 vs. day 5 | 1 | 0.0102 (*) |
| Delta pre-kainate | ||
| day 3 vs. day 4 | Wilcoxon matched-pairs signed rank test (2) | 0.6406 |
| day 4 vs. day 5 | 2 | 0.2969 |
| Delta post-kainate | ||
| day 3 vs. day 4 | 2 | 0.8438 |
| day 4 vs. day 5 | 2 | 0.2188 |
| Theta pre-kainate | ||
| day 3 vs. day 4 | 2 | 0.3828 |
| day 4 vs. day 5 | 2 | 0.5781 |
| Theta post-kainate | ||
| day 3 vs. day 4 | 2 | >0.9999 |
| day 4 vs. day 5 | 2 | 0.4375 |
| Beta pre-kainate | ||
| day 3 vs. day 4 | 2 | 0.7422 |
| day 4 vs. day 5 | 2 | 0.1094 |
| Beta post-kainate | ||
| day 3 vs. day 4 | 2 | >0.9999 |
| day 4 vs. day 5 | 2 | 0.3750 |
| Low gamma pre-kainate | ||
| day 3 vs. day 4 | 2 | 0.8438 |
| day 4 vs. day 5 | 2 | 0.4688 |
| Low gamma post-kainate | ||
| day 3 vs. day 4 | 2 | >0.9999 |
| day 4 vs. day 5 | 2 | 0.3750 |
| High gamma pre-kainate | ||
| day 3 vs. day 4 | 2 | 0.1953 |
| day 4 vs. day 5 | 2 | >0.9999 |
| High gamma post-kainate | ||
| day 3 vs. day 4 | 2 | 0.0156(*) |
| day 4 vs. day 5 | 2 | 0.0313(*) |
| Imaginary Interhemispheric Coherence between Primary Motor Cortices | ||
|---|---|---|
| Frequency Band | Test | p-Value |
| Delta post-kainate | Mixed-effects model (REML) F (0.9522, 7.617) = 0.3628 | 0.5541 |
| day 0 vs. day 1 | Tukey’s multiple comparisons test (3) | 0.8356 |
| day 0 vs. day 5 | 3 | 0.9514 |
| day 1 vs. day 5 | 3 | 0.9885 |
| Theta post-kainate | Mixed-effects model (REML) F (0.8259, 6.607) = 0.001127 | 0.9543 |
| day 0 vs. day 1 | 3 | 0.9019 |
| day 0 vs. day 5 | 3 | 0.9617 |
| day 1 vs. day 5 | 3 | 0.8733 |
| Beta post-kainate | Mixed-effects model (REML) F (1.316, 10.53) = 0.2092 | 0.7226 |
| day 0 vs. day 1 | 3 | 0.5070 |
| day 0 vs. day 5 | 3 | 0.9933 |
| day 1 vs. day 5 | 3 | 0.7590 |
| Low gamma post-kainate | Mixed-effects model (REML) F (0.9655, 7.724) = 2.428 | 0.1591 |
| day 0 vs. day 1 | 3 | 0.6644 |
| day 0 vs. day 5 | 3 | 0.5433 |
| day 1 vs. day 5 | 3 | 0.0896 |
| High gamma post-kainate | Mixed-effects model (REML) F (1.082, 8.658) = 17.85 | 0.0022 (**) |
| day 0 vs. day 1 | 3 | 0.7502 |
| day 0 vs. day 5 | 3 | 0.0093 (**) |
| day 1 vs. day 5 | 3 | 0.0028 (**) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgescu Margarint, E.L.; Georgescu, I.A.; Zahiu, C.-D.-M.; Șteopoaie, A.R.; Tirlea, S.-A.; Popa, D.; Zagrean, A.-M.; Zagrean, L. Reduced Interhemispheric Coherence after Cerebellar Vermis Output Perturbation. Brain Sci. 2020, 10, 621. https://doi.org/10.3390/brainsci10090621
Georgescu Margarint EL, Georgescu IA, Zahiu C-D-M, Șteopoaie AR, Tirlea S-A, Popa D, Zagrean A-M, Zagrean L. Reduced Interhemispheric Coherence after Cerebellar Vermis Output Perturbation. Brain Sciences. 2020; 10(9):621. https://doi.org/10.3390/brainsci10090621
Chicago/Turabian StyleGeorgescu Margarint, Elena Laura, Ioana Antoaneta Georgescu, Carmen-Denise-Mihaela Zahiu, Alexandru Răzvan Șteopoaie, Stefan-Alexandru Tirlea, Daniela Popa, Ana-Maria Zagrean, and Leon Zagrean. 2020. "Reduced Interhemispheric Coherence after Cerebellar Vermis Output Perturbation" Brain Sciences 10, no. 9: 621. https://doi.org/10.3390/brainsci10090621
APA StyleGeorgescu Margarint, E. L., Georgescu, I. A., Zahiu, C.-D.-M., Șteopoaie, A. R., Tirlea, S.-A., Popa, D., Zagrean, A.-M., & Zagrean, L. (2020). Reduced Interhemispheric Coherence after Cerebellar Vermis Output Perturbation. Brain Sciences, 10(9), 621. https://doi.org/10.3390/brainsci10090621