1. Introduction
In daily living, it is inevitable for an individual to be in a situation where distractions are not encountered. Thus, good control of focus is indeed important to achieve a particular goal in mind while eliminating interferences. Anatomically, it is the pre-frontal cortex (PFC), a part of the brain that is responsible for exercising executive function during this kind of situation [
1]. Executive functions or also known as cognitive or executive control are defined as the set of cognitive processes needed by an individual when focus and attention are required or simply when the time to think is needed before doing a particular action [
2]. Nowadays, many non-invasive modalities are being used to measure brain activity, however, one of those is the functional near-infrared spectroscopy (fNIRS) that is utilized in this study. It is considered as non-invasive, non-ionizing, easy to use, and low-cost that could improve patient’s quality of life [
3]. Aside from those characteristics, Schroeter and colleagues [
4] also added that it is flexible, portable, and can determine biochemical specificity as compared with other imaging that can be used to patients and children. Using the fNIRS device, this study aims to determine the hemodynamic activity or the vascular response (VR) of the brain when attention control is tested using the self-made color-word interference test (self-made CWIT) which is a modification of classic Stroop task.
Previous studies focused on within- and between-language differences, and not on the differences present between two sets of tests with a different language which was investigated in this study. In particular, this study would like to determine the mean scores and overall mean scores obtained in each condition per trial, if there are any significant differences per trial in each condition, between congruent and incongruent conditions using their native language, and lastly, between congruent conditions of subjects’ native and second languages. Moreover, the top 10 networks among fNIRS channels during the three conditions using the phase-locking value (PLV) will also be identified. It is hypothesized in this study that there are significant differences in mean scores and overall scores between self-made CWIT and VR, among trials per condition, trials between congruent conditions, and trials between congruent and incongruent conditions at a p-value of <0.05.
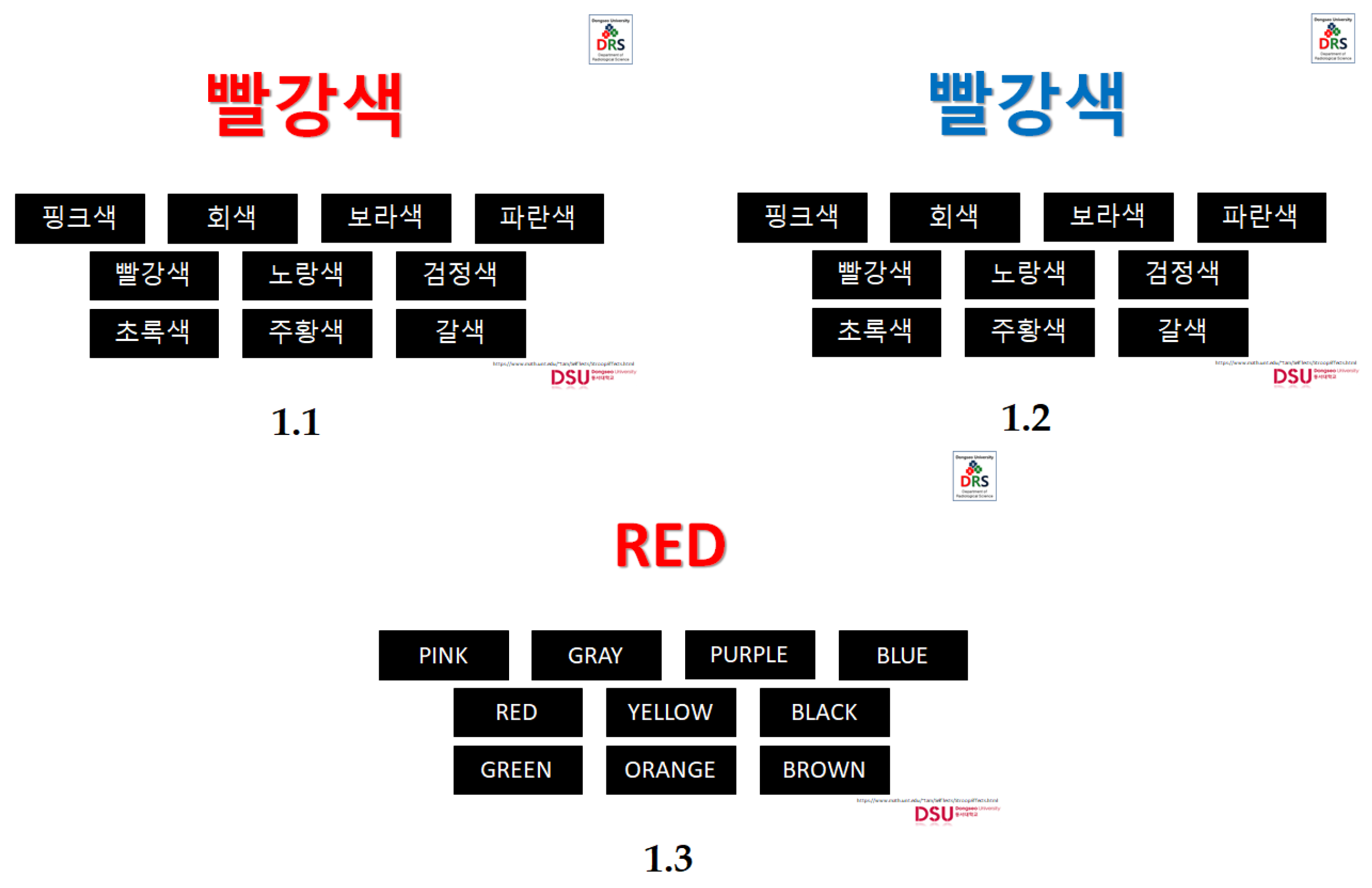
Specifically, the classic Stroop task [
5] is among the popular ones that are used to test the attention control of an individual that has three conditions, such as the neutral condition, the congruent condition, and the incongruent condition. In the neutral condition, the word shown has no relevance to the ink color (e.g., the word “xxx” with an ink color of blue). The congruent condition means that the color word in itself has the same color as its ink color when shown (e.g., the word “blue” shown by using a blue ink color) and lastly, the incongruent condition in which the color word in itself does not represent its ink color when shown (e.g., the word “blue” shown by using a red ink color). In this task, the neutral condition versus the congruent condition displays facilitation, while the incongruent condition versus the congruent condition displays interference [
6,
7].
Treisman [
8], proposed that the inability to focus attention in one dimension among several dimensions is what triggers the interference. Since interference is present in the incongruent task, it is said that the incongruent task is more difficult as compared with the congruent task. To enable us to understand the ability to counteract this interference and to execute the needed response during the Stroop task, various theories were developed. One of those is the theory of response competition by Eriksen and Eriksen [
9], stating that the two different responses, such as the correct and incorrect response compete for the single response channel which necessitates the inhibition of the incorrect response. In the case of an incongruent task, the word response must be suppressed, leaving the color response, to make a correct response. Another theory is the theory of automaticity by Stirling [
10]. This theory supports the idea of prolonged exposure to the same stimulus leads to the production of automatic response wherein attention control will no longer be needed. This implies that repeated exposure of a subject to certain stimuli will make the execution of a certain task easier.
In addition to the following mentioned above, there are studies conducted in the past to determine brain activity using the Stroop task. A study among Nepalese medical students showed that it took a long time for the subjects to read incongruent Stroop tasks and 60% of them made mistakes in reading, unlike in the congruent task where none of them committed a mistake [
11]. In support of this, another study written by Hiroyasu and colleagues [
12] presented that the number of correct responses among healthy young subjects was higher during congruent color-word Stroop task as compared in the incongruent color-word Stroop task. Aside from this, researches in the past focused on the determination of brain activity during the Stroop task. The specific parts of the brain that were activated are identified and most of them established the role of the prefrontal cortex (PFC) in attention control in the presence of interference [
1,
4,
12,
13,
14,
15,
16] which is also the focus in this study with the use of the fNIRS device.
Among healthy subjects, the anterior cingulate cortex (ACC) that is connected with PFC [
4,
13] has shown activation in fMRI studies during the incongruent task of the Stroop task. Also, greater activation of the inferior frontal region [
13] was observed when the subjects underwent the incongruent Stroop task, with more activation noted on the left inferior frontal region than the right inferior frontal region than during congruent task [
12]. In the study of Lague–Beauvais and colleagues [
15], the bilateral ventral prefrontal cortex (VLPFC) and dorsolateral prefrontal cortex (DLPFC), particularly the left posterior DLPFC and right anterior DLPFC has also shown activation during interference condition among older adults, as opposed to younger adults showing no significant activation of the prefrontal cortex.
Other studies demonstrated a more general activation of the lateral prefrontal cortex [
4] and left dorsolateral regions [
14]. Studies were also made regarding the use of the Stroop task in Chinese dyslexic children and those with prefrontal lesions. Sun and colleagues [
4] demonstrated that the bilateral PFC activated upon doing the difficult color task for healthy groups, while the dyslexic group showing no significant PFC activation. Their findings suggest that conflict resolution among Chinese dyslexic children is not functional, as shown by the deactivation of their PFC. Meanwhile, Vendrell and colleagues [
16] focused on determining if there are differences among healthy subjects and patients with prefrontal lesions when doing the Stroop task. It was found out that patients with lesions in the right lateral PFC made errors than healthy subjects, which suggests that the right lateral PFC is mostly associated with Stroop task errors. Also, patients who have undergone left lobectomy were able to perform the Stroop task normally. More importantly, the role of the right PFC has been noted in sustained attention, contradicting most of the studies indicating that the left PFC has a role in the inhibition of responses.
Likewise, in the previous studies, different modifications of the Stroop task have been made to get the desired outcome, which was also done by the authors of this study in which self-made CWIT using the native and second language of the students were employed. [
7]. Previously the classic and modified Stroop condition were administered using different languages.
In detail, the study of Fang, Tzeng, and Alva [
17] tested the Chinese-English (with Chinese as their native language) and Spanish-English (with Spanish as their native language) students using color-word incongruent Stroop task in Chinese, English, and Spanish language. It was found out that the response time in the Stroop test displayed in their second language is longer (1.431 s vs. 1.221 s for Chinese-English group; 1.169 s vs. 1.110 s for Spanish-English group). Furthermore, the prefrontal cortex was found out to have a role in encountering two different languages.
Moreover, in the study of Bialystok and colleagues [
18] concerning the control in the cognitive aspect and lexical access involving younger and the older individuals doing bilingualism, it was concluded that in the congruent tasks, individuals can depend on the inadvertent reading reaction. However, Bugg and colleagues [
19] stressed that for an individual to respond correctly in the incongruent tasks, the conflict existing between automatic reading and color-naming response must be resolved.
Also, in the study of Marian and colleagues [
20], it was mentioned that in comparison with an individual who only speaks one language, those who can do bilingualism proficiently are expected to encounter two types of interference such as the color-naming and the language. Thus, Stroop effect requires inhibiting the dominant automatic reading response and language suppression that are not used in the specific task.
Though the study of Kim and colleagues [
21] focused on verbal working memory (WM), the study discussed the active and relevant type of memory involved during language comprehension and problem-solving using the native language (Korean) and second language (English) for a short period. As proposed, the system of the brain temporarily stored and manipulate the essential information needed for several cognitive operations and one of those is linguistic. Also, it was proposed that there are three components for the WM, such as the central executive that is responsible for attention control and flow of information; second is the visuospatial sketch pad in which the visual images were set and maintained, and lastly is the phonological loop which the storage and rehearsal of verbal information happened. The findings of their study suggested that the right dorsolateral PFC is activated during the use of both languages.
In the study of Schroeter and colleagues [
4], the effect of the Stroop task in the hemodynamic response was specifically investigated and it was concluded in their study that in the incongruent tasks, the response is stronger as compared with the congruent task. Hemodynamic response means oxygenated- and total-hemoglobin increased while a decrease in deoxygenated hemoglobin happened which is the same definition in the present study.
As compared with the above-mentioned studies, the present study specifically investigated the hemodynamic response of the prefrontal cortex of the brain during the congruent and incongruent self-made CWIT among healthy Korean university students using their native and second language.
4. Discussion
The are several available modalities being used nowadays in determining the brain’s activity and in particular, this study focused on the PFC during the administration of self-made CWIT monitored by the fNIRS device to determine if there are any significant differences per trial, between trials in CKC-1 and IKC, between CKC-1 and CEC-3, and across trials using the test scores and VR. Moreover, it is hypothesized in this study that there are significant differences. Aside from the fact that this study focused on the differences present between two sets of tests instead of within- and between-language differences, the authors took the time to analyze brain connectivity.
The conducted experiment revealed that in
Table 1 both congruent conditions recorded the highest and higher scores, with overall mean scores of 92.02 and 85.05 than an incongruent condition with an overall score of 82.73. CKC-1 and CEC-3 respectively. This result is supported by the study of Hiroyasu and colleagues [
12] where higher correct responses were also attained in the congruent tasks. Moreover, in the study of Bialystok and colleagues [
18], it was concluded that the reason for better performance in the congruent task was due to inadvertent reading were both the reading and color-naming have are similar of having the true response. This is supported by the theory of Sterling [
10] which is the automaticity that is best to be applied in the congruent conditions since both the ink of the word and the word itself have the same response where control in the attention is no longer needed. Meanwhile, the reason for the incongruent tasks to record the lowest scores is that conflict between color naming was not addressed to get the true response as supported by Bugg and colleagues [
19]. Also, the response competition theory of Eriksen and Eriksen [
9] supported this for suppression of the word in the incongruent task that must be done to make the response correctly.
Likewise, the result of the native language involving VR in the congruent and incongruent was supported by the study of Schroeter and colleagues [
4]. The findings were particularly higher in the IKC-2 with a mean of 0.00027 as compared with CKC-1 with −0.31. It was mentioned that brain activation involved stronger hemodynamic response due to interference presented
In terms of the significant differences in the means of test scores, as shown in
Table 2,
Table 3 and
Table 4 the
p-value
s obtained were 0.58 and 0.82, 0.79 and 0.42, and lastly, 0.32 and 0.37 respectively. For VR responses in
Table 5,
Table 6 and
Table 7 per trial in each condition the
p-value
s were 0.77 and 0.12, and 0.07 and 0.09, and lastly 0.36 and 0.72. The findings showed that there are no significant differences when statistical treatment data was applied thus the hypothesis in this study is rejected. The reason is that no intervention is provided during rest time such as a review of the test items where the subjects got incorrect responses. As supported by Dweck’s Mindset Theory in which interventions give progressive and likely outcomes as mentioned in the study of Orosz and colleagues [
38].
In comparison with the significant difference of mean scores obtained per trial between CKC-1 and IKC-2, the hypothesis is accepted that there are significant differences. Across all trials, differences can be observed in
Table 8,
Table 9 and
Table 10 which scores in CKC-1 of 91.21, 91.12, and 92.73 are higher as compared with IKC-2 test scores of 81.21, 84.85, and 82.12. Aside from the studies of Bialystok and colleagues [
18] and Bugg and colleagues [
19] to support the findings of the present study, Triesman [
8] validated this result that incongruent conditions are difficult than incongruent conditions due to interferences presented, in particular the color itself. On the other hand, for the mean of the VR in
Table 11,
Table 12 and
Table 13, it is only in Trial 3 (
Table 13) where significant differences were observed having a
p-value of 0.027. A significant difference in this trial can be observed for the reason that when conferring with the mean responses as shown in
Table 1, the lowest mean of VR was recorded in this Trial across conditions except in IKC-2.
For CKC-1 and CEC-3 as shown in
Table 14,
Table 15 and
Table 16, despite their congruency conditions it is still evident that significant differences can be observed between them having
p-value
s of 0.001, 0.003, and 0.000 respectively. These findings are supported by the study of Marian and colleagues [
20] where color and language proficiency is expected to be encountered by individuals who are bilinguals. Meanwhile, in terms of the mean of the VR in
Table 17,
Table 18 and
Table 19 with
p-values of 0.939, 0.812, and 0.027 respectively, it is only in Trial 3 of
Table 19 where the hypothesis is accepted. The significant difference is most likely to be observed since brain activation is stronger in the incongruent conditions as compared with the congruent tasks causing the increase in VR as mentioned in the study of Schroeter [
4].
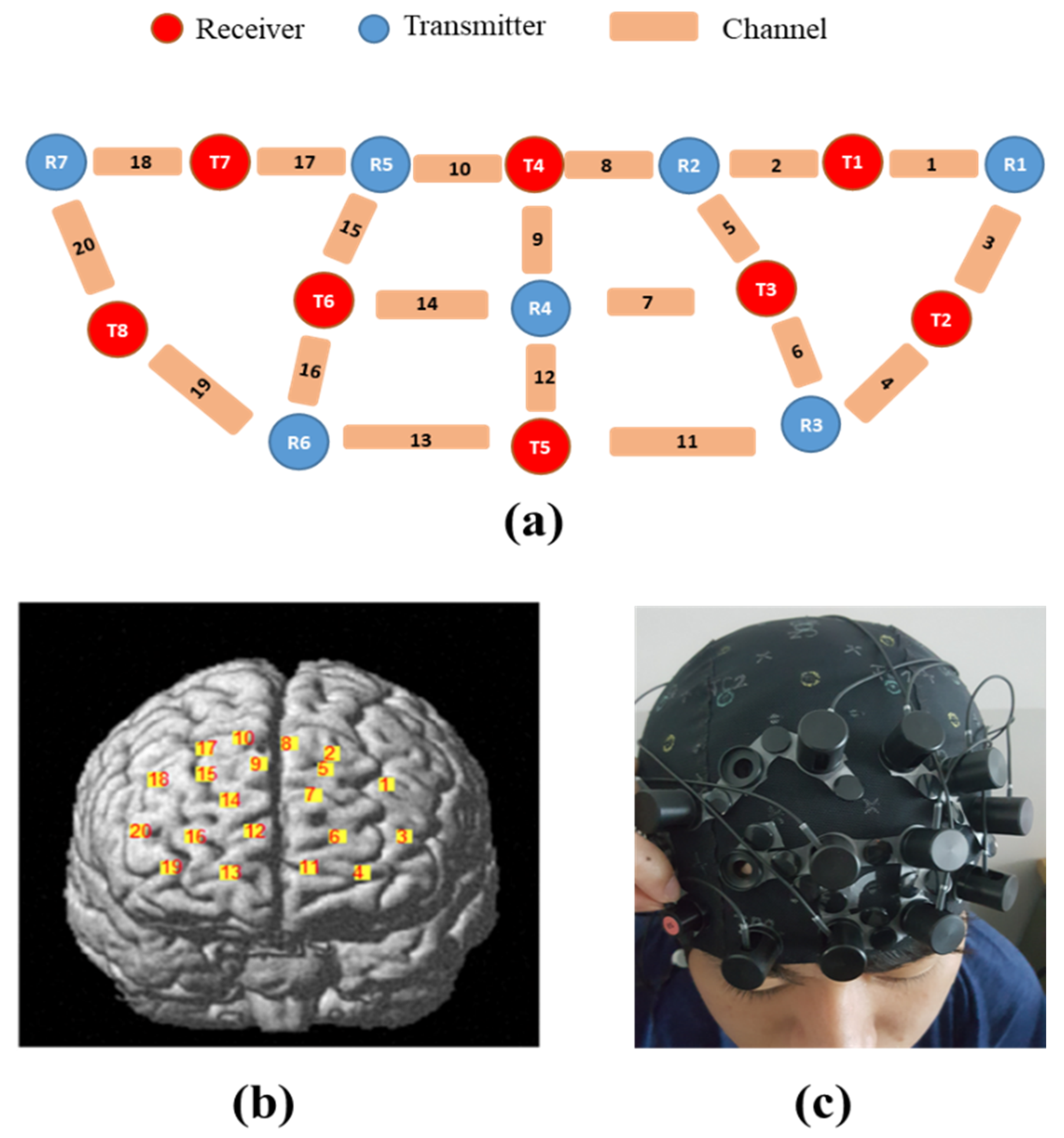
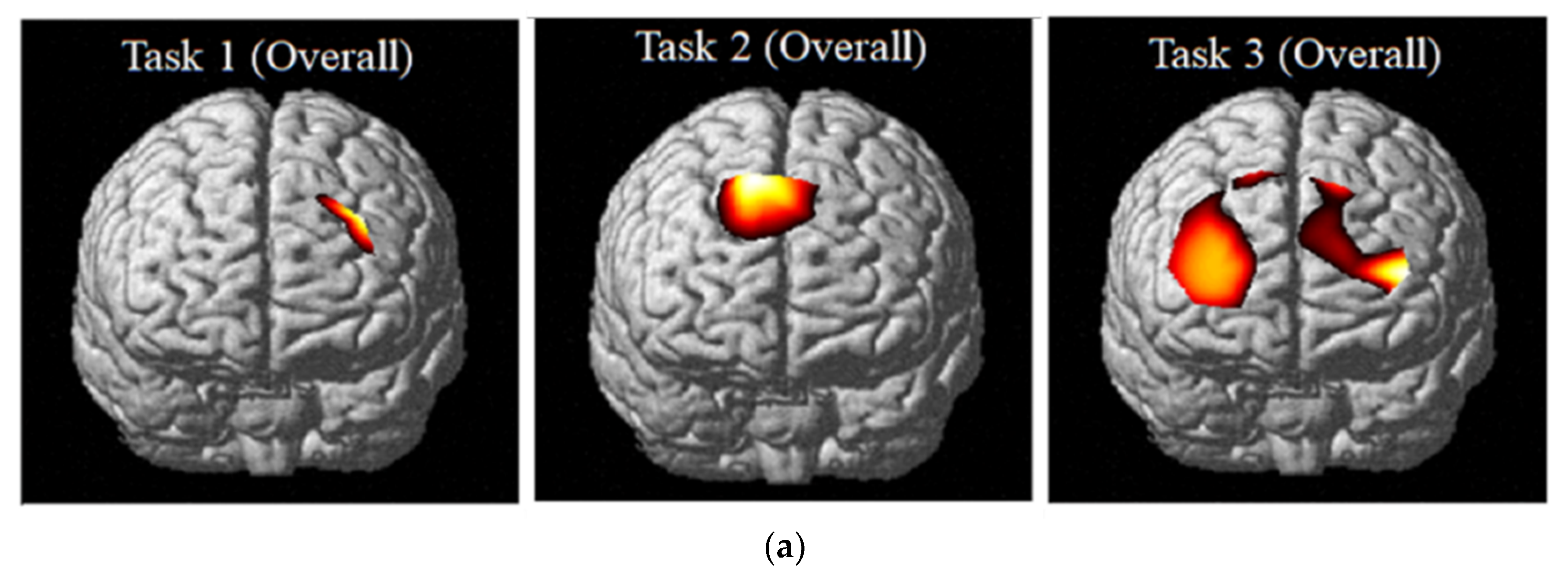
In terms of the analysis in the brain connectivity using the PLV that can identify the phase-locking between the two neuro-electric signals [
37], CEC-3 showed connectivities in the left and right of dorsomedial and dorsolateral of pre-frontal areas strongly. CKC-1 and IKC-2 were the same but less in CKC-1 due to less brain activation in the congruent conditions as compared with incongruent therefore less VR response [
4]. The entire activated parts of PFC can be seen on the figures in this study using the
t-values shown in
Table 20. PFC is activated since it is responsible for executive functions [
6] and language based on several studies conducted in the past [
1,
12,
13,
14,
15,
16].
In light of the above findings, it can be concluded that test scores and VR differ between the congruent and incongruent conditions due to interferences encountered. Moreover, in the connectivity analysis, right and left PFC are activated on ECC-3. In CKC-1 and IKC-2, encompassing to dorsomedial and dorsolateral although CKC-1 has less connection and connectivity due to less brain activation as compared.
Aside from color, the language was also considered a factor that must be suppressed in this experiment to give a correct response. Moreover, the t-values obtained showed specific parts of the brain that are activated that entirely focused on the PFC. Due to technical difficulties encountered, the data for IEC-4 were not presented. Moreover, response time was not included which the authors plan to address in future studies such as the automatic checking of answers using a developed software with corresponding response time and improved security in cap placement. However, the authors believe the result of this study could be of use as a reference among future researchers in using fNIRS to determine the brain connectivity among patients in a non-invasive way using non-ionizing radiation device without requiring the patient to go to the hospital since it is portable and at low-cost, thus promoting health, comfort, and financial advantages among patients towards a high quality of life.