Renal Contributions in the Pathophysiology and Neuropathological Substrates Shared by Chronic Kidney Disease and Alzheimer’s Disease
Abstract
1. Prevalence, Socio-Economic Aspects, and the Relationship between Chronic Kidney Disease and Alzheimer’s Disease
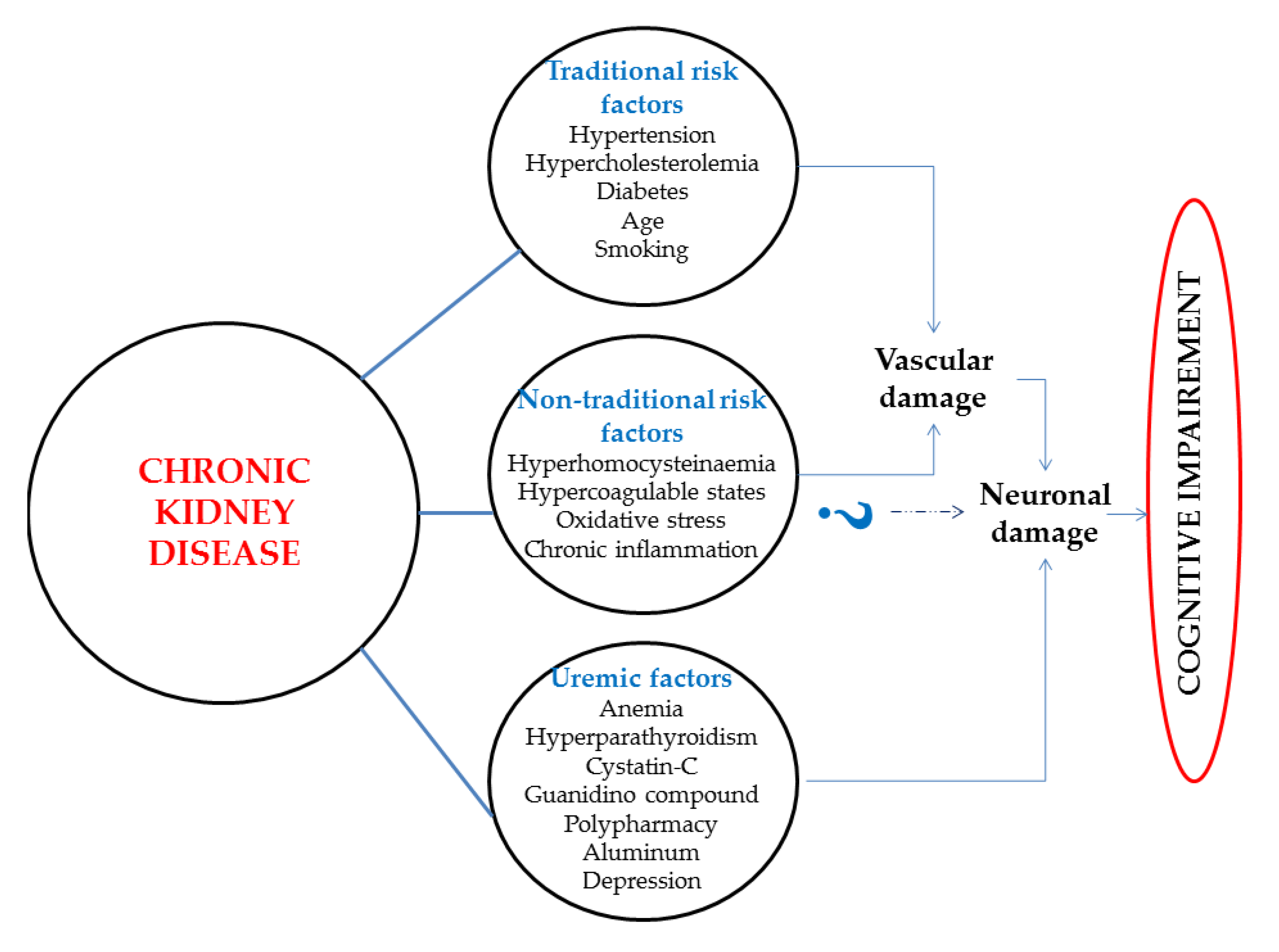
2. The Mechanisms Proposed for Cognitive Decline and Alzheimer’s Disease Associated with Chronic Kidney Disease
2.1. Vascular Dysfunction
2.2. Inflammation and Oxidative Stress
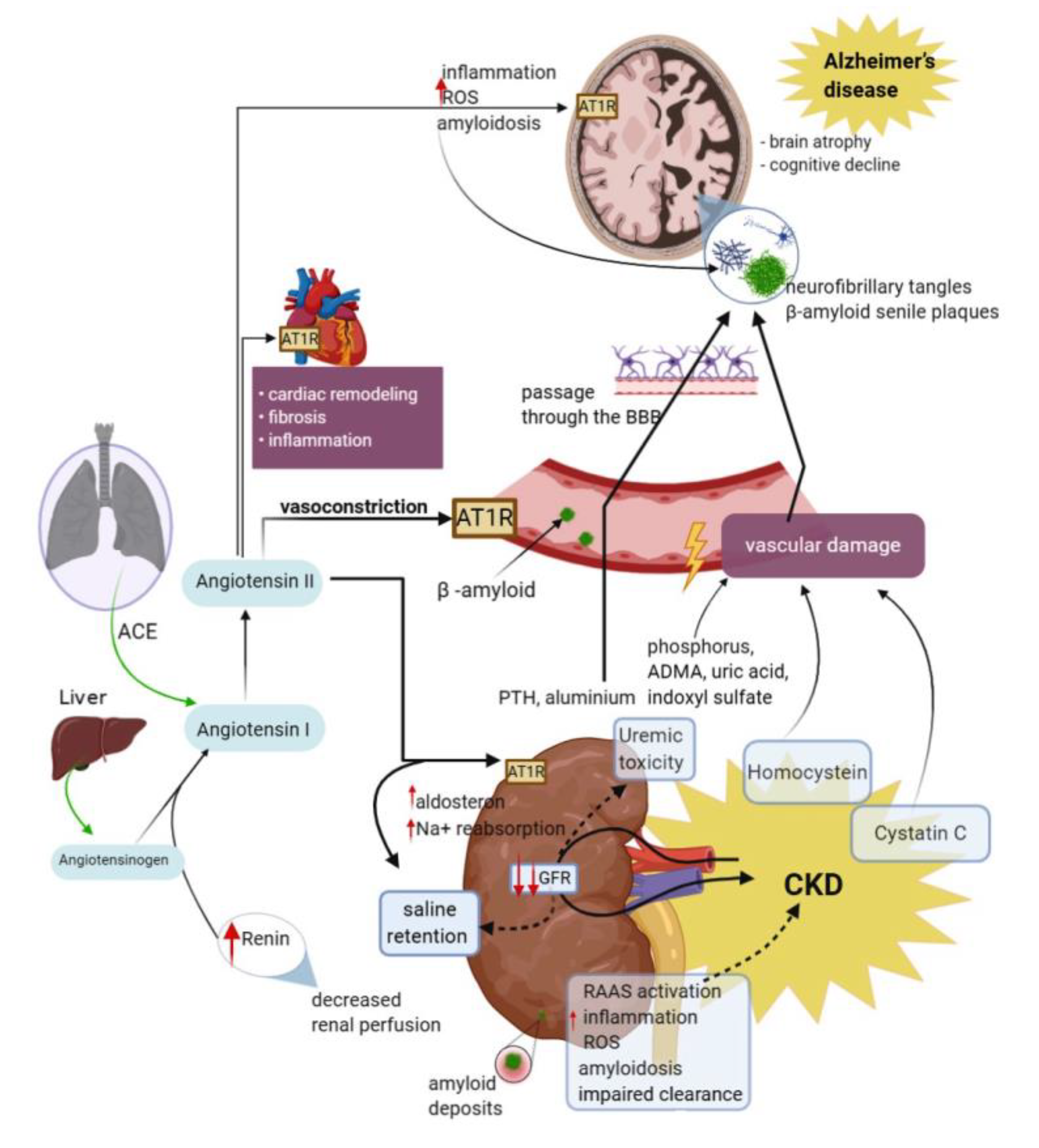
2.3. The Renin–Angiotensin–Aldosterone System (RAAS)
3. The Relationship between Biomarkers Related to Alzheimer’s Disease and Renal Function
3.1. The Potential Pathophysiological Markers Associated to Alzheimer’s Disease
3.2. Potential Use in the Diagnosis of Alzheimer’s Disease of the Biomarkers Associated with Chronic Kidney Disease
4. Effects of Renal Replacement Therapies on Amyloid-Beta Pathology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APP | amyloid precursor proteins |
| CKD | chronic kidney disease |
| ApoE4 | epsilon 4 allele of the apolipoprotein E gene |
| SorLA | sorting protein-related receptor |
| eGFR | estimated glomerular filtration rate |
| ESRD | end-stage renal disease |
| CVD | cerebrovascular disease |
| IL-1β | interleukines-1β |
| IL-6 | interleukine-6 |
| TNF | tumor necrosis factor |
| BBB | blood brain barrier |
| CNS | central nervous system |
| TGF-β | transforming growth factor β |
| ROS | reactive oxygen species |
| SOD | superoxide-dismutase |
| GPX | glutathione-peroxidase |
| CAT | catalase |
| GSH | glutathione |
| LPX | lipoperoxidation level |
| RAAS | renin–angiotensin–aldosterone system |
| AGT | angiotensinogen |
| ANG I, II | angiotensin I, II |
| Aβ | beta-amyloid |
| ACE | angiotensin converting enzyme |
| ADH | antidiuretic hormone |
| Hcy | homocysteine |
| PTH | parathyroid hormone |
| ADMA | asymmetric dimethylarginine |
| RAAS | renin–angiotensin–aldosterone system |
| vWF | von Willebrand factor |
| sVCAM-1 | soluble vascular cell adhesion molecule-1 |
| sICAM-1 | soluble intercellular cell adhesion molecule-1 |
| EA | endothelial activation |
| GSK3β | glycogen synthase kinase-3β |
| NfL | neurofilament light chain |
| FH | factor H |
| FB | factor B |
| iNOS | inducible nitric oxide synthase |
| COX-2 | cyclooxygenase-2 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells. |
References
- Patterson, C. World Alzheimer Report 2018-The State of the Art of Dementia Research: New frontiers; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Deture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wallin, K.; Boström, G.; Kivipelto, M.; Gustafson, Y. Risk factors for incident dementia in the very old. Int. Psychogeriatr. 2013, 25, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.B. Global glomerulosclerosis in primary nephrotic syndrome: Including age as a variable to predict renal outcomes. Kidney Int. 2018, 93, 1043–1044. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, C.; Von Strauss, E.; Winblad, B.; Fratiglioni, L. APOE genotype, family history of dementia, and alzheimer disease risk: A 6-year follow-up study. Arch. Neurol. 2004, 61, 1930–1934. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.J.; Zhang, M.; Xu, Z.Q.; Gao, C.Y.; Fang, C.Q.; Yan, J.C.; Zhou, H.D. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 2011, 76, 1485–1491. [Google Scholar] [CrossRef]
- Xue, L.; Lou, Y.; Feng, X.; Wang, C.; Ran, Z.; Zhang, X. Prevalence of chronic kidney disease and associated factors among the Chinese population in Taian, China Epidemiology and Health Outcomes. BMC Nephrol. 2014, 15, 205. [Google Scholar] [CrossRef]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Umegaki, H.; Hayashi, T.; Nomura, H.; Yanagawa, M.; Nonogaki, Z.; Nakshima, H.; Kuzuya, M. Cognitive dysfunction: An emerging concept of a new diabetic complication in the elderly. Geriatr. Gerontol. Int. 2013, 13, 28–34. [Google Scholar] [CrossRef]
- Kroner, Z. The relationship between Alzheimer’s disease and diabetes: Type 3 diabetes? Altern. Med. Rev. 2009, 14, 373–379. [Google Scholar] [PubMed]
- Stanciu, G.D.; Bild, V.; Ababei, D.C.; Rusu, R.N.; Cobzaru, A.; Paduraru, L.; Bulea, D. Link Between Diabetes and Alzheimer’s Disease due to the Shared Amyloid Aggregation and Deposition Involving both Neurodegenerative Changes and Neurovascular Damages. J. Clin. Med. 2020, 9, 1713. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, S.; Haynes, K.; Denburg, M.R.; Shin, D.B.; Gelfand, J.M. Risk of moderate to advanced kidney disease in patients with psoriasis: Population based cohort study. BMJ 2013, 347, f5961. [Google Scholar] [CrossRef]
- Mayer, F.; Di Pucchio, A.; Lacorte, E.; Bacigalupo, I.; Marzolini, F.; Ferrante, G.; Minardi, V.; Masocco, M.; Canevelli, M.; Di Fiandra, T.; et al. An Estimate of Attributable Cases of Alzheimer Disease and Vascular Dementia due to Modifiable Risk Factors: The Impact of Primary Prevention in Europe and in Italy. Dement. Geriatr. Cogn. Dis. Extra 2018, 8, 60–71. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Folstein, M.F. Insulin, insulin-degrading enzyme and amyloid-β peptide in Alzheimer’s disease: Review and hypothesis. Neurobiol. Aging 2006, 27, 190–198. [Google Scholar] [CrossRef]
- Yarbrough, C. Alzheimer’s and Kidney Disease: Common Molecular Culprit? Kidney News 2010, 2, 1–5. [Google Scholar]
- Wiinow, T.E.; Andersen, O.M. Sorting receptor SORLA-A trafficking path to avoid Alzheimer disease. J. Cell Sci. 2013, 126, 2751–2760. [Google Scholar]
- Nielsen, M.S.; Gustafsen, C.; Madsen, P.; Nyengaard, J.R.; Hermey, G.; Bakke, O.; Mari, M.; Schu, P.; Pohlmann, R.; Dennes, A.; et al. Sorting by the Cytoplasmic Domain of the Amyloid Precursor Protein Binding Receptor SorLA. Mol. Cell. Biol. 2007, 27, 6842–6851. [Google Scholar] [CrossRef]
- Scherzer, C.R.; Offe, K.; Gearing, M.; Rees, H.D.; Fang, G.; Heilman, C.J.; Schaller, C.; Bujo, H.; Levey, A.I.; Lah, J.J. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch. Neurol. 2004, 61, 1200–1205. [Google Scholar] [CrossRef]
- Jacobsen, L.; Madsen, P.; Moestrup, S.K.; Lund, A.H.; Tommerup, N.; Nykjær, A.; Sottrup-Jensen, L.; Gliemann, J.; Petersen, C.M. Molecular characterization of a novel human hybrid-type receptor that binds the α2-macroglobulin receptor-associated protein. J. Biol. Chem. 1996, 271, 31379–31383. [Google Scholar] [CrossRef] [PubMed]
- Motoi, Y.; Aizawa, T.; Haga, S.; Nakamura, S.; Namba, Y.; Ikeda, K. Neuronal localization of a novel mosaic apolipoprotein E receptor, LR11, in rat and human brain. Brain Res. 1999, 833, 209–215. [Google Scholar] [CrossRef]
- Foundation, N.K. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Etgen, T.; Chonchol, M.; Frstl, H.; Sander, D. Chronic kidney disease and cognitive impairment: A systematic review and meta-analysis. Am. J. Nephrol. 2012, 35, 474–482. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Kumar Agarwal, S.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Ikizler, T.A. Medical progress: Hemodialysis. N. Engl. J. Med. 2010, 363, 1833–1845. [Google Scholar] [CrossRef]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Tonelli, M.; Keech, A.; Shepherd, J.; Sacks, F.; Tonkin, A.; Packard, C.; Pfeffer, M.; Simes, J.; Isles, C.; Furberg, C.; et al. Effect of pravastatin in people with diabetes and chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 3748–3754. [Google Scholar] [CrossRef]
- Strachan, M.W.J.; Reynolds, R.M.; Marioni, R.E.; Price, J.F. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat. Rev. Endocrinol. 2011, 7, 108–114. [Google Scholar] [CrossRef]
- O’Lone, E.; Connors, M.; Masson, P.; Wu, S.; Kelly, P.J.; Gillespie, D.; Parker, D.; Whiteley, W.; Strippoli, G.F.M.; Palmer, S.C.; et al. Cognition in people with end-stage kidney disease treated with hemodialysis: A systematic review and meta-analysis. Am. J. Kidney Dis. 2016, 67, 925–935. [Google Scholar] [CrossRef]
- Madero, M.; Gul, A.; Sarnak, M.J. Cognitive function in chronic kidney disease. Semin. Dial. 2008, 21, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tilki, H.E.; Akpolat, T.; Tunali, G.; Kara, A.; Onar, M.K. Effects of haemodialysis and continuous ambulatory peritoneal dialysis on P300 cognitive potentials in uraemic patients. Ups. J. Med. Sci. 2004, 109, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Krishnan, A.V.; Pussell, B.A. Neurological complications in chronic kidney disease. J. R. Soc. Med. Cardiovasc. Dis. 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Tian, X.; Guo, X.; Xia, X.; Yu, H.; Li, X.; Jiang, A.; Zhan, Y. The comparison of cognitive function and risk of dementia in CKD patients under peritoneal dialysis and hemodialysis: A PRISMA-compliant systematic review and meta-analysis. Medicine 2019, 98, e14390. [Google Scholar] [CrossRef] [PubMed]
- Kurella Tamura, M.; Wadley, V.; Yaffe, K.; McClure, L.A.; Howard, G.; Go, R.; Allman, R.M.; Warnock, D.G.; McClellan, W. Kidney Function and Cognitive Impairment in US Adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am. J. Kidney Dis. 2008, 52, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ştefănescu, R.; Stanciu, G.D.; Luca, A.; Caba, I.C.; Tamba, B.I.; Mihai, C.T. Contributions of mass spectrometry to the identification of low molecular weight molecules able to reduce the toxicity of amyloid-β peptide to cell cultures and transgenic mouse models of Alzheimer’s disease. Molecules 2019, 24, 1167. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Chiriac, S.I.B.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef]
- Stefanescu, R.; Stanciu, G.D.; Luca, A.; Paduraru, L.; Tamba, B.-I. Secondary metabolites from plants possessing inhibitory properties against beta-amyloid aggregation as revealed by thioflavin-T assay and correlations with investigations on transgenic mouse models of Alzheimer’s disease. Biomolecules 2020, 10, 870. [Google Scholar] [CrossRef]
- Miranda, A.S.; Cordeiro, T.M.; Soares, T.M.; dos, S.L.; Ferreira, R.N.; Simões e Silva, A.C. Kidney-brain axis inflammatory cross-talk: From bench to bedside. Clin. Sci. 2017, 131, 1093–1105. [Google Scholar] [CrossRef]
- Weng, S.-C.; Wu, C.-L.; Kor, C.-T.; Chiu, P.-F.; Wu, M.-J.; Chang, C.-C.; Tarng, D.-C. Migraine and subsequent chronic kidney disease risk: A nationwide population-based cohort study. BMJ Open 2017, 7, 18483. [Google Scholar] [CrossRef]
- Wu, C.-L.; Kor, C.-T.; Chiu, P.-F.; Tsai, C.-C.; Lian, I.-B.; Yang, T.-H.; Tarng, D.-C.; Chang, C.-C. Long-term renal outcomes in patients with traumatic brain injury: A nationwide population-based cohort study. PLoS ONE 2017, 12, e0171999. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.Y.; Snyder, P.J.; Wu, W.C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.L.; Gillen, D.L.; Longstreth, W.T.; Kestenbaum, B.; Stehman-Breen, C.O. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003, 64, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Sorensen, E.P.; Giang, L.M.; Drew, D.A.; Shaffi, K.; Strom, J.A.; Singh, A.K.; et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 2013, 80, 471–480. [Google Scholar] [CrossRef]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Sandercock, P.A.G.; Dennis, M.S.; Starr, J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003, 34, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Lewis, S.C.; Keir, S.L.; Dennis, M.S.; Shenkin, S. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke 2006, 37, 2633–2636. [Google Scholar] [CrossRef]
- Helmer, C.; Stengel, B.; Metzger, M.; Froissart, M.; Massy, Z.A.; Tzourio, C.; Berr, C.; Dartigues, J.F. Chronic kidney disease, cognitive decline, and incident dementia: The 3C Study. Neurology 2011, 77, 2043–2051. [Google Scholar] [CrossRef]
- Bugnicourt, J.M.; Godefroy, O.; Chillon, J.M.; Choukroun, G.; Massy, Z.A. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J. Am. Soc. Nephrol. 2013, 24, 353–363. [Google Scholar] [CrossRef]
- Weiner, D.E.; Scott, T.M.; Giang, L.M.; Agganis, B.T.; Sorensen, E.P.; Tighiouart, H.; Sarnak, M.J. Cardiovascular disease and cognitive function in maintenance hemodialysis patients. Am. J. Kidney Dis. 2011, 58, 773–781. [Google Scholar] [CrossRef]
- Sorensen, E.P.; Sarnak, M.J.; Tighiouart, H.; Scott, T.; Giang, L.M.; Kirkpatrick, B.; Lou, K.; Weiner, D.E. The kidney disease quality of life cognitive function subscale and cognitive performance in maintenance hemodialysis patients. Am. J. Kidney Dis. 2012, 60, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Agganis, B.T.; Weiner, D.E.; Giang, L.M.; Scott, T.; Tighiouart, H.; Griffith, J.L.; Sarnak, M.J. Depression and cognitive function in maintenance hemodialysis patients. Am. J. Kidney Dis. 2010, 56, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Weiner, D.E.; Sarnak, M.J. Cognitive Impairment in CKD: Pathophysiology, Management, and Prevention. Am. J. Kidney Dis. 2019, 74, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Griva, K.; Thompson, D.; Jayasena, D.; Davenport, A.; Harrison, M.; Newman, S.P. Cognitive functioning pre-to post-kidney transplantation-A prospective study. Nephrol. Dial. Transplant. 2006, 21, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Harciarek, M.; Williamson, J.B.; Biedunkiewicz, B.; Lichodziejewska-Niemierko, M.; Dębska-Slizień, A.; Rutkowski, B. Memory performance in adequately dialyzed patients with end-stage renal disease: Is there an association with coronary artery bypass grafting? J. Clin. Exp. Neuropsychol. 2010, 32, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Harciarek, M.; Biedunkiewicz, B.; Lichodziejewska-Niemierko, M.; Debska-Ślizienñ, A.; Rutkowski, B. Cognitive performance before and after kidney transplantation: A prospective controlled study of adequately dialyzed patients with end-stage renal disease. J. Int. Neuropsychol. Soc. 2009, 15, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Radić, J.; Ljutić, D.; Radić, M.; Kovaić, V.; Dodig-Ćurković, K.; Šain, M. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am. J. Nephrol. 2011, 34, 399–406. [Google Scholar] [CrossRef]
- Chu, N.M.; Gross, A.L.; Shaffer, A.A.; Haugen, C.E.; Norman, S.P.; Xue, Q.L.; Sharrett, A.R.; Carlson, M.C.; Bandeen-Roche, K.; Segev, D.L.; et al. Frailty and changes in cognitive function after kidney transplantation. J. Am. Soc. Nephrol. 2019, 30, 336–345. [Google Scholar] [CrossRef]
- Silva, A.C.S.; Miranda, A.S.; Rocha, N.P.; Teixeira, A.L. Neuropsychiatric disorders in chronic kidney disease. Front. Pharmacol. 2019, 10, 1–11. [Google Scholar]
- Kalirao, P.; Pederson, S.; Foley, R.N.; Kolste, A.; Tupper, D.; Zaun, D.; Buot, V.; Murray, A.M. Cognitive impairment in peritoneal dialysis patients. Am. J. Kidney Dis. 2011, 57, 612–620. [Google Scholar] [CrossRef]
- Murray, A.M. Cognitive impairment in the aging dialysis and chronic cidney disease populations: An occult burden. Adv. Chronic Kidney Dis. 2009, 15, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T.; Takemoto, Y. New aspects of cerebrovascular diseases in dialysis patients. Contrib. Nephrol. 2015, 185, 138–146. [Google Scholar] [PubMed]
- Mukai, H.; Svedberg, O.; Lindholm, B.; Dai, L.; Heimbürger, O.; Barany, P.; Anderstam, B.; Stenvinkel, P.; Qureshi, A.R. Skin autofluorescence, arterial stiffness and Framingham risk score as predictors of clinical outcome in chronic kidney disease patients: A cohort study. Nephrol. Dial. Transplant. 2019, 34, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W. Is it time to utilize measurement of arterial stiffness to identify and reduce the risk of cognitive impairment? J. Clin. Hypertens. 2018, 20, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Kim, J.S.; Park, J.W.; An, J.Y.; Park, S.K.; Shim, Y.S.; Yang, D.W.; Lee, K.S. Arterial stiffness and impaired renal function in patients with Alzheimer’s disease. Neurol. Sci. 2016, 37, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Dimke, H.; Sparks, M.A.; Thomson, B.R.; Frische, S.; Coffman, T.M.; Quaggin, S.E. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J. Am. Soc. Nephrol. 2015, 26, 1027–1038. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Grammas, P. A damaged microcirculation contributes to neuronal cell death in Alzheimer’s disease. In Proceedings of the Neurobiology of Aging. Neurobiol. Aging 2000, 21, 199–205. [Google Scholar] [CrossRef]
- Heringa, S.M.; van den Berg, E.; Reijmer, Y.D.; Nijpels, G.; Stehouwer, C.D.A.; Schalkwijk, C.G.; Teerlink, T.; Scheffer, P.G.; van den Hurk, K.; Kappelle, L.J.; et al. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population—The Hoorn Study. Psychoneuroendocrinology 2014, 40, 108–118. [Google Scholar] [CrossRef]
- Zuliani, G.; Cavalieri, M.; Galvani, M.; Passaro, A.; Munari, M.R.; Bosi, C.; Zurlo, A.; Fellin, R. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J. Neurol. Sci. 2008, 272, 164–170. [Google Scholar] [CrossRef]
- Borroni, B.; Volpi, R.; Martini, G.; Del Bono, R.; Archetti, S.; Colciaghi, F.; Maalikjy Akkawi, N.; Di Luca, M.; Romanelli, G.; Caimi, L.; et al. Peripheral blood abnormalities in Alzheimer disease: Evidence for early endothelial dysfunction. Alzheimer Dis. Assoc. Disord. 2002, 16, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Kooman, J.P.; Dekker, M.J.; Usvyat, L.A.; Kotanko, P.; van der Sande, F.M.; Schalkwijk, C.G.; Shiels, P.G.; Stenvinkel, P. Inflammation and premature aging in advanced chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2017, 313, F938–F950. [Google Scholar] [CrossRef]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007, 9, 2277–2293. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; De Vinuesa, S.G.; Oubĩa, P.; Lahera, V.; Lũo, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 74, S1–S9. [Google Scholar] [CrossRef]
- Putri, A.Y.; Thaha, M. Role of oxidative stress on chronic kidney disease progression. Acta Med. Indones. 2014, 46, 244–252. [Google Scholar]
- Vinothkumar, G.; Kedharnath, C.; Krishnakumar, S.; Sreedhar, S.; Preethikrishnan, K.; Dinesh, S.; Sundaram, A.; Balakrishnan, D.; Shivashekar, G.; Kumar, S.; et al. Abnormal amyloid β42 expression and increased oxidative stress in plasma of CKD patients with cognitive dysfunction: A small scale case control study comparison with Alzheimer’s disease. BBA Clin. 2017, 8, 20–27. [Google Scholar] [CrossRef]
- Roses, A.D. Apolipoprotein E genotyping in the differential diagnosis, not prediction, of Alzheimer’s disease. Ann. Neurol. 1995, 38, 6–14. [Google Scholar] [CrossRef]
- Roses, A.D. Apolipoprotein E and Alzheimer’s disease: A rapidly expanding field with medical and epidemiological consequences. In Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Malden, MA, USA, 1996; Volume 802, pp. 50–57. [Google Scholar]
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, O.A.; Ola, M.S. Role of brain renin angiotensin system in neurodegeneration: An update. Saudi J. Biol. Sci. 2020, 27, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Nanjundaiah, S.; Chidambaram, H.; Chandrashekar, M.; Chinnathambi, S. Role of Microglia in Regulating Cholesterol and Tau Pathology in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2020, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.; Anton, E.; Chirita, R.; Bild, V.; Ciobica, A.; Alexinschi, O.; Arcan, O.; Popescu, R.; Paduraru, L.; Timofte, D. Current aspects of the interactions between dementia, the brain renin-angiotensin system and oxidative stress. Arch. Biol. Sci. 2015, 67, 903–907. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. Brain renin-angiotensin-A new look at an old system. Prog. Neurobiol. 2011, 95, 49–67. [Google Scholar] [CrossRef]
- Singh, K.D.; Karnik, S.S. Angiotensin Receptors: Structure, Function, Signaling and Clinical Applications. J. cell Signal. 2016, 1, 111. [Google Scholar]
- McKinley, M.J.; Albiston, A.L.; Allen, A.M.; Mathai, M.L.; May, C.N.; McAllen, R.M.; Oldfield, B.J.; Mendelsohn, F.A.O.; Chai, S.Y. The brain renin-angiotensin system: Location and physiological roles. Int. J. Biochem. Cell Biol. 2003, 35, 901–918. [Google Scholar] [CrossRef]
- Saavedra, J.M. Beneficial effects of Angiotensin II receptor blockers in brain disorders. Pharmacol. Res. 2017, 125, 91–103. [Google Scholar] [CrossRef]
- Torika, N.; Asraf, K.; Danon, A.; Apte, R.N.; Fleisher-Berkovich, S. Telmisartan Modulates Glial Activation: In Vitro and In Vivo Studies. PLoS ONE 2016, 11, e0155823. [Google Scholar] [CrossRef]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Quitterer, U.; AbdAlla, S. Improvements of symptoms of Alzheimer‘s disease by inhibition of the angiotensin system. Pharmacol. Res. 2020, 154, 104230. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, K.; Kanemaru, A.; Murayama, S. Association between Renal Functions and CSF Biomarkers in Alzheimer’s Disease. Alzheimer’s Dement. 2016, 12, P665. [Google Scholar] [CrossRef]
- Hansson, O.; Zetterberg, H.; Vanmechelen, E.; Vanderstichele, H.; Andreasson, U.; Londos, E.; Wallin, A.; Minthon, L.; Blennow, K. Evaluation of plasma Aβ40 and Aβ42 as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol. Aging 2010, 31, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shi, J.; Chu, D.; Hu, W.; Guan, Z.; Gong, C.X.; Iqbal, K.; Liu, F. Relevance of phosphorylation and truncation of tau to the etiopathogenesis of Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 27. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Y.; Hu, W.; Tung, Y.C.; Dai, C.; Chu, D.; Gong, C.X.; Iqbal, K.; Liu, F. Pathological Alterations of Tau in Alzheimer’s Disease and 3xTg-AD Mouse Brains. Mol. Neurobiol. 2019, 56, 6168–6183. [Google Scholar] [CrossRef]
- Harrington, C.R.; Wischik, C.M.; McArthur, F.K.; Taylor, G.A.; Edwardson, J.A.; Candy, J.M. Alzheimer’s-disease-like changes in tau protein processing: Association with aluminium accumulation in brains of renal dialysis patients. Lancet 1994, 343, 993–997. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimer’s Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Solcan, G. Acute idiopathic polyradiculoneuritis concurrent with acquired myasthenia gravis in a West Highland white terrier dog. BMC Vet. Res. 2016, 12, 111. [Google Scholar] [CrossRef]
- Lai, W.K.C.; Kan, M.Y. Homocysteine-induced endothelial dysfunction. Ann. Nutr. Metab. 2015, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Karmin, O.; Siow, Y.L. Metabolic Imbalance of Homocysteine and Hydrogen Sulfide in Kidney Disease. Curr. Med. Chem. 2017, 25, 367–377. [Google Scholar]
- Ohishi, T.; Fujita, T.; Suzuki, D.; Nishida, T.; Asukai, M.; Matsuyama, Y. Serum homocysteine levels are affected by renal function during a 3-year period of minodronate therapy in female osteoporotic patients. J. Bone Miner. Metab. 2019, 37, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33, S141–S144. [Google Scholar] [CrossRef]
- Johnson, K.A.; Schultz, A.; Betensky, R.A.; Becker, J.A.; Sepulcre, J.; Rentz, D.; Mormino, E.; Chhatwal, J.; Amariglio, R.; Papp, K.; et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 2016, 79, 110–119. [Google Scholar] [CrossRef]
- Reddy, P.H. Amyloid beta-induced glycogen synthase kinase 3β phosphorylated VDAC1 in Alzheimer’s disease: Implications for synaptic dysfunction and neuronal damage. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1913–1921. [Google Scholar] [CrossRef]
- Vinothkumar, G.; Krishnakumar, S.; Venkataraman, P. Correlation between abnormal GSK3β, β Amyloid, total Tau, p-Tau 181 levels and neuropsychological assessment total scores in CKD patients with cognitive dysfunction: Impact of rHuEPO therapy. J. Clin. Neurosci. 2019, 69, 38–42. [Google Scholar] [CrossRef]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, A.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef]
- Weston, P.S.J.; Poole, T.; Ryan, N.S.; Nair, A.; Liang, Y.; Macpherson, K.; Druyeh, R.; Malone, I.B.; Ahsan, R.L.; Pemberton, H.; et al. Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology 2017, 89, 2167–2175. [Google Scholar] [CrossRef]
- Stevenson, A.; Lopez, D.; Khoo, P.; Kalaria, R.N.; Mukaetova-Ladinska, E.B. Exploring Erythrocytes as Blood Biomarkers for Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 845–857. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Muramatsu, T.; Matsushita, S.; Arai, H.; Sasaki, H.; Higuchi, S. α1-Antichymotrypsin gene polymorphism and risk for Alzheimer’s disease. J. Neural Transm. 1996, 103, 1205–1210. [Google Scholar] [CrossRef]
- Pirttila, T.; Mehta, P.D.; Frey, H.; Wisniewski, H.M. α1-Antichymotrypsin and IL-1β are not increased in CSF or serum in Alzheimer’s disease. Neurobiol. Aging 1994, 15, 313–317. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Movilli, E.; Camerini, C.; Gaggia, P.; Poiatti, P.; Pola, A.; Viola, B.F.; Zubani, R.; Jeannin, G.; Cancarini, G. Effect of post-dilutional on-line haemodiafiltration on serum calcium, phosphate and parathyroid hormone concentrations in uraemic patients. Nephrol. Dial. Transpl. 2011, 26, 4032–4037. [Google Scholar] [CrossRef][Green Version]
- Neirynck, N.; Vanholder, R.; Schepers, E.; Eloot, S.; Pletinck, A.; Glorieux, G. An update on uremic toxins. Int. Urol. Nephrol. 2013, 45, 139–150. [Google Scholar] [CrossRef]
- Souberbielle, J.C.P.; Roth, H.; Fouque, D.P. Parathyroid hormone measurement in CKD. Kidney Int. 2010, 77, 93–100. [Google Scholar] [CrossRef]
- Lishmanov, A.; Dorairajan, S.; Pak, Y.; Chaudhary, K.; Chockalingam, A. Elevated serum parathyroid hormone is a cardiovascular risk factor in moderate chronic kidney disease. Int. Urol. Nephrol. 2012, 44, 541–547. [Google Scholar] [CrossRef]
- Anderson, J.L.; Vanwoerkom, R.C.; Horne, B.D.; Bair, T.L.; May, H.T.; Lappé, D.L.; Muhlestein, J.B. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am. Heart J. 2011, 162, 331–339. [Google Scholar] [CrossRef]
- Bhuriya, R.; Li, S.; Chen, S.C.; McCullough, P.A.; Bakris, G.L. Plasma Parathyroid Hormone Level and Prevalent Cardiovascular Disease in CKD Stages 3 and 4: An Analysis From the Kidney Early Evaluation Program (KEEP). Am. J. Kidney Dis. 2009, 53, S3–S10. [Google Scholar] [CrossRef]
- Lourida, I.; Thompson-Coon, J.; Dickens, C.M.; Soni, M.; Kuźma, E.; Kos, K.; Llewellyn, D.J. Parathyroid hormone, cognitive function and dementia: A systematic review. PLoS ONE 2015, 10, e0127574. [Google Scholar] [CrossRef] [PubMed]
- Çermik, T.F.; Kaya, M.; Uğur-Altun, B.; Bedel, D.; Berkarda, Ş.; Yiğitbaşı, Ö.N. Regional cerebral blood flow abnormalities in patients with primary hyperparathyroidism. Neuroradiology 2007, 49, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.M.; Cummings, J.L. Frontal-Subcortical Dementias. Neurologist 2008, 14, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Bild, V.; Ababei, D.C.; Neamtu, M.; Vasincu, A.; Bild, W.; Stanciu, G.D.; Tamba, B.I.; Solcan, G.; Beschea Chiriac, S. Isobolar analysis of the binary fixed-ratio combination of acetylsalicilic acid-acetaminophen. Farmacia 2017, 65, 563–566. [Google Scholar]
- Filiopoulos, V.; Hadjiyannakos, D.; Vlassopoulos, D. New insights into uric acid effects on the progression and prognosis of chronic kidney disease. Ren. Fail. 2012, 34, 510–520. [Google Scholar] [CrossRef]
- Nashar, K.; Fried, L.F. Hyperuricemia and the Progression of Chronic Kidney Disease: Is Uric Acid a Marker or an Independent Risk Factor? Adv. Chronic Kidney Dis. 2012, 19, 386–391. [Google Scholar] [CrossRef]
- Dousdampanis, P.; Trigka, K.; Musso, C.G.; Fourtounas, C. Hyperuricemia and chronic kidney disease: An enigma yet to be solved. Ren. Fail. 2014, 36, 1351–1359. [Google Scholar] [CrossRef]
- Liu, W.C.; Tomino, Y.; Lu, K.C. Impacts of indoxyl sulfate and p-Cresol sulfate on chronic kidney disease and mitigating effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Goto, S.; Fukagawa, M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins 2018, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Cao, X.; Zou, J.; Shen, B.; Zhang, X.; Liu, Z.; Lv, W.; Teng, J.; Ding, X. Indoxyl sulfate, a valuable biomarker in chronic kidney disease and dialysis. Hemodial. Int. 2017, 21, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, J.A.; Liftman, C.; Glickman, J.D. Frequency of elevated serum aluminum levels in adult dialysis patients. Am. J. Kidney Dis. 2005, 46, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Fang, Y.W.; Liou, H.H.; Leu, J.G.; Lin, B.S. Association of Serum Aluminum Levels with Mortality in Patients on Chronic Hemodialysis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Katz, R.; Sarnak, M.J.; Ix, J.; Fried, L.F.; De Boer, I.; Palmas, W.; Siscovick, D.; Levey, A.S.; Shlipak, M.G. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J. Am. Soc. Nephrol. 2011, 22, 147–155. [Google Scholar] [CrossRef] [PubMed]
- DSa, J.; Shetty, S.; Bhandary, R.R.; Rao, A. V Association Between Serum Cystatin C and Creatinine in Chronic Kidney Disease Subjects Attending a Tertiary Health Care Centre. J. Clin. Diagn. Res. 2017, 11, BC09. [Google Scholar] [CrossRef]
- Sastre, M.; Calero, M.; Pawlik, M.; Mathews, P.M.; Kumar, A.; Danilov, V.; Schmidt, S.D.; Nixon, R.A.; Frangione, B.; Levy, E. Binding of cystatin C to Alzheimer’s amyloid β inhibits in vitro amyloid fibril formation. Neurobiol. Aging 2004, 25, 1033–1043. [Google Scholar] [CrossRef]
- Mathews, P.M.; Levy, E. Cystatin C in aging and in Alzheimer’s disease. Ageing Res. Rev. 2016, 32, 38–50. [Google Scholar] [CrossRef]
- Wang, C.; Sun, B.; Zhou, Y.; Grubb, A.; Gan, L. Cathepsin B degrades amyloid-β in mice expressing wild-type human amyloid precursor protein. J. Biol. Chem. 2012, 287, 39834–39841. [Google Scholar] [CrossRef]
- Wang, B.; Xie, Y.; Yang, Z.; Peng, D.; Wang, J.; Zhou, S.; Li, S.; Ma, X. Lack of an Association between Alzheimer’s Disease and the Cystatin C (CST3) Gene G73A Polymorphism in Mainland Chinese. Dement. Geriatr. Cogn. Disord. 2008, 25, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.; Lao, J.I.; Gómez, M.; Riutort, N.; Latorre, P.; Mate, J.L.; Ariza, A. Alzheimer’s disease and the cystatin C gene polymorphism: An association study. Neurosci. Lett. 2001, 315, 17–20. [Google Scholar] [CrossRef]
- Stanciu, G.-D.; Packer, R.M.A.; Pakozdy, A.; Solcan, G.; Volk, H.A. Clinical reasoning in feline epilepsy: Which combination of clinical information is useful? Vet. J. 2017, 225, 9–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oh, S.W.; Kim, S.; Na, K.Y.; Kim, K.W.; Chae, D.W.; Chin, H.J. Glomerular filtration rate and proteinuria: Association with mortality and renal progression in a prospective cohort of a community-based elderly population. PLoS ONE 2014, 9, e94120. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef]
- Yuede, C.M.; Lee, H.; Restivo, J.L.; Davis, T.A.; Hettinger, J.C.; Wallace, C.E.; Young, K.L.; Hayne, M.R.; Bu, G.; Li, C.; et al. Rapid in vivo measurement of ß-amyloid reveals biphasic clearance kinetics in an Alzheimer’s mouse model. J. Exp. Med. 2016, 213, 677–685. [Google Scholar] [CrossRef]
- Qosa, H.; Abuasal, B.S.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Keller, J.N.; Kaddoumi, A. Differences in amyloid-β clearance across mouse and human blood-brain barrier models: Kinetic analysis and mechanistic modeling. Neuropharmacology 2014, 79, 668–678. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhao, Y.; Marshall, C.; Wu, T.; Xiao, M. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 2019, 29, 176–192. [Google Scholar] [CrossRef]
- Jin, W.-S.; Shen, L.-L.; Bu, X.-L.; Zhang, W.-W.; Chen, S.-H.; Huang, Z.-L.; Xiong, J.-X.; Gao, C.-Y.; Dong, Z.; He, Y.-N.; et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol. 2017, 134, 207–220. [Google Scholar] [CrossRef]
- Liu, Y.H.; Xiang, Y.; Wang, Y.R.; Jiao, S.S.; Wang, Q.H.; Bu, X.-L.; Zhu, C.; Yao, X.Q.; Giunta, B.; Tan, J.; et al. Association Between Serum Amyloid-Beta and Renal Functions: Implications for Roles of Kidney in Amyloid-Beta Clearance. Mol. Neurobiol. 2015, 52, 115–119. [Google Scholar] [PubMed]
- Stanciu, G.D.; Musteaţă, M.; Armaşu, M.; Solcan, G. Evaluation of central vestibular syndrome in dogs using brainstem auditory evoked responses recorded with surface electrodes. Arq. Bras. Med. Vet. e Zootec. 2016, 68. [Google Scholar] [CrossRef][Green Version]
- Xiang, Y.; Bu, X.-L.; Liu, Y.-H.; Zhu, C.; Shen, L.-L.; Jiao, S.-S.; Zhu, X.-Y.; Giunta, B.; Tan, J.; Song, W.-H.; et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol. 2015, 130, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, N.; Hasegawa, M.; Ito, S.; Kawaguchi, K.; Hiki, Y.; Nakai, S.; Suzuki, N.; Shimano, Y.; Ishida, O.; Kushimoto, H.; et al. A prospective study on blood Aβ levels and the cognitive function of patients with hemodialysis: A potential therapeutic strategy for Alzheimer’s disease. J. Neural Transm. 2015, 122, 1593–1607. [Google Scholar] [CrossRef]
- Kitaguchi, N.; Kawaguchi, K.; Nakai, S.; Murakami, K.; Ito, S.; Hoshino, H.; Hori, H.; Ohashi, A.; Shimano, Y.; Suzuki, N.; et al. Reduction of Alzheimer’s disease amyloid-β in plasma by hemodialysis and its relation to cognitive functions. Blood Purif. 2011, 32, 57–62. [Google Scholar] [CrossRef]
- Kato, M.; Kawaguchi, K.; Nakai, S.; Murakami, K.; Hori, H.; Ohashi, A.; Hiki, Y.; Ito, S.; Shimano, Y.; Suzuki, N.; et al. Potential therapeutic system for Alzheimer’s disease: Removal of blood Aβs by hemodialzyers and its effect on the cognitive functions of renal-failure patients. J. Neural Transm. 2012, 119, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Tholen, S.; Schmaderer, C.; Chmielewski, S.; Förstl, H.; Heemann, U.; Baumann, M.; Steubl, D.; Grimmer, T. Reduction of Amyloid-β Plasma Levels by Hemodialysis: An Anti-Amyloid Treatment Strategy? J. Alzheimer’s Dis. 2016, 50, 791–796. [Google Scholar] [CrossRef] [PubMed]
| Chronic Kidney Disease Stages | Description | Potential Sign/Symptoms |
|---|---|---|
| Stages 1 and 2 | minimal kidney damage with normal eGFR >60 mL/min/1.73 m2, ACR ≥30 mg/g | usually urea and creatinine levels are normal or slightly raised |
| Stage 3 | moderate reduced eGFR 30–59 mL/min/1.73 m2 | early signs occur and may comprise fatigue and weakness, loss of appetite, itching, rising levels of urea and creatinine, anemia, nausea, vomiting, hypertension |
| Stage 4 | severe reduced eGFR 15–29 mL/min/1.73 m2 | anemia, hypertension, nausea, vomiting, reduction in calcium absorption, dyslipidemia, heart failure, metabolic acidosis |
| Stage 5 | kidney failure, eGFR <15 mL/min/1.73 m2 | anemia, hypertension, nausea, vomiting hypertrophy of left ventricular, hyperparathyroidism, hyperphosphatemia, hyperkalemia |
| End-stage renal disease (ESRD) | renal transplant and dialysis | Anemia, cardiovascular dysfunction, hyperparathyroidism, hyperphosphatemia, hyperkalemia |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciu, G.D.; Ababei, D.C.; Bild, V.; Bild, W.; Paduraru, L.; Gutu, M.M.; Tamba, B.-I. Renal Contributions in the Pathophysiology and Neuropathological Substrates Shared by Chronic Kidney Disease and Alzheimer’s Disease. Brain Sci. 2020, 10, 563. https://doi.org/10.3390/brainsci10080563
Stanciu GD, Ababei DC, Bild V, Bild W, Paduraru L, Gutu MM, Tamba B-I. Renal Contributions in the Pathophysiology and Neuropathological Substrates Shared by Chronic Kidney Disease and Alzheimer’s Disease. Brain Sciences. 2020; 10(8):563. https://doi.org/10.3390/brainsci10080563
Chicago/Turabian StyleStanciu, Gabriela Dumitrita, Daniela Carmen Ababei, Veronica Bild, Walther Bild, Luminita Paduraru, Mihai Marius Gutu, and Bogdan-Ionel Tamba. 2020. "Renal Contributions in the Pathophysiology and Neuropathological Substrates Shared by Chronic Kidney Disease and Alzheimer’s Disease" Brain Sciences 10, no. 8: 563. https://doi.org/10.3390/brainsci10080563
APA StyleStanciu, G. D., Ababei, D. C., Bild, V., Bild, W., Paduraru, L., Gutu, M. M., & Tamba, B.-I. (2020). Renal Contributions in the Pathophysiology and Neuropathological Substrates Shared by Chronic Kidney Disease and Alzheimer’s Disease. Brain Sciences, 10(8), 563. https://doi.org/10.3390/brainsci10080563






