The Role of Hp-NCL Network in Goal-Directed Routing Information Encoding of Bird: A Review
Abstract
1. Introduction
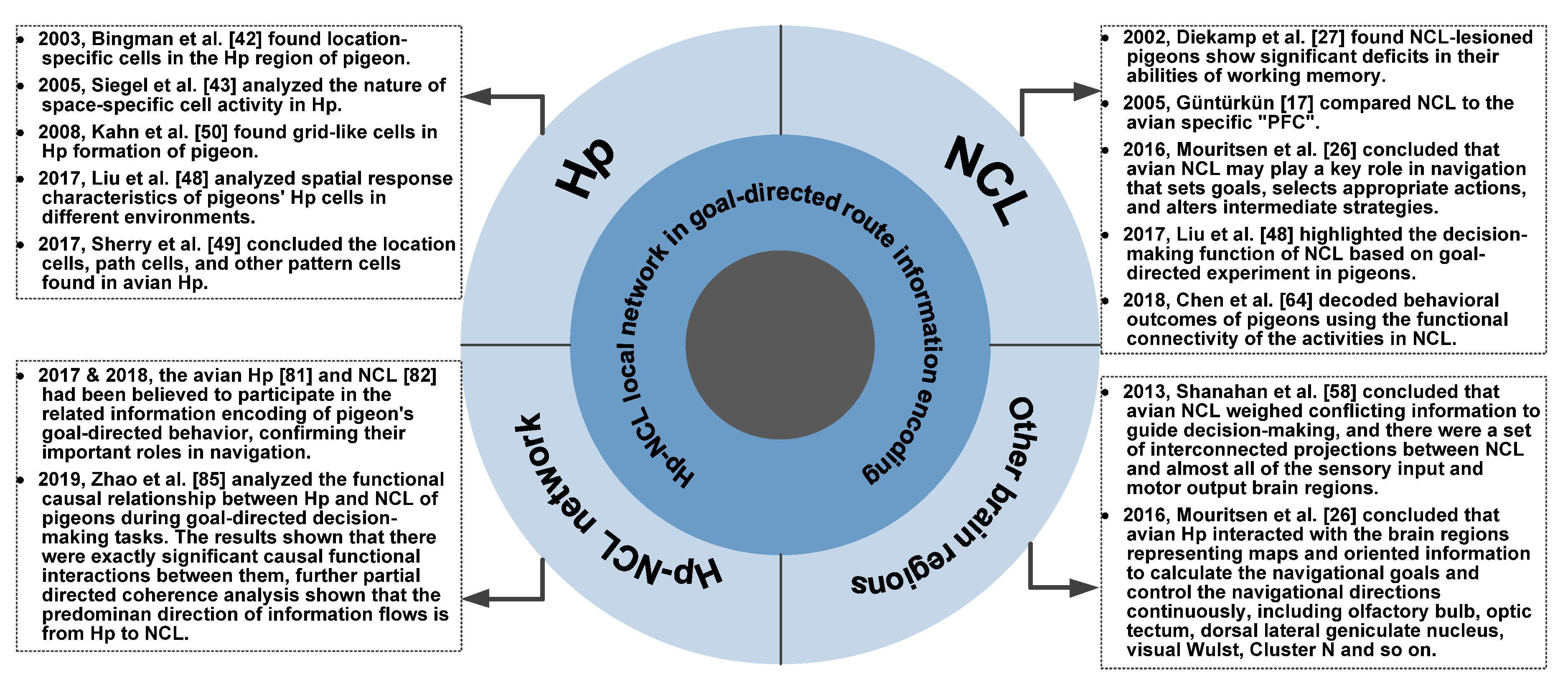
2. The Role of Hp in Goal-Directed Route Information Encoding
3. The Role of NCL in Goal-Directed Route Information Encoding
4. The Role of Hp-NCL Local Network in Goal-Directed Route Information Encoding
5. The Interactions Between Hp-NCL Network and Other Brain Regions
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Buschman, T.J.; Miller, E.K. Goal-direction and top-down control. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130471. [Google Scholar] [CrossRef] [PubMed]
- Milford, M.; Schulz, R. Principles of goal-directed spatial robot navigation in biomimetic models. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130484. [Google Scholar] [CrossRef] [PubMed]
- Verschure, P.F.; Pennartz, C.M.; Pezzulo, G. The why, what, where, when and how of goal-directed choice: Neuronal and computational principles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130483. [Google Scholar] [CrossRef] [PubMed]
- Tolman, E.C. Cognitive maps in rats and men. Psychol. Rev. 1948, 55, 189–208. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Fyhn, M.; Molden, S.; Witter, M.; Moser, E.; Moser, M. Spatial representation in the entorhinal cortex. Science 2004, 305, 1258–1264. [Google Scholar] [CrossRef]
- Taube, J.S.; Muller, R.U.; Ranck, J.J. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 1990, 10, 420–435. [Google Scholar] [CrossRef]
- Solstad, T.; Boccara, C.N.; Kropff, E.; Moser, M.; Moser, E.I. Representation of geometric borders in the entorhinal cortex. Science 2008, 322, 1865–1868. [Google Scholar] [CrossRef]
- Kropff, E.; Carmichael, J.E.; Moser, M.; Moser, E.I. Speed cells in the medial entorhinal cortex. Nature 2015, 523, 419–424. [Google Scholar] [CrossRef]
- Ólafsdóttir, H.F.; Bush, D.; Barry, C. The role of hippocampal replay in memory and planning. Curr. Biol. 2018, 28, R37–R50. [Google Scholar] [CrossRef]
- Wirt, R.; Hyman, J. Integrating spatial working memory and remote memory: Interactions between the medial prefrontal cortex and hippocampus. Brain Sci. 2017, 7, 43. [Google Scholar] [CrossRef]
- Spiers, H.J.; Burgess, N.; Hartley, T.; Vargha-Khadem, F.; O’Keefe, J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus 2001, 11, 715–725. [Google Scholar] [CrossRef]
- Winocur, G.; Moscovitch, M.; Rosenbaum, R.S.; Sekeres, M. An investigation of the effects of hippocampal lesions in rats on pre- and postoperatively acquired spatial memory in a complex environment. Hippocampus 2010, 20, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Hirel, J.; Gaussier, P.; Quoy, M.; Banquet, J.P.; Save, E.; Poucet, B. The hippocampo-cortical loop: Spatio-temporal learning and goal-oriented planning in navigation. Neural Netw. 2013, 43, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.T. Prefrontal-hippocampal interactions for spatial navigation. Neurosci. Res. 2018, 129, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Case, J.; Crone, N.E.; Chang, E.F.; King-Stephens, D.; Laxer, K.D.; Weber, P.B.; Parvizi, J.; Knight, R.T.; Shestyuk, A.Y. Persistent neuronal activity in human prefrontal cortex links perception and action. Nat. Hum. Behav. 2018, 2, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O. The avian ‘prefrontal cortex’ and cognition. Curr. Opin. Neurobiol. 2005, 15, 686–693. [Google Scholar] [CrossRef]
- Poucet, B.; Chaillan, F.; Truchet, B.; Save, E.; Sargolini, F.; Hok, V. Is there a pilot in the brain? Contribution of the self-positioning system to spatial navigation. Front. Behav. Neurosci. 2015, 9, 292. [Google Scholar] [CrossRef]
- Hyman, J.M.; Ma, L.; Balaguer-Ballester, E.; Durstewitz, D.; Seamans, J.K. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc. Natl. Acad. Sci. USA 2012, 109, 5086–5091. [Google Scholar] [CrossRef]
- Lengersdorf, D.; Pusch, R.; Güntürkün, O.; Stüttgen, M.C. Neurons in the pigeon nidopallium caudolaterale signal the selection and execution of perceptual decisions. Eur. J. Neurosci. 2014, 40, 3316–3327. [Google Scholar] [CrossRef]
- Herold, C.; Bingman, V.P.; Ströckens, F.; Letzner, S.; Sauvage, M.; Palomero-Gallagher, N.; Zilles, K.; Güntürkün, O. Distribution of neurotransmitter receptors and zinc in the pigeon (Columba livia) hippocampal formation: A basis for further comparison with the mammalian hippocampus. J. Comp. Neurol. 2014, 522, 2553–2575. [Google Scholar] [CrossRef] [PubMed]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef]
- Niessner, C.; Denzau, S.; Peichl, L.; Wiltschko, W.; Wiltschko, R. Magnetoreception in birds: I. immunohistochemical studies concerning the cryptochrome cycle. J. Exp. Biol. 2014, 217, 4221–4224. [Google Scholar] [CrossRef] [PubMed]
- Wiltschko, R.; Gehring, D.; Denzau, S.; Niessner, C.; Wiltschko, W. Magnetoreception in birds: II. behavioural experiments concerning the cryptochrome cycle. J. Exp. Biol. 2014, 217, 4225–4228. [Google Scholar] [CrossRef] [PubMed]
- Walcott, C.; Wiltschko, W.; Wiltschko, R.; Zupanc, G.K.H. Olfactory navigation versus olfactory activation: A controversy revisited. J. Comp. Physiol. A 2018, 204, 703–706. [Google Scholar] [CrossRef]
- Mouritsen, H.; Heyers, D.; Güntürkün, O. The neural basis of long-distance navigation in birds. Annu. Rev. Physiol. 2016, 78, 133–154. [Google Scholar] [CrossRef]
- Diekamp, B.; Gagliardo, A.; Güntürkün, O. Nonspatial and subdivision-specific working memory deficits after selective lesions of the avian prefrontal cortex. J. Neurosci. 2002, 22, 9573–9580. [Google Scholar] [CrossRef]
- Gagliardo, A.; Ioalè, P.; Savini, M.; Dell Omo, G.; Bingman, V.P. Hippocampal-dependent familiar area map supports corrective re-orientation following navigational error during pigeon homing: A GPS-tracking study. Eur. J. Neurosci. 2009, 29, 2389–2400. [Google Scholar] [CrossRef]
- O’Keefe, J. Place units in the hippocampus of the freely moving rat. Exp. Neurol. 1976, 51, 78–109. [Google Scholar] [CrossRef]
- O’Keefe, J.; Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 1993, 3, 317–330. [Google Scholar] [CrossRef]
- Ainge, J.A.; Tamosiunaite, M.; Wörgötter, F.; Dudchenko, P.A. Hippocampal place cells encode intended destination, and not a discriminative stimulus, in a conditional T-maze task. Hippocampus 2012, 22, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Stachenfeld, K.L.; Botvinick, M.M.; Gershman, S.J. The hippocampus as a predictive map. Nat. Neurosci. 2017, 20, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.R.; Dannenberg, H.; Alexander, A.S.; Hasselmo, M.E. Neural mechanisms of navigation involving interactions of cortical and subcortical structures. J. Neurophysiol. 2018, 119, 2007–2029. [Google Scholar] [CrossRef]
- Porter, B.S.; Schmidt, R.; Bilkey, D.K. Hippocampal place cell encoding of sloping terrain. Hippocampus 2018, 28, 767–782. [Google Scholar] [CrossRef]
- Mattar, M.G.; Daw, N.D. Prioritized memory access explains planning and hippocampal replay. Nat. Neurosci. 2018, 21, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Lopes-dos-Santos, V.; van de Ven, G.M.; Morley, A.; Trouche, S.; Campo-Urriza, N.; Dupret, D. Parsing hippocampal theta oscillations by nested spectral components during spatial exploration and memory-guided behavior. Neuron 2018, 100, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Maboudi, K.; Ackermann, E.; de Jong, L.W.; Pfeiffer, B.E.; Foster, D.; Diba, K.; Kemere, C. Uncovering temporal structure in hippocampal output patterns. eLife 2018, 7, e34467. [Google Scholar] [CrossRef] [PubMed]
- Haga, T.; Fukai, T. Recurrent network model for learning goal-directed sequences through reverse replay. eLife 2018, 7, e34171. [Google Scholar] [CrossRef]
- Bingman, V.P.; Muzio, R.N. Reflections on the Structural-Functional Evolution of the Hippocampus: What Is the Big Deal about a Dentate Gyrus? Brain Behav. Evol. 2017, 90, 53–61. [Google Scholar] [CrossRef]
- Herold, C.; Schloemer, P.; Mafoppa-Fomat, I.; Mehlhorn, J.; Amunts, K.; Axer, M. The hippocampus of birds in a view of evolutionary connectomics. Cortex 2019, 118, 165–187. [Google Scholar] [CrossRef]
- Mayer, U.; Bhushan, R.; Vallortigara, G.; Lee, S.A. Representation of environmental shape in the hippocampus of domestic chicks (Gallus gallus). Brain Struct. Funct. 2018, 223, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Bingman, V.P.; Hough, G.E., II; Kahn, M.C.; Siegel, J.J. The homing pigeon hippocampus and space: In search of adaptive specialization. Brain Behav. Evol. 2003, 62, 117–127. [Google Scholar] [CrossRef]
- Siegel, J.J.; Nitz, D.; Bingman, V.P. Spatial-specificity of single-units in the hippocampal formation of freely moving homing pigeons. Hippocampus 2005, 15, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Coppola, V.J.; Bingman, V.P. The maturation of research into the avian hippocampal formation: Recent discoveries from one of the nature’s foremost navigators. Hippocampus 2015, 25, 1193–1211. [Google Scholar] [CrossRef] [PubMed]
- Mayer, U.; Watanabe, S.; Bischof, H.J. Hippocampal activation of immediate early genes Zenk and c-Fos in zebra finches (Taeniopygia guttata) during learning and recall of a spatial memory task. Neurobiol. Learn. Mem. 2010, 93, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Mayer, U.; Bischof, H.J. Visual Wulst analyses “where” and entopallium analyses “what” in the zebra finch visual system. Behav. Brain Res. 2011, 222, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Mayer, U.; Pecchia, T.; Bingman, V.P.; Flore, M.; Vallortigara, G. Hippocampus and medial striatum dissociation during goal navigation by geometry or features in the domestic chick: An immediate early gene study. Hippocampus 2016, 40, 27–40. [Google Scholar] [CrossRef]
- Liu, X.; Wan, H.; Chen, X.; Shang, Z.; Shi, L.; Li, S.; Chen, Y.; Nie, J. Response properties of place cells in the hippocampus of freely moving pigeons. Sci. Sin. Vitae 2017, 47, 292–304. (In Chinese) [Google Scholar]
- Sherry, D.F.; Grella, S.L.; Guigueno, M.F.; White, D.J.; Marrone, D.F. Are there place cells in the avian hippocampus? Brain Behav. Evol. 2017, 90, 73–80. [Google Scholar] [CrossRef]
- Kahn, M.C.; Siegel, J.J.; Jechura, T.J.; Bingman, V.P. Response properties of avian hippocampal formation cells in an environment with unstable goal locations. Behav. Brain Res. 2008, 191, 153–163. [Google Scholar] [CrossRef]
- Lormant, F.; Cornilleau, F.; Constantin, P.; Meurisse, M.; Lansade, L.; Leterrier, C.; Levy, F.; Calandreau, L. Research note: Role of the hippocampus in spatial memory in Japanese quail. Poult. Sci. 2020, 99, 61–66. [Google Scholar] [CrossRef]
- Bingman, V.P.; Cheng, K. Mechanisms of animal global navigation: Comparative perspectives and enduring challenges. Ethol. Ecol. Evol. 2005, 17, 295–318. [Google Scholar] [CrossRef]
- Floresco, S.B.; Seamans, J.K.; Phillips, A.G. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci. 1997, 17, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Horst, N.K.; Laubach, M. Working with memory: Evidence for a role for the medial prefrontal cortex in performance monitoring during spatial delayed alternation. J. Neurophysiol. 2012, 108, 3276–3288. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hyman, J.M.; Durstewitz, D.; Phillips, A.G.; Seamans, J.K. A quantitative analysis of context-dependent remapping of medial frontal cortex neurons and ensembles. J. Neurosci. 2016, 36, 8258–8272. [Google Scholar] [CrossRef] [PubMed]
- Negrón-Oyarzo, I.; Espinosa, N.; Aguilar-Rivera, M.; Fuenzalida, M.; Aboitiz, F.; Fuentealba, P. Coordinated prefrontal-hippocampal activity and navigation strategy-related prefrontal firing during spatial memory formation. Proc. Natl. Acad. Sci. USA 2018, 115, 7123–7128. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Perkel, D.J.; Bruce, L.L.; Butler, A.B.; Csillag, A.; Kuenzel, W.; Medina, L.; Paxinos, G.; Shimizu, T.; Striedter, G.; et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004, 473, 377–414. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, M.; Bingman, V.P.; Shimizu, T.; Wild, M.; Güntürkün, O. Large-scale network organization in the avian forebrain: A connectivity matrix and theoretical analysis. Front. Comput. Neurosci. 2013, 7, 89. [Google Scholar] [CrossRef]
- Hartmann, B.; Güntürkün, O. Selective deficits in reversal learning after neostriatum caudolaterale lesions in pigeons: Possible behavioral equivalencies to the mammalian prefrontal system. Behav. Brain Res. 1998, 96, 125–133. [Google Scholar] [CrossRef]
- Mouritsen, H. Long-distance navigation and magnetoreception in migratory animals. Nature 2018, 558, 50–59. [Google Scholar] [CrossRef]
- Rinnert, P.; Kirschhock, M.E.; Nieder, A. Neuronal correlates of spatial working memory in the endbrain of crows. Curr. Biol. 2019, 29, 2616–2624. [Google Scholar] [CrossRef]
- Liu, X.; Wan, H.; Li, S.; Shang, Z.; Shi, L. The role of nidopallium caudolaterale in the goal-directed behavior of pigeons. Behav. Brain Res. 2017, 326, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, K.; Wang, D.; Ping, Y.; Wan, H. Goal-directed behavior elevates gamma oscillations in nidopallium caudolaterale of pigeon. Brain Res. Bull. 2018, 137, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Li, S.; Wan, H. Decoding pigeon behavior outcomes using functional connections among local field potentials. Comput. Intell. Neurosci. 2018, 2018, 3505371. [Google Scholar] [CrossRef] [PubMed]
- Dykes, M.; Klarer, A.; Porter, B.; Rose, J.; Colombo, M. Neurons in the pigeon nidopallium caudolaterale display value-related activity. Sci. Rep. 2018, 8, 5377. [Google Scholar] [CrossRef]
- Dykes, M.; Porter, B.; Colombo, M. Neurons in the pigeon nidopallium caudolaterale, but not the corticoidea dorsolateralis, display value and effort discounting activity. Sci. Rep. 2019, 9, 15677. [Google Scholar] [CrossRef]
- Johnston, M.; Clarkson, A.N.; Gowing, E.K.; Scarf, D.; Colombo, M. Effects of nidopallium caudolaterale inactivation on serial-order behavior in pigeons (Columba livia). J. Neurophysiol. 2018, 120, 1143–1152. [Google Scholar] [CrossRef]
- Johnston, M.; Porter, B.; Colombo, M. Nidopallium caudolaterale neuronal responses during serial-order behaviour in pigeons. Behav. Brain Res. 2020, 378, 112269. [Google Scholar] [CrossRef]
- Goodroe, S.C.; Starnes, J.; Brown, T.I. The complex nature of hippocampal-striatal interactions in spatial navigation. Front. Hum. Neurosci. 2018, 12, 250. [Google Scholar] [CrossRef]
- Cholvin, T.; Hok, V.; Giorgi, L.; Chaillan, F.A.; Poucet, B. Ventral midline thalamus is necessary for hippocampal place field stability and cell firing modulation. J. Neurosci. 2017, 38, 158–172. [Google Scholar] [CrossRef]
- Jones, M.W.; Wilson, M.A. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005, 3, e402. [Google Scholar] [CrossRef] [PubMed]
- Avigan, P.D.; Cammack, K.; Shapiro, M.L. Flexible spatial learning requires both the dorsal and ventral hippocampus and their functional interactions with the prefrontal cortex. Hippocampus 2020, 30, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Tuscher, J.J.; Taxier, L.R.; Fortress, A.M.; Frick, K.M. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol. Learn. Mem. 2018, 156, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Hok, V.; Save, E.; Lenck-Santini, P.P.; Poucet, B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc. Natl. Acad. Sci. USA 2005, 102, 4602–4607. [Google Scholar] [CrossRef] [PubMed]
- Hok, V.; Lenck-Santini, P.P.; Roux, S.; Save, E.; Muller, R.U.; Poucet, B. Goal-related activity in hippocampal place cells. J. Neurosci. 2007, 27, 472–482. [Google Scholar] [CrossRef]
- Liu, T.; Bai, W.; Xia, M.; Tian, X. Directional hippocampal-prefrontal interactions during working memory. Behav. Brain Res. 2018, 338, 1–8. [Google Scholar] [CrossRef]
- Zielinski, M.C.; Shin, J.D.; Jadhav, S.P. Coherent coding of spatial position mediated by theta oscillations in the hippocampus and prefrontal cortex. J. Neurosci. 2019, 39, 4550–4565. [Google Scholar] [CrossRef]
- Shin, J.D.; Tang, W.; Jadhav, S.P. Dynamics of awake hippocampal-prefrontal replay for spatial learning and memory-guided decision making. Neuron 2019, 104, 1110–1125.e7. [Google Scholar] [CrossRef]
- Zielinski, M.C.; Tang, W.; Jadhav, S.P. The role of replay and theta sequences in mediating hippocampal-prefrontal interactions for memory and cognition. Hippocampus 2018, 30, 60–72. [Google Scholar] [CrossRef]
- Taufique, S.K.T.; Prabhat, A.; Kumar, V. Constant light environment suppresses maturation and reduces complexity of new born neuron processes in the hippocampus and caudal nidopallium of a diurnal corvid: Implication for impairment of the learning and cognitive performance. Neurobiol. Learn. Mem. 2018, 147, 120–127. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, K.; Li, M.; Wan, H. Local field potential functional network analysis of the left and right hippocampus of pigeons in goal-directed task. In Proceedings of the 2018 11th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Beijing, China, 13–15 October 2018; IEEE: Washington, DC, USA, 2018. [Google Scholar]
- Liu, X.; Nie, J.; Ping, Y.; Wan, H. Local field potential oscillations induced by goal-directed behavior of pigeon. In Proceedings of the 2017 10th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Shanghai, China, 14–16 October 2017; IEEE: Washington, DC, USA, 2019. [Google Scholar]
- Atoji, Y.; Wild, J.M.; Yamamoto, Y.; Suzuki, Y. Intratelencephalic connections of the hippocampus in pigeons (Columba livia). J. Comp. Neurol. 2002, 447, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Rattenborg, N.C.; Martinez-Gonzalez, D.; Roth, T.C.; Pravosudov, V.V. Hippocampal memory consolidation during sleep: A comparison of mammals and birds. Biol. Rev. 2011, 86, 658–691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Nie, J.; Yang, L.; Liu, X.; Shang, Z.; Wan, H. Hippocampus-nidopallium caudolaterale interactions exist in the goal-directed behavior of pigeon. Brain Res. Bull. 2019, 153, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zelikowsky, M.; Hersman, S.; Chawla, M.K.; Barnes, C.A.; Fanselow, M.S. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J. Neurosci. 2014, 34, 8462–8466. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; White, N.M. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993, 107, 3–22. [Google Scholar] [CrossRef]
- Gruber, A.J.; McDonald, R.J. Context, emotion, and the strategic pursuit of goals: Interactions among multiple brain systems controlling motivated behavior. Front. Behav. Neurosci. 2012, 6, 50. [Google Scholar] [CrossRef]
- Xiong, Y.; Cai, T.; Lei, F. How do migrating birds find their way? Chin. Sci. Bull. 2017, 62, 1204–1213. (In Chinese) [Google Scholar] [CrossRef]

| No. | Description of the Question |
|---|---|
| 1 | Do any other mammalian like functional specific cells (such as grid cells) exist in the avian brain to support spatial representation except location cells and some other pattern cells? |
| 2 | Are there any navigation-related functional specific cells in avian NCL to support spatial cue integration or navigational behavioral response? |
| 3 | How do different types of cells support spatial perception, memory, and decision-making? |
| 4 | How is goal-directed spatial perception information processed and transmitted in avian Hp? |
| 5 | How is the multisensory navigational information integrated and weighted to guide the decision-making action output in NCL? |
| 6 | How do different brain regions coordinated encode the goal-directed navigational-related information in the process of route learning? |
| 7 | How can different brain regions interact with each other to dynamically represent the routing information to adjust the path when obstacles are encountered? |
| 8 | How do the more widely distributed brain networks cooperate and interact with each other to support goal-directed navigation except avian Hp and NCL? |
| 9 | Is the spatial navigational mechanism of the limited space concluded in the laboratory environment consistent with the large-scale mechanism in the natural environment? |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Shang, Z.; Zhao, K.; Cheng, S.; Wan, H. The Role of Hp-NCL Network in Goal-Directed Routing Information Encoding of Bird: A Review. Brain Sci. 2020, 10, 617. https://doi.org/10.3390/brainsci10090617
Li M, Shang Z, Zhao K, Cheng S, Wan H. The Role of Hp-NCL Network in Goal-Directed Routing Information Encoding of Bird: A Review. Brain Sciences. 2020; 10(9):617. https://doi.org/10.3390/brainsci10090617
Chicago/Turabian StyleLi, Mengmeng, Zhigang Shang, Kun Zhao, Shuguan Cheng, and Hong Wan. 2020. "The Role of Hp-NCL Network in Goal-Directed Routing Information Encoding of Bird: A Review" Brain Sciences 10, no. 9: 617. https://doi.org/10.3390/brainsci10090617
APA StyleLi, M., Shang, Z., Zhao, K., Cheng, S., & Wan, H. (2020). The Role of Hp-NCL Network in Goal-Directed Routing Information Encoding of Bird: A Review. Brain Sciences, 10(9), 617. https://doi.org/10.3390/brainsci10090617




