Pathophysiology of Swallowing Dysfunction in Parkinson Disease and Lack of Dopaminergic Impact on the Swallow Function and on the Effect of Thickening Agents
Abstract
1. Introduction
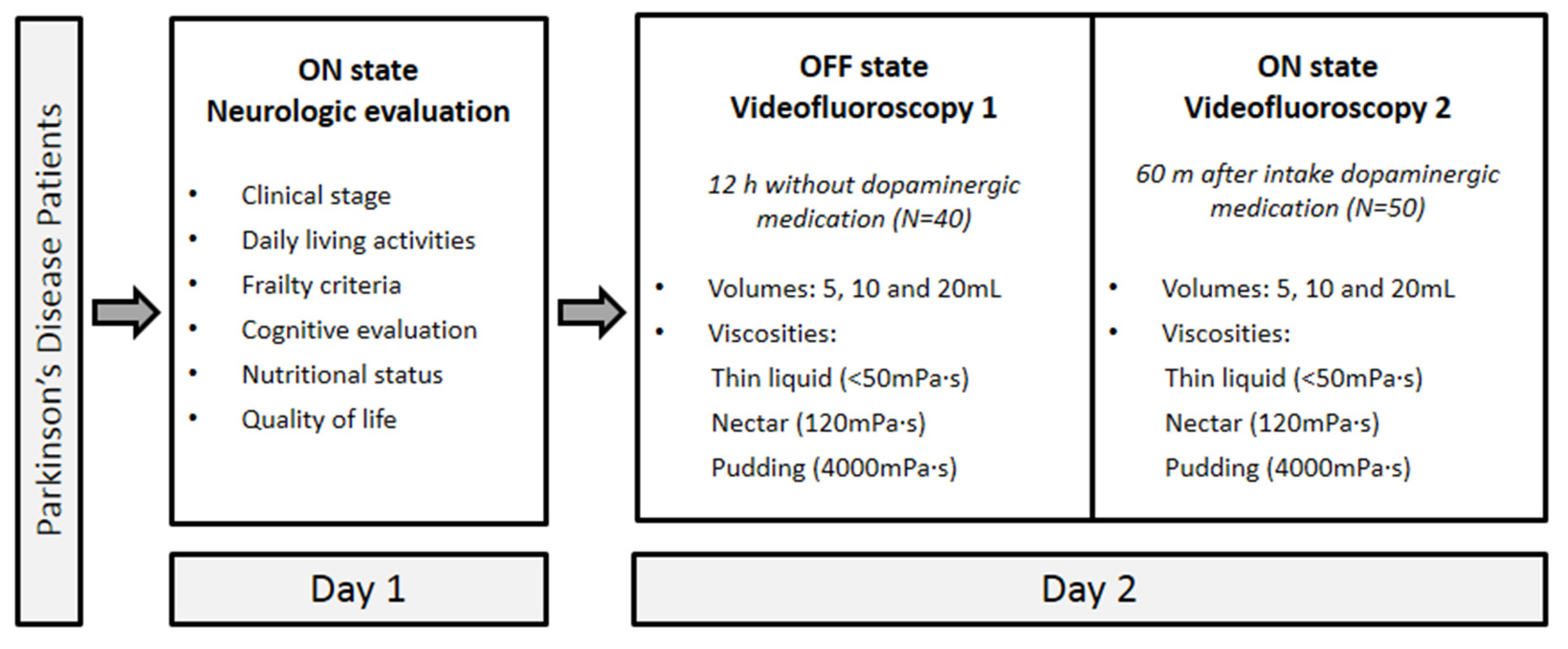
2. Materials and Methods
2.1. Study Population
2.2. Drug Analysis
2.3. Experimental Design
2.3.1. Day 1
- (1)
- PD clinical stage, following Hoehn-Yahr (1967) criteria [27];
- (2)
- Autonomy, according to the Schwab-England Activities of Daily Living (ADL) scale [31];
- (3)
- Non-motor and motor experiences of daily living and motor complications, according to the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [32];
- (4)
- Frailty status, according to Fried criteria [33];
- (5)
- Cognitive evaluation, according to the Montreal Cognitive Assessment (MoCA) [34];
- (6)
- Nutritional status, according to Mini Nutritional Assessment Short Form (MNA-SF) [35]; and
- (7)
- Health status and quality of life, according to the Parkinson’s Disease Questionnaire-8 (PDQ-8) [36].
2.3.2. Day 2
2.3.3. Videofluoroscopy (VFS)
- -
- Efficacy was evaluated by the presence of oral, vallecular, and pyriform sinus residues, piecemeal deglutition, oral apraxia, pumping, and impaired oral bolus control. Residue was categorized as coating or pooling residue [38].
- -
2.3.4. Bolus Rheology
2.4. Data Analysis and Statistical Methods
3. Results
3.1. Demographics and Clinical Inventory Scores
3.2. Pathophysiology
3.2.1. Videofluoroscopy
3.2.2. Oropharyngeal Physiology
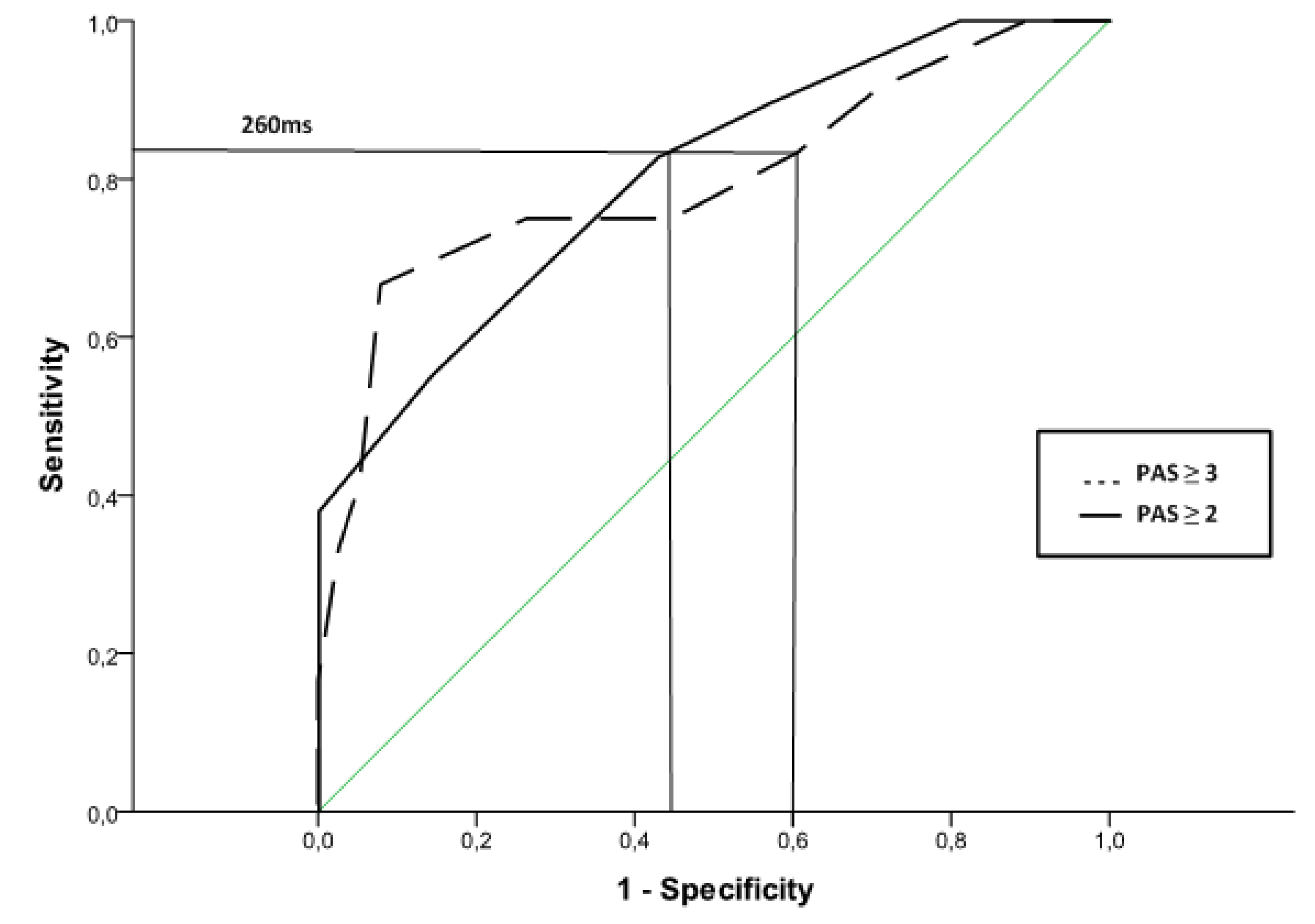
3.2.3. ROC Curves to Predict Unsafe Swallows
4. Impact of Dopaminergic Treatment
4.1. Effect of Dopaminergic Treatment on VFS Signs
4.2. Effect of Dopaminergic Treatment on Swallow Response
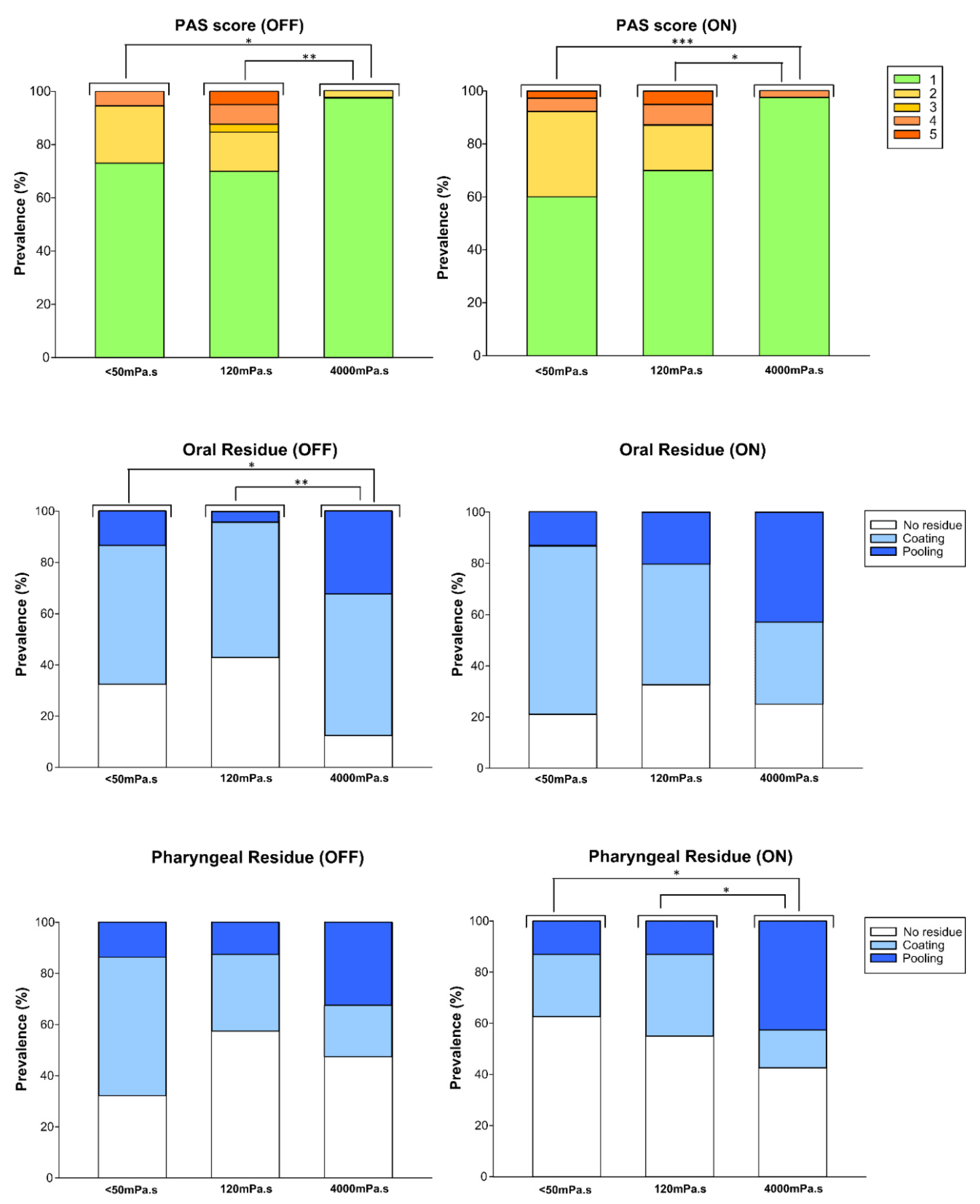
4.3. Dopaminergic Impact and the Therapeutic Effects of Thickening Agents
4.3.1. Viscosity Effect: PAS and Residues
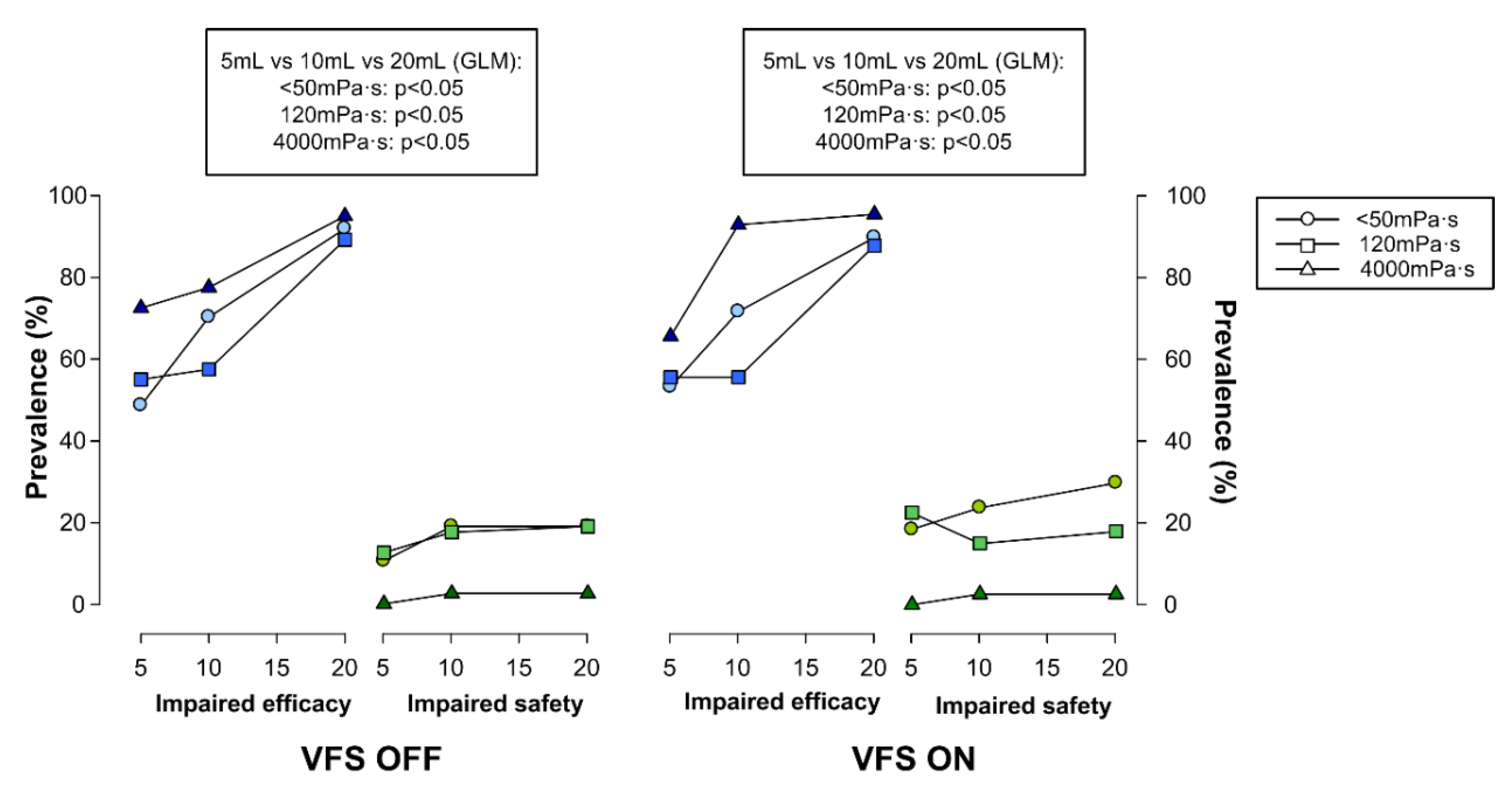
4.3.2. Effect of Bolus Volume on Swallow (Safety and Efficacy)
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michou, E.; Baijens, L.; Rofes, L.; Sanz Cartagena, P.; Clavé, P. Oropharyngeal Swallowing Disorders in Parkinson’s Disease: Revisited. Int. J. Speech Lang. Pathol. Audiol. 2013, 1, 76–88. [Google Scholar] [CrossRef][Green Version]
- Kalf, J.G.; de Swart, B.J.M.; Bloem, B.R.; Munneke, M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: A meta-analysis. Park. Relat. Disord. 2012, 18, 311–315. [Google Scholar] [CrossRef]
- Skodda, S.; Grönheit, W.; Mancinelli, N.; Schlegel, U. Progression of Voice and Speech Impairment in the Course of Parkinson’s Disease: A Longitudinal Study. Park. Dis. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- van Hooren, M.R.A.; Baijens, L.W.J.; Vos, R.; Pilz, W.; Kuijpers, L.M.F.; Kremer, B.; Michou, E. Voice- and swallow-related quality of life in idiopathic Parkinson’s disease. Laryngoscope 2016, 126, 408–414. [Google Scholar] [CrossRef]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A Systematic Review of the Prevalence of Oropharyngeal Dysphagia in Stroke, Parkinson’s Disease, Alzheimer’s Disease, Head Injury, and Pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef]
- Leow, L.P.; Beckert, L.; Anderson, T.; Huckabee, M.-L. Changes in Chemosensitivity and Mechanosensitivity in Aging and Parkinson’s Disease. Dysphagia 2012, 27, 106–114. [Google Scholar] [CrossRef]
- Ali, G.; Wallace, K.; Schwartz, R.; DeCarle, D.; Zagami, A.; Cook, I. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology 1996, 110, 383–392. [Google Scholar] [CrossRef]
- Clavé, P.; de Kraa, M.; Arreola, V.; Girvent, M.; Farré, R.; Palomera, E.; Serra-Prat, M. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment. Pharmacol. Ther. 2006, 24, 1385–1394. [Google Scholar] [CrossRef]
- Teskey, W.J.E.; Elhabiby, M.; El-Sheimy, N. Inertial Sensing to Determine Movement Disorder Motion Present before and after Treatment. Sensors 2012, 12, 3512–3527. [Google Scholar] [CrossRef]
- Schrag, A.; Jahanshahi, M.; Quinn, N. What contributes to quality of life in patients with Parkinson’s disease? J. Neurol. Neurosurg. Psychiatry 2000, 69, 308–312. [Google Scholar] [CrossRef]
- Fernandez, H.H.; Lapane, K.L. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med. Sci. Monit. 2002, 8, CR241–CR246. [Google Scholar]
- Bushmann, M.; Dobmeyer, S.M.; Leeker, L.; Perlmutter, J.S. Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology 1989, 39, 1309. [Google Scholar] [CrossRef]
- Fuh, J.L.; Lee, R.C.; Wang, S.J.; Lin, C.H.; Wang, P.N.; Chiang, J.H.; Liu, H.C. Swallowing difficulty in Parkinson’s disease. Clin. Neurol. Neurosurg. 1997, 99, 106–112. [Google Scholar] [CrossRef]
- Hunter, P.C.; Crameri, J.; Austin, S.; Woodward, M.C.; Hughes, A.J. Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. J. Neurol. Neurosurg. Psychiatry 1997, 63, 579–583. [Google Scholar] [CrossRef]
- Warnecke, T.; Suttrup, I.; Schröder, J.B.; Osada, N.; Oelenberg, S.; Hamacher, C.; Suntrup, S.; Dziewas, R. Levodopa responsiveness of dysphagia in advanced Parkinson’s disease and reliability testing of the FEES-Levodopa-test. Park. Relat. Disord. 2016, 28, 100–106. [Google Scholar] [CrossRef]
- Sutton, J.P. Dysphagia in Parkinson’s disease is responsive to levodopa. Park. Relat. Disord. 2013, 19, 282–284. [Google Scholar] [CrossRef]
- Menezes, C.; Melo, A. Does levodopa improve swallowing dysfunction in Parkinson’s disease patients? J. Clin. Pharm. Ther. 2009, 34, 673–676. [Google Scholar] [CrossRef]
- Lim, A.; Leow, L.; Huckabee, M.-L.; Frampton, C.; Anderson, T. A Pilot Study of Respiration and Swallowing Integration in Parkinson’s Disease: “On” and “Off” Levodopa. Dysphagia 2008, 23, 76–81. [Google Scholar] [CrossRef]
- Cereda, E.; Cilia, R.; Klersy, C.; Canesi, M.; Zecchinelli, A.L.; Mariani, C.B.; Tesei, S.; Sacilotto, G.; Meucci, N.; Zini, M.; et al. Swallowing disturbances in Parkinson’s disease: A multivariate analysis of contributing factors. Park. Relat. Disord. 2014, 20, 1382–1387. [Google Scholar] [CrossRef]
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31, 232–249. [Google Scholar] [CrossRef]
- Michou, E.; Hamdy, S.; Harris, M.; Vania, A.; Dick, J.; Kellett, M.; Rothwell, J. Characterization fo Corticobulbar Pharyngeal Neurophysiology in Dysphagic Patients with Parkinson’s Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2037–2045. [Google Scholar] [CrossRef]
- Troche, M.S.; Sapienza, C.M.; Rosenbek, J.C. Effects of Bolus Consistency on Timing and Safety of Swallow in Patients with Parkinson’s Disease. Dysphagia 2008, 23, 26–32. [Google Scholar] [CrossRef]
- Nagaya, M.; Kachi, T.; Yamada, T.; Sumi, Y. Videofluorographic observations on swallowing in patients with dysphagia due to neurodegenerative diseases. Nagoya J. Med. Sci. 2004, 67, 17–23. [Google Scholar]
- Logemann, J.A.; Gensler, G.; Robbins, J.; Lindblad, A.S.; Brandt, D.; Hind, J.A.; Kosek, S.; Dikeman, K.; Kazandjian, M.; Gramigna, G.D.; et al. A Randomized Study of Three Interventions for Aspiration of Thin Liquids in Patients with Dementia or Parkinson’s Disease. J. Speech Lang. Hear. Res. 2008, 51, 173–183. [Google Scholar] [CrossRef]
- Robbins, J.; Gensler, G.; Hind, J.; Logemann, J.A.; Lindblad, A.S.; Brandt, D.; Baum, H.; Lilienfeld, D.; Kosek, S.; Lundy, D.; et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: A randomized trial. Ann. Intern. Med. 2008, 148, 509–518. [Google Scholar] [CrossRef]
- Ortega, O.; Bolívar-Prados, M.; Arreola, V.; Nascimento, W.V.; Tomsen, N.; Gallegos, C.; Brito-de La Fuente, E.; Clavé, P. Therapeutic Effect, Rheological Properties and α-Amylase Resistance of a New Mixed Starch and Xanthan Gum Thickener on Four Different Phenotypes of Patients with Oropharyngeal Dysphagia. Nutrients 2020, 12, 1873. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Arriaga, A.; Rodróguez-Violante, M.; Villar-Velarde, A.; Corona, T. Cálculo de unidades de equivalencia de levodopa en enfermedad de Parkinson. Arch. Neurocienc. 2009, 14, 116–119. [Google Scholar]
- Schwab, R.; England, A. Projection techniques for evaluating surgery in Parkinson’s Disease. In Proceedings of the Projection Techniques for Evaluating Surgery in Parkinson’s Disease, Third Symposium on Parkinson’s Disease; Royal College of Surgeons in Edinburgh: Edinburgh, UK; E. & S. Livingstone Ltd.: Edinburgh, UK, 1968. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA®-SF): A practical tool for identification of nutritional status. J. Nutr. Heath Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The PDQ-8: Development and validation of a short-form parkinson’s disease questionnaire. Psychol. Health 1997, 12, 805–814. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Romea, M.; Palomera, E.; Almirall, J.; Cabré, M.; Serra-Prat, M.; Clavé, P. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol. Motil. 2010, 22, 851-e230. [Google Scholar] [CrossRef]
- Hind, J.A.; Nicosia, M.A.; Roecker, E.B.; Carnes, M.L.; Robbins, J.A. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch. Phys. Med. Rehabil. 2001, 82, 1661–1665. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Clavé, P. The Volume-Viscosity Swallow Test for Clinical Screening of Dysphagia and Aspiration. In Stepping Stones to Living Well with Dysphagia; Karger Publishers: Basel, Switzerland, 2012; pp. 33–42. [Google Scholar]
- Popa Nita, S.; Murith, M.; Chisholm, H.; Engmann, J. Matching the Rheological Properties of Videofluoroscopic Contrast Agents and Thickened Liquid Prescriptions. Dysphagia 2013, 28, 245–252. [Google Scholar] [CrossRef]
- La Fuente, E.B.; Turcanu, M.; Ekberg, O.; Gallegos, C. Rheological Aspects of Swallowing and Dysphagia: Shear and Elongational Flows. In Dysphagia. Medical Radiology; Ekberg, O., Ed.; Springer: Berlin, Heidelberg, 2017; pp. 687–716. [Google Scholar]
- Baijens, L.W.J.; Speyer, R.; Passos, V.L.; Pilz, W.; Roodenburg, N.; Clave, P. Swallowing in Parkinson Patients versus Healthy Controls: Reliability of Measurements in Videofluoroscopy. Gastroenterol. Res. Pract. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Pflug, C.; Bihler, M.; Emich, K.; Niessen, A.; Nienstedt, J.C.; Flügel, T.; Koseki, J.C.; Plaetke, R.; Hidding, U.; Gerloff, C.; et al. Critical Dysphagia is Common in Parkinson Disease and Occurs Even in Early Stages: A Prospective Cohort Study. Dysphagia 2018, 33, 41–50. [Google Scholar] [CrossRef]
- Ertekin, C.; Tarlaci, S.; Aydogdu, I.; Kiylioglu, N.; Yuceyar, N.; Turman, A.B.; Secil, Y.; Esmeli, F. Electrophysiological evaluation of pharyngeal phase of swallowing in patients with Parkinson’s disease. Mov. Disord. 2002, 17, 942–949. [Google Scholar] [CrossRef]
- Miarons, M.; Clavé, P.; Wijngaard, R.; Ortega, O.; Arreola, V.; Nascimento, W.; Rofes, L. Pathophysiology of Oropharyngeal Dysphagia Assessed by Videofluoroscopy in Patients with Dementia Taking Antipsychotics. J. Am. Med. Dir. Assoc. 2018, 19, 812.e1–812.e10. [Google Scholar] [CrossRef]
- Vilardell, N.; Rofes, L.; Arreola, V.; Martin, A.; Muriana, D.; Palomeras, E.; Ortega, O.; Clavé, P. Videofluoroscopic assessment of the pathophysiology of chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 2017, 29, e13111. [Google Scholar] [CrossRef]
- Suttrup, I.; Warnecke, T. Dysphagia in Parkinson’s Disease. Dysphagia 2016, 31, 24–32. [Google Scholar] [CrossRef]
- Clavé, P.; Arreola, V.; Romea, M.; Medina, L.; Palomera, E.; Serra-Prat, M. Accuracy of the volume-viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clin. Nutr. 2008, 27, 806–815. [Google Scholar] [CrossRef]

| N (%) /Mean ± SD (Range) | ||
|---|---|---|
| Gender (N = 50) | Men | 26 (52.00) |
| Women | 24 (48.00) | |
| Comorbidities (N = 40) | Arthrosis/rheumatism | 21 (52.50) |
| Depression | 7 (17.50) | |
| Hypertension | 17 (42.50) | |
| Dyslipidemia | 14 (35.00) | |
| Hoehn-Yahr clinical stage (N = 50) | 1 | 8 (16.00) |
| 1.5 | 2 (4.00) | |
| 2 | 14 (28.00) | |
| 2.5 | 8 (16.00) | |
| 3 | 15 (30.00) | |
| 4 | 3 (6.00) | |
| MNA-SF (N = 40) | At risk of malnutrition | 4 (10.00) |
| Normal nutrition | 36 (90.00) | |
| Frailty criteria (N = 40) | Robust | 33 (82.50) |
| Pre-frail | 7 (17.50) | |
| ADL (N = 40) | 87.25 ± 11.09 (60–100) | |
| PDQ-8 (N = 40) | 4.8 ± 5.01 (0–19) | |
| MDS-UPDRS-III (N = 40) | 20.12 ± 8.60 (7–39) | |
| MoCA (N = 40) | 24.12 ± 4.32 (13–30) | |
| Oropharyngeal Swallow Response (Mean ± SD) | HVs | PD (N = 50) | p-Value |
|---|---|---|---|
| LVC (ms) | 156.67 ± 46.58 | 311.20 ± 102.95 | <0.0001 |
| UESO (ms) | 200.00 ± 38.14 | 257.60 ± 200.43 | 0.2009 |
| LVO (ms) | 753.33 ± 79.70 | 969.60 ± 216.17 | <0.0001 |
| Kinematics (mJ) | 0.34 ± 0.07 | 0.33 ± 0.17 | 0.2811 |
| Force (mJ) | 1.41 ± 0.63 | 1.62 ± 1.99 | 0.2444 |
| Mean bolus velocity (m/s) | 21.50 ± 8.54 | 23.79 ± 27.98 | 0.3141 |
| Patients with PD | |||
|---|---|---|---|
| OFF (N = 40) | ON (N = 40) | p-Value | |
| Impaired swallow efficacy (%) | 97.50 (39) | 100.00 (40) | 0.4937 |
| Liquid (<50 mPa·s) | 91.89 (34) | 86.84 (33) | 1.000 |
| Nectar (120 mPa·s) | 85.00 (34) | 85.00 (34) | 1.000 |
| Pudding (4000 mPa·s) | 92.50 (37) | 94.87 (37) | 1.000 |
| Oral residue | |||
| Coating | 60.00 (24) | 42.50 (17) | 0.6830 |
| Pooling | 32.50 (13) | 47.50 (19) | 1.0000 |
| Pharyngeal residue | |||
| Coating | 20.00 (8) | 20.00 (8) | 1.0000 |
| Pooling | 40.00 (16) | 45.00 (18) | 0.7205 |
| Impaired swallow safety (%) | 37.50 (15) | 47.50 (19) | 0.4978 |
| Liquid (<50 mPa·s) | 27.03 (10) | 39.47 (15) | 0.3289 |
| Nectar (120 mPa·s) | 30.00 (12) | 30.00 (12) | 1.000 |
| Pudding (4000 mPa·s) | 2.50 (1) | 2.50 (1) | 1.000 |
| Penetrations (%) | 37.50 (15) | 47.50 (19) | 0.4978 |
| Aspirations (%) | 0 | 0 | - |
| Silent aspirations (PAS = 8) (%) | 0 | 0 | - |
| Higher mean PAS | 1.73 ± 1.20 | 1.90 ± 1.28 | >0.05 |
| Oropharyngeal swallow response (mean ± SD) | |||
| LVC (ms) | 293.33 ± 90.07 | 305.00 ± 105.90 | >0.05 |
| UESO (ms) | 211.28 ± 55.02 | 219.00 ± 77.92 | >0.05 |
| LVO (ms) | 953.85 ± 166.59 | 938.00 ± 122.52 | >0.05 |
| Kinematics (mJ) | 0.34 ± 0.11 | 0.35 ± 0.14 | >0.05 |
| Force (mJ) | 1.54 ± 1.17 | 1.65 ± 1.53 | >0.05 |
| Mean bolus velocity (m/s) | 22.45 ± 15.15 | 24.53 ± 23.57 | >0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, W.V.; Arreola, V.; Sanz, P.; Necati, E.; Bolivar-Prados, M.; Michou, E.; Ortega, O.; Clavé, P. Pathophysiology of Swallowing Dysfunction in Parkinson Disease and Lack of Dopaminergic Impact on the Swallow Function and on the Effect of Thickening Agents. Brain Sci. 2020, 10, 609. https://doi.org/10.3390/brainsci10090609
Nascimento WV, Arreola V, Sanz P, Necati E, Bolivar-Prados M, Michou E, Ortega O, Clavé P. Pathophysiology of Swallowing Dysfunction in Parkinson Disease and Lack of Dopaminergic Impact on the Swallow Function and on the Effect of Thickening Agents. Brain Sciences. 2020; 10(9):609. https://doi.org/10.3390/brainsci10090609
Chicago/Turabian StyleNascimento, Weslania Viviane, Viridiana Arreola, Pilar Sanz, Ediz Necati, Mireia Bolivar-Prados, Emilia Michou, Omar Ortega, and Pere Clavé. 2020. "Pathophysiology of Swallowing Dysfunction in Parkinson Disease and Lack of Dopaminergic Impact on the Swallow Function and on the Effect of Thickening Agents" Brain Sciences 10, no. 9: 609. https://doi.org/10.3390/brainsci10090609
APA StyleNascimento, W. V., Arreola, V., Sanz, P., Necati, E., Bolivar-Prados, M., Michou, E., Ortega, O., & Clavé, P. (2020). Pathophysiology of Swallowing Dysfunction in Parkinson Disease and Lack of Dopaminergic Impact on the Swallow Function and on the Effect of Thickening Agents. Brain Sciences, 10(9), 609. https://doi.org/10.3390/brainsci10090609






