Differential Expression of Multiple Disease-Related Protein Groups Induced by Valproic Acid in Human SH-SY5Y Neuroblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and VPA Treatment
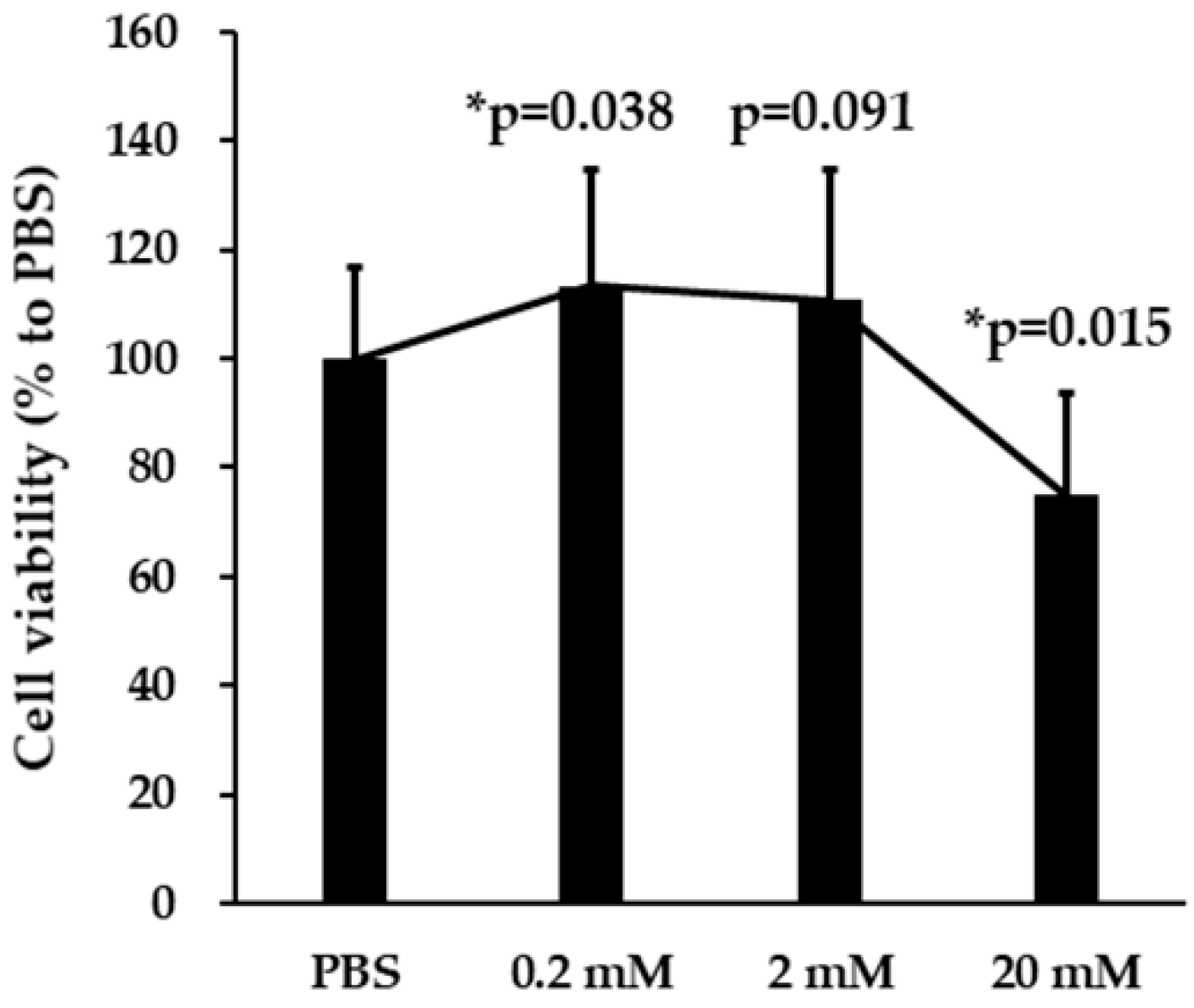
2.2. Cell Viability Assay
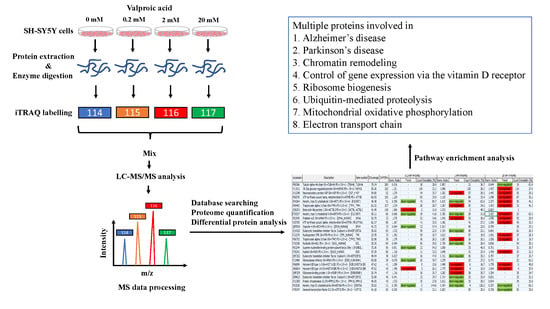
2.3. Protein Sample Preparation and iTRAQ Peptide Labeling
2.4. LC-MS/MS Analysis
2.5. Protein Identification
2.6. Pathway Analysis
2.7. Immunoblotting Analysis
2.8. Total RNA Preparation and RT-qPCR
3. Results
3.1. Effects of VPA on SH-SY5Y Cell Viability
3.2. Identification of Differentially Expressed Proteins by the iTRAQ Analysis
3.3. Pathway Enrichment Analysis
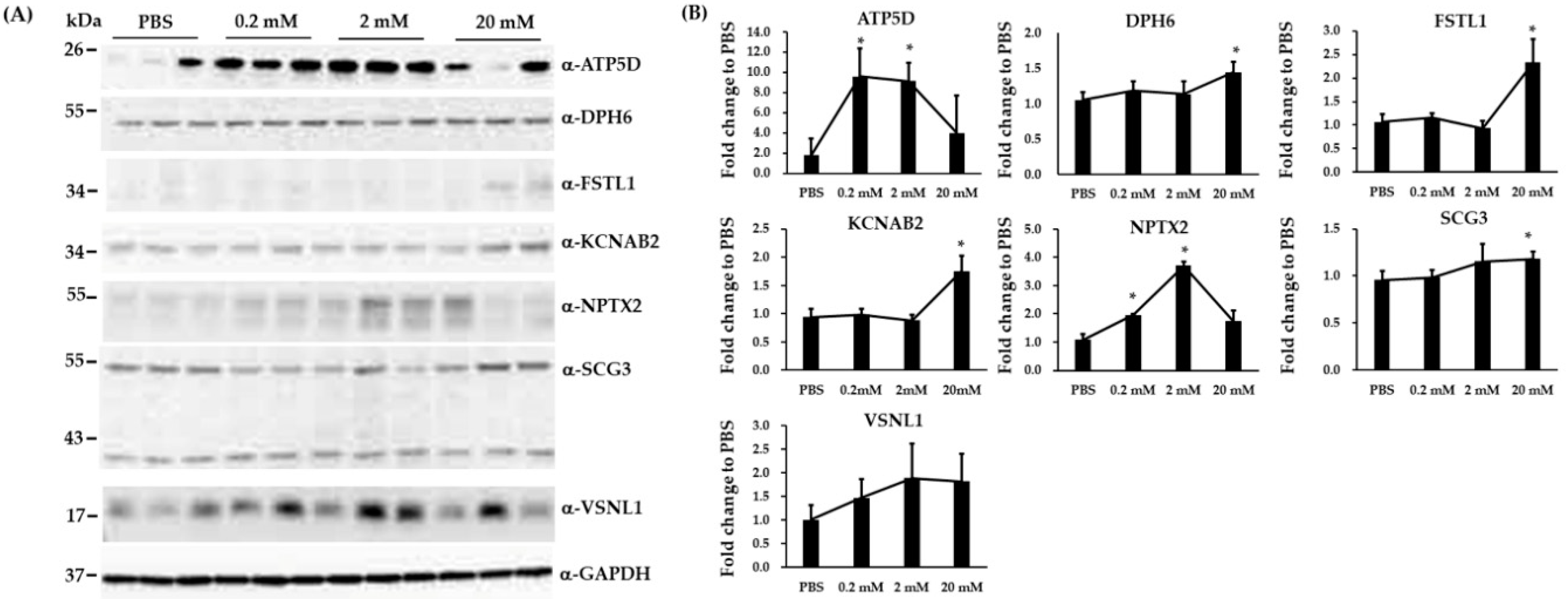
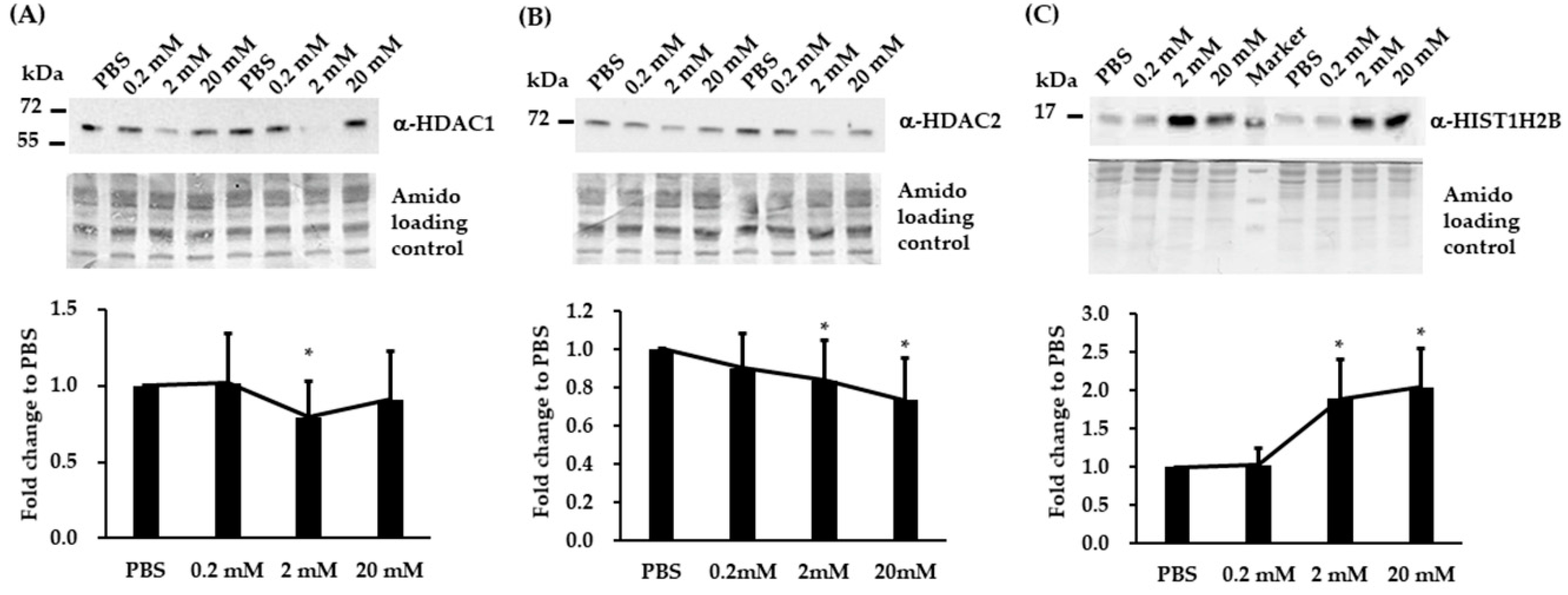
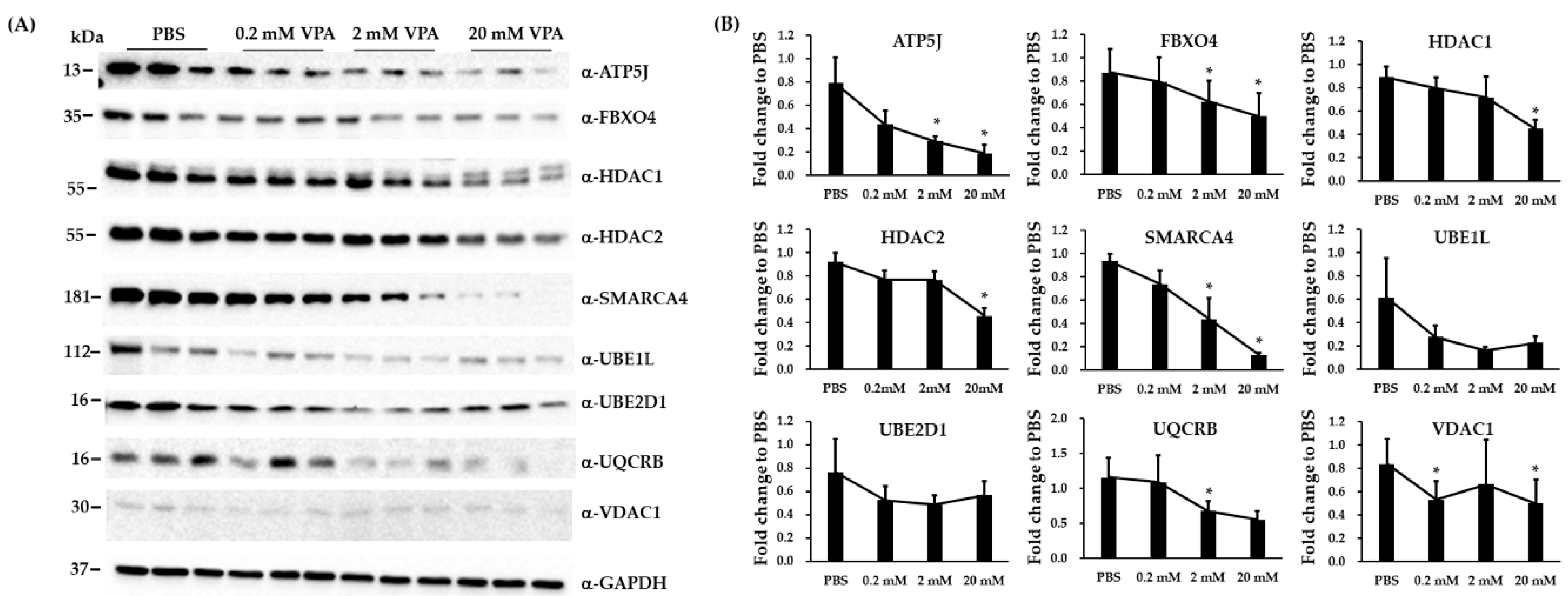
3.4. Validation of Three Differentially Expressed Epigenetic Proteins by Immunoblotting
3.5. Immunoblotting and RT-qPCR Analysis in Biological Replicated SH-SY5Y Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haddad, P.M.; Das, A.; Ashfaq, M.; Wieck, A. A review of valproate in psychiatric practice. Expert Opin. Drug Metab. Toxicol. 2009, 5, 539–551. [Google Scholar] [CrossRef]
- Timmer, R.T.; Sands, J.M. Lithium intoxication. J. Am. Soc. Nephrol. 1999, 10, 666–674. [Google Scholar]
- Dreifuss, F.E.; Langer, D.H. Side effects of valproate. Am. J. Med. 1988, 84, 31–41. [Google Scholar] [CrossRef]
- Peterson, G.M.; Naunton, M. Valproate: A simple chemical with so much to offer. J. Clin. Pharm. Ther. 2005, 30, 417–421. [Google Scholar] [CrossRef]
- Blaheta, R.A.; Cinatl, J., Jr. Anti-tumor mechanisms of valproate: A novel role for an old drug. Med. Res. Rev. 2002, 22, 492–511. [Google Scholar] [CrossRef]
- Cincarova, L.; Zdrahal, Z.; Fajkus, J. New perspectives of valproic acid in clinical practice. Expert Opin. Investig. Drugs 2013, 22, 1535–1547. [Google Scholar] [CrossRef]
- Owens, M.J.; Nemeroff, C.B. Pharmacology of valproate. Psychopharmacol. Bull. 2003, 37 (Suppl. 2), 17–24. [Google Scholar]
- Farber, N.B.; Jiang, X.P.; Heinkel, C.; Nemmers, B. Antiepileptic drugs and agents that inhibit voltage-gated sodium channels prevent nmda antagonist neurotoxicity. Mol. Psychiatry 2002, 7, 726–733. [Google Scholar] [CrossRef]
- Bosetti, F.; Bell, J.M.; Manickam, P. Microarray analysis of rat brain gene expression after chronic administration of sodium valproate. Brain Res. Bull. 2005, 65, 331–338. [Google Scholar] [CrossRef]
- Du, T.; Rong, Y.; Feng, R.; Verkhratsky, A.; Peng, L. Chronic treatment with anti-bipolar drugs down-regulates gene expression of TRPC1 in neurones. Front. Cell. Neurosci. 2016, 10, 305. [Google Scholar] [CrossRef]
- Jiang, H.Z.; Wang, S.Y.; Yin, X.; Jiang, H.Q.; Wang, X.D.; Wang, J.; Wang, T.H.; Qi, Y.; Yang, Y.Q.; Wang, Y.; et al. Downregulation of Homer1b/c in SOD1 G93A models of ALS: A novel mechanism of neuroprotective effect of lithium and valproic acid. Int. J. Mol. Sci. 2016, 17, 2129. [Google Scholar] [CrossRef] [PubMed]
- Wittmann-Liebold, B.; Graack, H.R.; Pohl, T. Two-dimensional gel electrophoresis as tool for proteomics studies in combination with protein identification by mass spectrometry. Proteomics 2006, 6, 4688–4703. [Google Scholar] [CrossRef] [PubMed]
- Rauniyar, N.; Yates, J.R., 3rd. Isobaric labeling-based relative quantification in shotgun proteomics. J. Proteome Res. 2014, 13, 5293–5309. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.M.; Khan, J.M.; Bringans, S.D.; Koudelka, T.; Takle, P.S.; Downs, R.A.; Livk, A.; Syme, R.A.; Tan, K.C.; Lipscombe, R.J. Analysis of reproducibility of proteome coverage and quantitation using isobaric mass tags (iTRAQ and TMT). J. Proteome Res. 2017, 16, 384–392. [Google Scholar] [CrossRef]
- Velasquez, E.; Nogueira, F.C.S.; Velasquez, I.; Schmitt, A.; Falkai, P.; Domont, G.B.; Martins-de-Souza, D. Synaptosomal proteome of the orbitofrontal cortex from schizophrenia patients using quantitative label-free and iTRAQ-based shotgun proteomics. J. Proteome Res. 2017, 16, 4481–4494. [Google Scholar] [CrossRef]
- Biedler, J.L.; Roffler-Tarlov, S.; Schachner, M.; Freedman, L.S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978, 38, 3751–3757. [Google Scholar]
- Vaughan, P.F.; Peers, C.; Walker, J.H. The use of the human neuroblastoma SH-SY5Y to study the effect of second messengers on noradrenaline release. Gen. Pharmacol. 1995, 26, 1191–1201. [Google Scholar] [CrossRef]
- Liguori, L.; Andolfo, I.; de Antonellis, P.; Aglio, V.; di Dato, V.; Marino, N.; Orlotti, N.I.; De Martino, D.; Capasso, M.; Petrosino, G.; et al. The metallophosphodiesterase Mpped2 impairs tumorigenesis in neuroblastoma. Cell Cycle 2012, 11, 569–581. [Google Scholar] [CrossRef]
- Di Daniel, E.; Mudge, A.W.; Maycox, P.R. Comparative analysis of the effects of four mood stabilizers in SH-SY5Y cells and in primary neurons. Bipolar Disord. 2005, 7, 33–41. [Google Scholar] [CrossRef]
- Park, S.W.; Seo, M.K.; Cho, H.Y.; Lee, J.G.; Lee, B.J.; Seol, W.; Kim, Y.H. Differential effects of amisulpride and haloperidol on dopamine D2 receptor-mediated signaling in SH-SY5Y cells. Neuropharmacology 2011, 61, 761–769. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Storey, J.D.; MacCoss, M.J.; Noble, W.S. Posterior error probalilities and false discovery rates: Two sides of the same coin. J. Proteome Res. 2008, 7, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- DAVID v6.8. Available online: https://david.ncifcrf.gov/home.jsp (accessed on 6 August 2020).
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef]
- Becker, K.G.; White, S.L.; Muller, J.; Engel, J. Bbid: The biological biochemical image database. Bioinformatics 2000, 16, 745–746. [Google Scholar] [CrossRef]
- Nishimura, D. Biocarta. Biotech Softw. Internet Rep. 2001, 2, 117–120. [Google Scholar] [CrossRef]
- NIH Image. Available online: http://rsb.info.nih.gov/nih-image/ (accessed on 6 August 2020).
- Mestdagh, P.; Van Vlierberghe, P.; De Weer, A.; Muth, D.; Westermann, F.; Speleman, F.; Vandesompele, J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009, 10, R64. [Google Scholar] [CrossRef]
- Electron Transprot Chain. Available online: https://data.broadinstitute.org/gsea-msigdb/msigdb/biocarta/human/h_etcPathway.gif (accessed on 6 August 2020).
- Control of Gene Expression by Vitamin D Receptor. Available online: https://data.broadinstitute.org/gsea-msigdb/msigdb/biocarta/human/h_vdrPathway.gif (accessed on 6 August 2020).
- Makarevic, J.; Rutz, J.; Juengel, E.; Maxeiner, S.; Tsaur, I.; Chun, F.K.; Bereiter-Hahn, J.; Blaheta, R.A. Influence of the hdac inhibitor valproic acid on the growth and proliferation of temsirolimus-resistant prostate cancer cells in vitro. Cancers 2019, 11, 566. [Google Scholar] [CrossRef]
- Monti, B.; Polazzi, E.; Contestabile, A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr. Mol. Pharmacol. 2009, 2, 95–109. [Google Scholar] [CrossRef]
- Soria-Castro, R.; Schcolnik-Cabrera, A.; Rodriguez-Lopez, G.; Campillo-Navarro, M.; Puebla-Osorio, N.; Estrada-Parra, S.; Estrada-Garcia, I.; Chacon-Salinas, R.; Chavez-Blanco, A.D. Exploring the drug repurposing versatility of valproic acid as a multifunctional regulator of innate and adaptive immune cells. J. Immunol. Res. 2019, 2019, 9678098. [Google Scholar] [CrossRef]
- Riva, G.; Cilibrasi, C.; Bazzoni, R.; Cadamuro, M.; Negroni, C.; Butta, V.; Strazzabosco, M.; Dalpra, L.; Lavitrano, M.; Bentivegna, A. Valproic acid inhibits proliferation and reduces invasiveness in glioma stem cells through wnt/beta catenin signalling activation. Genes 2018, 9, 522. [Google Scholar] [CrossRef]
- Lee, B.S.; Kim, Y.S.; Kim, H.J.; Kim, D.H.; Won, H.R.; Kim, Y.S.; Kim, C.H. HDAC4 degradation by combined TRAIL and valproic acid treatment induces apoptotic cell death of TRAIL-resistant head and neck cancer cells. Sci. Rep. 2018, 8, 12520. [Google Scholar] [CrossRef]
- Li, X.N.; Shu, Q.; Su, J.M.; Perlaky, L.; Blaney, S.M.; Lau, C.C. Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol. Cancer Ther. 2005, 4, 1912–1922. [Google Scholar] [CrossRef]
- Shukla, S.; Tekwani, B.L. Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation. Front. Pharmacol. 2020, 11, 537. [Google Scholar] [CrossRef]
- Mazzocchi, M.; Collins, L.M.; Sullivan, A.M.; O’Keefee, G.W. The class II histone deacetylases as therapeutic targets for Parkinson’s disease. Neuronal Signal. 2020, 4, NS20200001. [Google Scholar] [CrossRef]
- Kamarudin, M.N.A.; Parhar, I. Emerging therapeutic potential of anti-psychotic drugs in the management of human glioma: A comprehensive review. Oncotarget 2019, 10, 3952–3977. [Google Scholar] [CrossRef]
- Goh, W.W.; Sergot, M.J.; Sng, J.C.; Wong, L. Comparative network-based recovery analysis and proteomic profiling of neurological changes in valproic acid-treated mice. J. Proteome Res. 2013, 12, 2116–2127. [Google Scholar] [CrossRef]
- Wu, L.; Peng, J.; Wei, C.; Liu, G.; Wang, G.; Li, K.; Yin, F. Characterization, using comparative proteomics, of differentially expressed proteins in the hippocampus of the mesial temporal lobe of epileptic rats following treatment with valproate. Amino Acids 2011, 40, 221–238. [Google Scholar] [CrossRef]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analysis. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Creson, T.; Zhang, L.; Li, P.; Du, F.; Yuan, P.; Gould, T.D.; Manji, H.K.; Chen, G. Mood stabilizer valproate promotes erk pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 2004, 24, 6590–6599. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Long, Z.; Feng, M.; Zhao, Y.; Luo, S.; Wang, K.; Wang, Y.; Yang, G.; He, G. Valproic acid stimulates hippocampal neurogenesis via activating the Wnt/-Catenin signaling pathway in the APP/PS1/Nestin-GFP triple transgenic mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.M.; Zhao, L.; Jiang, R.; Wang, K.J.; Luo, S.F.; Zheng, M.; Li, X.F.; He, G.Q. Valproic acid modifies synaptic structure and accelerates neurite outgrowth via the glycogen synthase Kinase-3 signaling pathway in an Alzheimer’s disease model. CNS Neurosci. Ther. 2015, 21, 887–897. [Google Scholar] [CrossRef]
- Mizukami, K.; Hatanaka, K.; Ishii, T.; Iwakiri, M.; Sodeyama, N.; Tanaka, Y.; Asada, T. Effects of sodium valproate on behavioral disturbances in elderly outpatients with dementia. Geriatr. Gerontol. Int. 2010, 10, 324–326. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Tariot, P.N.; Jakimovich, L.J.; Kowalski, N.; Holt, C.; Erb, R.; Cox, C. Valproate therapy for agitation in dementia: Open-label extension of a double-blind trial. Am. J. Geriatr. Psychiatry 2003, 11, 434–440. [Google Scholar] [CrossRef]
- Tariot, P.N.; Loy, R.; Ryan, J.M.; Porsteinsson, A.; Ismail, S. Mood stabilizers in Alzheimer’s disease: Symptomatic and neuroprotective rationales. Adv. Drug Deliv. Rev. 2002, 54, 1567–1577. [Google Scholar] [CrossRef]
- Loy, R.; Tariot, P.N. Neuroprotective properties of valproate: Potential benefit for ad and tauopathies. J. Mol. Neurosci. 2002, 19, 303–307. [Google Scholar] [CrossRef]
- Carriere, C.H.; Kang, N.H.; Niles, L.P. Neuroprotection by valproic acid in an intrastriatal rotenone model of Parkinson’s disease. Neuroscience 2014, 267, 114–121. [Google Scholar] [CrossRef]
- Warren, N.; O’Gorman, C.; Lehn, A.; Siskind, D. Dopamine dysregulation syndrome in Parkinson’s disease: A systematic review of published cases. J. Neurol. Neurosurg. Psychiatry 2017, 88, 1060–1064. [Google Scholar] [CrossRef]
- Adiele, R.C.; Adiele, C.A. Mitochondrial regulatory pathways in the pathogenesis of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 53, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Wallace, D.C. Mitochondrial etiology of neuropsychiatric disorders. Biol. Psychiatry 2018, 83, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Masliah-Planchon, J.; Bieche, I.; Guinebretiere, J.M.; Bourdeaut, F.; Delattre, O. SWI/SNF chromatin remodeling and human malignancies. Annu. Rev. Pathol. 2015, 10, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef]
- Dobnig, H. A review of the health consequences of the vitamin d deficiency pandemic. J. Neurol. Sci. 2011, 311, 15–18. [Google Scholar] [CrossRef]
- Nakken, K.O.; Tauboll, E. Bone loss associated with use of antiepileptic drugs. Expert Opin. Drug Saf. 2010, 9, 561–571. [Google Scholar] [CrossRef]
- Albaghdadi, O.; Alhalabi, M.S.; Alourfi, Z.; Youssef, L.A. Bone health and vitamin d status in young epilepsy patients on valproate monotherapy. Clin. Neurol. Neurosurg. 2016, 146, 52–56. [Google Scholar] [CrossRef]
- Fuller, H.R.; Man, N.T.; Lam le, T.; Shamanin, V.A.; Androphy, E.J.; Morris, G.E. Valproate and bone loss: iTRAQ proteomics show that valproate reduces collagens and osteonectin in sma cells. J. Proteome Res. 2010, 9, 4228–4233. [Google Scholar] [CrossRef]
- Seuter, S.; Pehkonen, P.; Heikkinen, S.; Carlberg, C. Dynamics of 1α,25-dihydroxyvitamin D3-dependent chromatin accessibility of early vitamin D receptor target genes. Biochim. Biophys. Acta 2013, 1829, 1266–1275. [Google Scholar] [CrossRef]
- Battaglia, S.; Karasik, E.; Gillard, B.; Williams, J.; Winchester, T.; Moser, M.T.; Smiraglia, D.J.; Foster, B.A. LSD1 dual function in mediating epigenetic corruption of the vitamin D signaling in prostate cancer. Clin. Epigenetics 2017, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Duenas-Gonzalez, A.; Candelaria, M.; Perez-Plascencia, C.; Perez-Cardenas, E.; de la Cruz-Hernandez, E.; Herrera, L.A. Valproic acid as epigenetic cancer drug: Preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat. Rev. 2008, 34, 206–222. [Google Scholar] [CrossRef] [PubMed]

| # Proteins | 0.2 mM VPA/PBS | 2 mM VPA/PBS | 20 mM VPA/PBS |
|---|---|---|---|
| Total Quantification | 5293 | 5295 | 5294 |
| ≥mean + 1.5 × s.d. (fold change) | 213 (≥1.35) | 261 (≥1.28) | 302 (≥1.39) |
| ≤mean − 1.5 × s.d. (fold change) | 229 (≤0.73) | 332 (≤0.76) | 280 (≤0.72) |
| ≥1.5 fold change | 83 | 49 | 176 |
| ≤1.5 fold change | 122 | 117 | 153 |
| Group | Trend | Category | Term | Count | Proteins |
|---|---|---|---|---|---|
| 0.2 mM/PBS | Down | KEGG_pathway: map04120 | Ubiquitin mediated proteolysis | 10 | UBE2E3, NEDD4, UBE2G2, ANAPC4, FBXO2, CDC23, FBXO4, TRAF6, UBE2D1, UBE2E1 |
| 2 mM/PBS | Up | KEGG_pathway: map05012 | Parkinson’s disease | 10 | UQCRC2, ATP5D, UQCRH, ATP5F1, UBA7, UQCRFS1, VDAC3, VDAC1, ATP5J, UQCRB |
| 2 mM/PBS | Down | BIOCARTA | Control of Gene Expression by Vitamin D Receptor # | 9 | NCOA2, BAZ1B, SMARCE1, HDAC1, SMARCC1, SMARCD1, SUPT16H, TOP2B, SMARCA4 |
| 2 mM/PBS | Down | KEGG_pathway: map03008 | Ribosome biogenesis in eukaryotes | 10 | WDR75, GTPBP4, WDR36, DKC1, TCOF1, LSG1, GNL2, RIOK2, BMS1, NOP10 |
| 20 mM/PBS | Up | BIOCARTA | Electron Transport Reaction in Mitochondria $ | 5 | SDHA, SDHB, UQCRC1, SLC25A6, ATP5A1 |
| 20 mM/PBS | Up | KEGG_pathway: map00190 | Oxidative phosphorylation | 13 | ATP5D, UQCRC2, UQCRC1, ATP5B, ATP5F1, ATP6V1G1, SDHA, SDHB, UQCRH, ATP5L, ATP5A1, UQCRB, ATP5J |
| 20 mM/PBS | Up | KEGG_pathway: map05012 | Parkinson’s disease | 12 | UQCRC2, SDHA, ATP5D, SDHB, UQCRC1, UQCRH, ATP5B, SLC25A6, ATP5F1, ATP5A1, ATP5J, UQCRB |
| 20 mM/PBS | Up | KEGG_pathway: map05010 | Alzheimer’s disease | 11 | UQCRC2, SDHA, ATP5D, SDHB, UQCRC1, UQCRH, ATP5B, ATP5F1, ATP5A1, ATP5J, UQCRB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.-M.; Chung, H.-S.; Ping, L.-Y.; Hsu, S.-H.; Tsai, H.-Y.; Chen, S.-J.; Cheng, M.-C. Differential Expression of Multiple Disease-Related Protein Groups Induced by Valproic Acid in Human SH-SY5Y Neuroblastoma Cells. Brain Sci. 2020, 10, 545. https://doi.org/10.3390/brainsci10080545
Hu T-M, Chung H-S, Ping L-Y, Hsu S-H, Tsai H-Y, Chen S-J, Cheng M-C. Differential Expression of Multiple Disease-Related Protein Groups Induced by Valproic Acid in Human SH-SY5Y Neuroblastoma Cells. Brain Sciences. 2020; 10(8):545. https://doi.org/10.3390/brainsci10080545
Chicago/Turabian StyleHu, Tsung-Ming, Hsiang-Sheng Chung, Lieh-Yung Ping, Shih-Hsin Hsu, Hsin-Yao Tsai, Shaw-Ji Chen, and Min-Chih Cheng. 2020. "Differential Expression of Multiple Disease-Related Protein Groups Induced by Valproic Acid in Human SH-SY5Y Neuroblastoma Cells" Brain Sciences 10, no. 8: 545. https://doi.org/10.3390/brainsci10080545
APA StyleHu, T.-M., Chung, H.-S., Ping, L.-Y., Hsu, S.-H., Tsai, H.-Y., Chen, S.-J., & Cheng, M.-C. (2020). Differential Expression of Multiple Disease-Related Protein Groups Induced by Valproic Acid in Human SH-SY5Y Neuroblastoma Cells. Brain Sciences, 10(8), 545. https://doi.org/10.3390/brainsci10080545