Explaining Age at Autism Spectrum Diagnosis in Children with Migrant and Non-Migrant Background in Austria
Abstract
1. Introduction
Aim and Hypotheses
2. Methods
2.1. Study Design
2.2. Study Variables
2.3. Procedures
2.4. Statistical Analysis
3. Results
3.1. Descriptive Results
3.2. Bivariate Analysis
3.3. Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Christensen, D.L.; Maenner, M.J.; Bilder, D.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; Pettygrove, S.D.; Robinson, C.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 4 Years-Early Autism and Developmental Disabilities Monitoring Network, Seven Sites, United States, 2010, 2012, and 2014. MMWR. Surveill. Summ. 2019, 68, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Atladottir, H.O.; Schendel, D.E.; Henriksen, T.B.; Hjort, L.; Parner, E.T. Gestational Age and Autism Spectrum Disorder: Trends in Risk Over Time. Autism Res. 2016, 9, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Boilson, A.M.; Staines, A.; Ramirez, A.; Posada, M.; Sweeney, M.R. Operationalisation of the European Protocol for Autism Prevalence (EPAP) for Autism Spectrum Disorder Prevalence Measurement in Ireland. J. Autism Dev. Disord. 2016, 46, 3054–3067. [Google Scholar] [CrossRef]
- Reichow, B. Overview of meta-analyses on early intensive behavioral intervention for young children with autism spectrum disorders. J. Autism Dev. Disord. 2012, 42, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Rydzewska, E.; Hughes-McCormack, L.A.; Gillberg, C.; Henderson, A.; MacIntyre, C.; Rintoul, J.; Cooper, S.-A. Age at identification, prevalence and general health of children with autism: Observational study of a whole country population. BMJ Open 2019, 9, e025904. [Google Scholar] [CrossRef]
- Bejarano-Martin, A.; Canal-Bedia, R.; Magan-Maganto, M.; Fernandez-Alvarez, C.; Cilleros-Martin, M.V.; Sanchez-Gomez, M.C.; Garcia-Primo, P.; Rose-Sweeney, M.; Boilson, A.; Linertova, R.; et al. Early Detection, Diagnosis and Intervention Services for Young Children with Autism Spectrum Disorder in the European Union (ASDEU): Family and Professional Perspectives. J. Autism Dev. Disord. 2019. [Google Scholar] [CrossRef]
- Allison, C.; Williams, J.; Scott, F.; Stott, C.; Bolton, P.; Baron-Cohen, S.; Brayne, C. The Childhood Asperger Syndrome Test (CAST): Test-Retest Reliability in a High Scoring Sample. Autism Int. J. Res. Pract. 2007, 11, 173–185. [Google Scholar] [CrossRef]
- Bolton, S.; McDonald, D.; Curtis, E.; Kelly, S.; Gallagher, L. Autism in a recently arrived immigrant population. Eur. J. Pediatr. 2014, 173, 337–343. [Google Scholar] [CrossRef]
- Haglund, N.G.S.; Kallen, K.B.M. Risk factors for autism and Asperger syndrome. Perinatal factors and migration. Autism Int. J. Res. Pract. 2011, 15, 163–183. [Google Scholar] [CrossRef]
- Lehti, V.; Hinkka-Yli-Salomaki, S.; Cheslack-Postava, K.; Gissler, M.; Brown, A.S.; Sourander, A. The risk of childhood autism among second-generation migrants in Finland: A case-control study. BMC Pediatr. 2013, 13, 171. [Google Scholar] [CrossRef]
- Fuentes, J.; Basurko, A.; Isasa, I.; Galende, I.; Muguerza, M.D.; García-Primo, P.; García, J.; Fernández-Álvarez, C.J.; Canal-Bedia, R.; La Posada de Paz, M. The ASDEU autism prevalence study in northern Spain. Eur. Child Adolesc. Psychiatry 2020. [Google Scholar] [CrossRef]
- Kawa, R.; Saemundsen, E.; Loa Jonsdottir, S.; Hellendoorn, A.; Lemcke, S.; Canal-Bedia, R.; Garcia-Primo, P.; Moilanen, I. European studies on prevalence and risk of autism spectrum disorders according to immigrant status-a review. Eur. J. Public Health 2017, 27, 101–110. [Google Scholar] [CrossRef]
- Croen, L.A.; Grether, J.K.; Hoogstrate, J.; Selvin, S. The changing prevalence of autism in California. J. Autism Dev. Disord. 2002, 32, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Yeargin-Allsopp, M.; Rice, C.; Karapurkar, T.; Doernberg, N.; Boyle, C.; Murphy, C. Prevalence of autism in a US metropolitan area. JAMA 2003, 289, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Nevison, C.; Zahorodny, W. Race/Ethnicity-Resolved Time Trends in United States ASD Prevalence Estimates from IDEA and ADDM. J. Autism Dev. Disord. 2019, 49, 4721–4730. [Google Scholar] [CrossRef]
- EUROSTAT. Migration and Migrant Population Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Migration_and_migrant_population_statistics#Migrant_population:_22.3_million_non-EU_citizens_living_in_the_EU_on_1_January_2018 (accessed on 4 April 2020).
- Bundesministerium Europäische und Internationale Angelegenheiten. Statistisches Jahrbuch “migration & Integration 2019”: Integrationsbericht 2019. Available online: https://www.bmeia.gv.at/integration/integrationsbericht/ (accessed on 4 April 2020).
- Bundesministerium Europäische und Internationale Angelegenheiten. Österreich Integrationsbericht 2019: Integrationsbericht 2019: Integration in Österreich-Zahlen, Entwicklungen, Schwerpunkte. Available online: https://www.bmeia.gv.at/fileadmin/user_upload/Zentrale/Integration/Integrationsbericht_2019/Migration-Integration-2019.pdf (accessed on 4 April 2020).
- Hertz-Picciotto, I.; Delwiche, L. The rise in autism and the role of age at diagnosis. Epidemiology 2009, 20, 84–90. [Google Scholar] [CrossRef]
- Russell, G.; Norwich, B. Dilemmas, diagnosis and de-stigmatization: Parental perspectives on the diagnosis of autism spectrum disorders. Clin. Child Psychol. Psychiatry 2012, 17, 229–245. [Google Scholar] [CrossRef]
- Dawson, G. Recent advances in research on early detection, causes, biology, and treatment of autism spectrum disorders. Curr. Opin. Neurol. 2010, 23, 95–96. [Google Scholar] [CrossRef]
- Warren, Z.; McPheeters, M.L.; Sathe, N.; Foss-Feig, J.H.; Glasser, A.; Veenstra-Vanderweele, J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics 2011, 127, e1303–e1311. [Google Scholar] [CrossRef]
- Hayes, S.A.; Watson, S.L. The impact of parenting stress: A meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J. Autism Dev. Disord. 2013, 43, 629–642. [Google Scholar] [CrossRef]
- Koegel, L.K.; Koegel, R.L.; Ashbaugh, K.; Bradshaw, J. The importance of early identification and intervention for children with or at risk for autism spectrum disorders. Int. J. Speech-Lang. Pathol. 2014, 16, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Horlin, C.; Falkmer, M.; Parsons, R.; Albrecht, M.A.; Falkmer, T. The cost of autism spectrum disorders. PLoS ONE 2014, 9, e106552. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.W.; Mulick, J.A. System and cost research issues in treatments for people with autistic disorders. J. Autism Dev. Disord. 2000, 30, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.B.; Hsu, J.; Boerma, T. Universal health coverage and universal access. Bull. World Health Organ. 2013, 91, 546–546A. [Google Scholar] [CrossRef]
- Mandell, D.S.; Novak, M.M.; Zubritsky, C.D. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics 2005, 116, 1480–1486. [Google Scholar] [CrossRef]
- Szymanski, C.A.; Brice, P.J.; Lam, K.H.; Hotto, S.A. Deaf children with autism spectrum disorders. J. Autism Dev. Disord. 2012, 42, 2027–2037. [Google Scholar] [CrossRef]
- Bickel, J.; Bridgemohan, C.; Sideridis, G.; Huntington, N. Child and family characteristics associated with age of diagnosis of an autism spectrum disorder in a tertiary care setting. J. Dev. Behav. Pediatr. 2015, 36, 1–7. [Google Scholar] [CrossRef]
- Eriksson, M.A.; Westerlund, J.; Hedvall, Å.; Åmark, P.; Gillberg, C.; Fernell, E. Medical conditions affect the outcome of early intervention in preschool children with autism spectrum disorders. Eur. Child Adolesc. Psychiatry 2013, 22, 23–33. [Google Scholar] [CrossRef]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef]
- Harstad, E.; Huntington, N.; Bacic, J.; Barbaresi, W. Disparity of care for children with parent-reported autism spectrum disorders. Acad. Pediatr. 2013, 13, 334–339. [Google Scholar] [CrossRef]
- Delobel-Ayoub, M.; Ehlinger, V.; Klapouszczak, D.; Maffre, T.; Raynaud, J.-P.; Delpierre, C.; Arnaud, C. Socioeconomic Disparities and Prevalence of Autism Spectrum Disorders and Intellectual Disability. PLoS ONE 2015, 10, e0141964. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Meaney, F.J.; Levy, S.E.; DiGuiseppi, C.; Nicholas, J.S.; Kirby, R.S.; Pinto-Martin, J.A.; Schieve, L.A. Socioeconomic inequality in the prevalence of autism spectrum disorder: Evidence from a U.S. cross-sectional study. PLoS ONE 2010, 5, e11551. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Autism spectrum disorders in Hispanics and non-Hispanics. Autism Int. J. Res. Pract. 2012, 16, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.M.; Mandell, D.S. Explaining differences in age at autism spectrum disorder diagnosis: A critical review. Autism Int. J. Res. Pract. 2014, 18, 583–597. [Google Scholar] [CrossRef]
- Mandell, D.S.; Listerud, J.; Levy, S.E.; Pinto-Martin, J.A. Race differences in the age at diagnosis among medicaid-eligible children with autism. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 1447–1453. [Google Scholar] [CrossRef]
- Mandell, D.S.; Morales, K.H.; Xie, M.; Lawer, L.J.; Stahmer, A.C.; Marcus, S.C. Age of diagnosis among Medicaid-enrolled children with autism, 2001–2004. Psychiatr. Serv. 2010, 61, 822–829. [Google Scholar] [CrossRef]
- Rivard, M.; Millau, M.; Magnan, C.; Mello, C.; Boulé, M. Snakes and Ladders: Barriers and Facilitators Experienced by Immigrant Families when Accessing an Autism Spectrum Disorder Diagnosis. J. Dev. Phys. Disabil. 2019, 31, 519–539. [Google Scholar] [CrossRef]
- Valicenti-McDermott, M.; Hottinger, K.; Seijo, R.; Shulman, L. Age at diagnosis of autism spectrum disorders. J. Pediatr. 2012, 161, 554–556. [Google Scholar] [CrossRef]
- Samadi, S.A.; McConkey, R. Autism in developing countries: Lessons from iran. Autism Res. Treat. 2011, 2011, 145359. [Google Scholar] [CrossRef]
- VanDenHeuvel, A.; Fitzgerald, M.; Greiner, B.; Perry, I.J. Screening for autistic spectrum disorder at the 18-month developmental assessment: A population-based study. Ir. Med. J 2007, 100, 565–567. [Google Scholar]
- Liptak, G.S.; Benzoni, L.B.; Mruzek, D.W.; Nolan, K.W.; Thingvoll, M.A.; Wade, C.M.; Fryer, G.E. Disparities in diagnosis and access to health services for children with autism: Data from the National Survey of Children’s Health. J. Dev. Behav. Pediatr. 2008, 29, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.M.; Pellicano, E.; Crane, L. Understanding and awareness of autism among Somali parents living in the United Kingdom. Autism Int. J. Res. Pract. 2019, 23, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, B.; Koola, M.M. Barriers faced by immigrant families of children with autism: A program to address the challenges. Asian J. Psychiatry 2019, 39, 53–57. [Google Scholar] [CrossRef]
- Zuckerman, K.E.; Sinche, B.; Mejia, A.; Cobian, M.; Becker, T.; Nicolaidis, C. Latino parents’ perspectives on barriers to autism diagnosis. Acad. Pediatr. 2014, 14, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.B.; Skinner, D.; Correa, V.; Arcia, E.; Reyes-Blanes, M.E.; Rodriguez, P.; Vázquez-Montilla, E.; Skinner, M. Needs and supports reported by Latino families of young children with developmental disabilities. Am. J. Ment. Retard. 1999, 104, 437–451. [Google Scholar] [CrossRef]
- Dyches, T.T.; Wilder, L.K.; Sudweeks, R.R.; Obiakor, F.E.; Algozzine, B. Multicultural issues in autism. J. Autism Dev. Disord. 2004, 34, 211–222. [Google Scholar] [CrossRef]
- Rogers-Dulan, J.; Blacher, J. African American families, religion, and disability: A conceptual framework. Ment. Retard. 1995, 33, 226–238. [Google Scholar]
- Matson, J.L.; Matheis, M.; Burns, C.O.; Esposito, G.; Venuti, P.; Pisula, E.; Misiak, A.; Kalyva, E.; Tsakiris, V.; Kamio, Y.; et al. Examining cross-cultural differences in autism spectrum disorder: A multinational comparison from Greece, Italy, Japan, Poland, and the United States. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2017, 42, 70–76. [Google Scholar] [CrossRef]
- Pondé, M.P.; Rousseau, C.; Carlos, M.A.C. Pervasive developmental disorder in the children of immigrant parents: Comparison of different assessment instruments. Arq. Neuropsiquiatr. 2013, 71, 877–882. [Google Scholar] [CrossRef]
- Carruthers, S.; Kinnaird, E.; Rudra, A.; Smith, P.; Allison, C.; Auyeung, B.; Chakrabarti, B.; Wakabayashi, A.; Baron-Cohen, S.; Bakolis, I.; et al. A cross-cultural study of autistic traits across India, Japan and the UK. Mol. Autism 2018, 9, 52. [Google Scholar] [CrossRef]
- American Psychological Association. Ethical Principles of Psychologists and Code of Conduct (2002, Amended Effective 1 June 2010, and 1 January 2017). 2017. Available online: https://www.apa.org/ethics/code/ethics-code-2017.pdf (accessed on 14 July 2020).
- STATcube- Statistical Database of STATISCS AUSTRIA. Available online: http://www.statistik.at/web_en/publications_services/statcube/index.html (accessed on 14 July 2020).
- Fellinger, J.; Holzinger, D. Creating innovative clinical and service models for communication: Institut fuer Sinnes- und Sprachneurologie. J. Dev. Behav. Pediatr. 2014, 35, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Laister, D.; Vivanti, G.; Barbaresi, W.J.; Fellinger, J. Feasibility and Outcomes of the Early Start Denver Model Implemented with Low Intensity in a Community Setting in Austria. J. Dev. Behav. Pediatr. 2019, 40, 354–363. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Ebrary, Inc. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993; ISBN 9789241544559. [Google Scholar]
- Poustka, L. ADOS-2: Diagnostische Beobachtungsskala für Autistische Störungen 2; Hogrefe: Göttingen, Germany, 2015. [Google Scholar]
- Mullen, E.M. Mullen Scales of Early Learning (AGS ed.); American Guidance Service Inc.: Circle Pines, MN, USA, 1995. [Google Scholar]
- Bayley scales of infant and toddler development. In Harcourt Assessment; Springer: Boston, MA, USA, 2006.
- Petermann, F.; Petermann, U.J. Hamburg Wechsler Intelligenztest für Kinder IV (HAWIK-IV); Huber: Bern, Switzerland, 2008. [Google Scholar]
- Gotham, K.; Pickles, A.; Lord, C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; Le, C.A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Gearing, R.E.; Mian, I.A.; Barber, J.; Ickowicz, A. A methodology for conducting retrospective chart review research in child and adolescent psychiatry. J. Can. Acad. Child Adolesc. Psychiatry = J. de l’Academie canadienne de psychiatrie de l’enfant et de l’adolescent 2006, 15, 126–134. [Google Scholar]
- Vassar, M.; Holzmann, M. The retrospective chart review: Important methodological considerations. J. Educ. Eval. Health Prof. 2013, 10, 12. [Google Scholar] [CrossRef]
- The World Medical Association, Inc. (WMA). WMA DoH Übersetzung DE_Rev 190905: Declaration of Helsinki of 1975, 2013 Reviewed 2013. Available online: https://www.wma.net/policy/hb-e-version-2019/ (accessed on 14 July 2020).
- Cohen, J.; Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed.; Routledge, Taylor & Francis Group: New York, NY, USA; London, UK, 2003; ISBN 9780805822236. [Google Scholar]
- Kelley, K.; Preacher, K.J. On effect size. Psychol. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef]
- Peterson, R.A.; Brown, S.P. On the use of beta coefficients in meta-analysis. J. Appl. Psychol. 2005, 90, 175–181. [Google Scholar] [CrossRef]
- Enders, C.K. Multiple imputation as a flexible tool for missing data handling in clinical research. Behav. Res. Ther. 2017, 98, 4–18. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys, [Book on Demand]; John Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-471-65574-9. [Google Scholar]
- Keller, B.T.; Enders, C.K. Blimp User’s Manual (Version 1.0), Los Angeles, CA, USA. 2017. Available online: http://www.appliedmissingdata.com/blimpuserguide-4.pdf (accessed on 14 July 2020).
- von Hippel, P.T. How Many Imputations Do You Need? A Two-stage Calculation Using a Quadratic Rule. Sociol. Methods Res. 2018. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide, 8th ed.; Muthén & Muthén: Los Angeles, CA, USA, 1998–2017. [Google Scholar]
- The Jamovi Project. Jamovi. (Version 1.1), 2019, Sydney, Australia. Available online: https://www.jamovi.org/about.html (accessed on 14 July 2020).
- Schenk, L.; Kamtsiuris, P.; Ellert, U. Ambulante Versorgung von Kindern mit Migrationshintergrund–Inanspruchnahme und Zufriedenheit. Gesundheitswesen 2009, 71. [Google Scholar] [CrossRef]
- Zuckerman, K.E.; Mattox, K.; Donelan, K.; Batbayar, O.; Baghaee, A.; Bethell, C. Pediatrician identification of Latino children at risk for autism spectrum disorder. Pediatrics 2013, 132, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.; Rai, D.; Goodman, A.; Lundberg, M.; Idring, S.; Svensson, A.; Koupil, I.; Serlachius, E.; Dalman, C. Migration and autism spectrum disorder: Population-based study. Br. J. Psychiatry 2012, 201, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Rasmussen, F.; Sundelin, C. Early identification of children with communication disabilities--evaluation of a screening programme in a Swedish county. Acta Paediatr. 1996, 85, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Lampi, K.M.; Lehtonen, L.; Tran, P.L.; Suominen, A.; Lehti, V.; Banerjee, P.N.; Gissler, M.; Brown, A.S.; Sourander, A. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J. Pediatr. 2012, 161, 830–836. [Google Scholar] [CrossRef]
- Kiing, J.S.H.; Neihart, M.; Chan, Y.-H. Teachers’ role in identifying young children at risk for developmental delay and disabilities: Usefulness of the Parents Evaluation of Developmental Status tool. Child Care Health Dev. 2019, 45, 637–643. [Google Scholar] [CrossRef]
- Henrichs, J.; Rescorla, L.; Donkersloot, C.; Schenk, J.J.; Raat, H.; Jaddoe, V.W.V.; Hofman, A.; Verhulst, F.C.; Tiemeier, H. Early Vocabulary Delay and Behavioral/Emotional Problems in Early Childhood: The Generation R Study. J. Speech Lang. Hear. Res. 2013, 56, 553–566. [Google Scholar] [CrossRef]
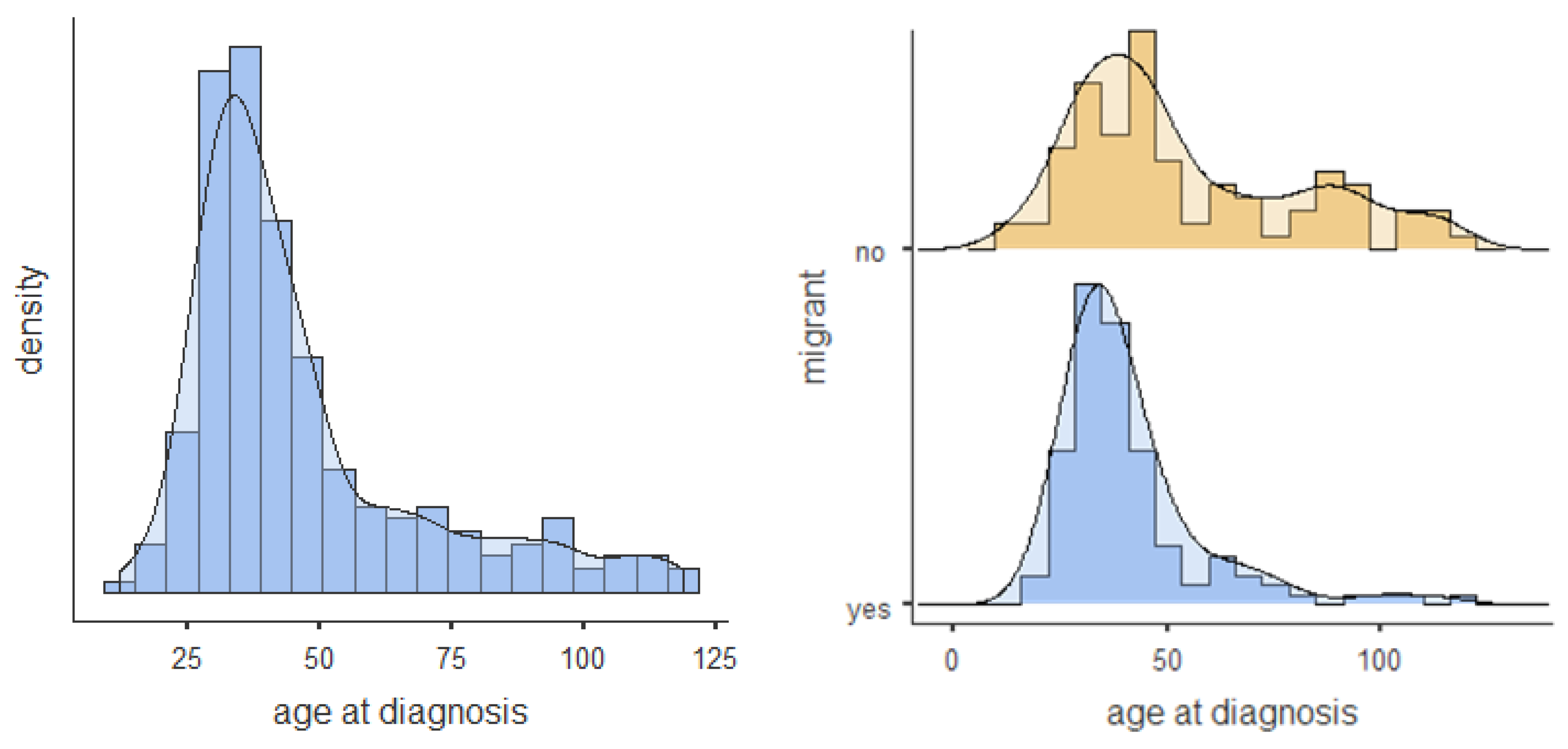
| Total (n = 211) | Non-Migrants (n = 91) | Migrants (n = 120) | Difference | ||||
|---|---|---|---|---|---|---|---|
| Values | %M | M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | ES r a | p | |
| Age at Diagnosis | 12–119 m.o. | 0% | 46.7 (22.80) | 53.96 (26.86) | 41.19 (17.33) | 0.278 | <0.001 |
| Sociodemographic Characteristics | |||||||
| Family Residence in Urban Area | No/Yes | 0% | 148 (70.1%) | 51 (56.0%) | 97 (80.8%) | 0.268 | <0.001 |
| Distance to Hospital | 0–187 km | 0% | 38.27 (37.50) | 49.27 (41.67) | 29.93 (31.73) | 0.256 | <0.001 |
| Parental Level of Education above High School | No/Yes | 41% | 37 (30.1%) | 23 (42.6%) | 14 (20.3%) | 0.241 | 0.007 |
| Clinical Characteristics | |||||||
| Male Gender | No/Yes | 0% | 174 (82.5%) | 77 (84.6%) | 97 (80.8%) | 0.049 | 0.474 |
| Non-Verbal Developmental Quotient, (D/IQ) | 16–122 | 13% | 62.26 (18.89) | 65.5 (21.15) | 59.67 (16.56) | 0.155 | 0.036 |
| D/IQ 50 or Below | No/Yes | 13% | 37 (20.1%) | 16 (19.8%) | 21 (20.4%) | 0.008 | 0.915 |
| Expressive Language Quotient (ELQ) | 13–128 | 15% | 46.38 (24.44) | 57.42 (28.93) | 39.34 (18.46) | 0.340 | <0.001 |
| ELQ 50 or Below | No/Yes | 15% | 118 (65.9%) | 37 (49.3%) | 81 (77.90%) | 0.297 | <0.001 |
| Receptive Language Quotient (RLQ) | 15–133 | 15% | 48.25 (24.65) | 59.6 (29.04) | 40.75 (18.23) | 0.355 | <0.001 |
| RLQ Score 50 or Below | No/Yes | 15% | 116 (64.8%) | 36 (47.4%) | 80 (77.70%) | 0.314 | <0.001 |
| ADOS-Calibrated Severity Scores—Total CSS | |||||||
| Social Affect—CSS | 1–10 | 18% | 6.35 (2.19) | 5.83 (2.16) | 6.71 (2.15) | 0.591. | 0.041 |
| Repetitive Behaviour—CSS | 1–10 | 18% | 5.85 (1.8) | 5.76 (1.86) | 5.91 (1.86) | 0.009. | 0.198 |
| ICD-10 ASD Code | |||||||
| Autism Disorder | 0% | 122 (57.80%) | 38 (41.8%) | 84 (70.00%) | 0.385 | <0.001 | |
| Asperger‘s Disorder | 21 (10%) | 20 (22%) | 1 (0.80%) | ||||
| PDD-nos | 68 (32.20%) | 33 (36.2%) | 35 (29.20%) | ||||
| Referral to Diagnosis | |||||||
| Referred by Paediatrician | No/Yes | 27% | 76 (36%) | 23 (25.3%) | 53 (44.2%) | 0.289 | <0.001 |
| Total | Non-Migrants | Migrants | Correlation Difference between Groups a | |
|---|---|---|---|---|
| Sociodemographic Characteristics | r | r | r | p |
| Family Residence in Urban Area | −0.132 | −0.073 | −0.047 | 0.684 |
| Distance Between Home And Hospital in Km | 0.031 | −0.050 | −0.034 | 0.740 |
| Parental Level of Education above High School | 0.100 | 0.198 | −0.169 * | 0.035 |
| Clinical Characteristics | ||||
| Male Gender | 0.011 | 0.095 | −0.048 | 0.252 |
| Non-Verbal Developmental Quotient (IQ) | 0.065 | 0.123 | −0.131 | 0.136 |
| Expressive Language Quotient (ELQ) | 0.423 *** | 0.443 *** | 0.216 | 0.014 |
| Receptive Language Quotient (RLQ) | 0.355 *** | 0.341 *** | 0.177 | 0.059 |
| ADOS-Calibrated Severity Scores—Total CSS | ||||
| Social Affect—CSS | −0.158 * | 0.011 | −0.263 ** | 0.206 |
| Repetitive Behaviour—CSS | −0.370 *** | −0.446 *** | −0.326 *** | 0.186 |
| Referred by Paediatrician | −0.255 *** | −0.195 | −0.220 * | 0.707 |
| Age at Diagnosis < 48 Months | Age at Diagnosis ≥ 48 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| (1) Non-Migrants (n = 50) | (2) Migrants (n = 97) | (3) Non-Migrants (n = 41) | (4) Migrants (n = 23) | Correlation Differences between Groups (p-Values) a | ||||
| Sociodemographic Characteristics | r | r | r | r | 1, 2 | 3, 4 | 1, 3 | 2, 4 |
| Family Residence in Urban Area | −0.079 | 0.167 | 0.033 | −0.114 | 0.244 | 0.597 | 0.700 | 0.461 |
| Home Distance to Hospital in km | −0.009 | −0.122 | −0.282* | 0.179 | 0.711 | 0.059 | 0.118 | 0.265 |
| Parental Level of Education above High School | 0.037 | −0.094 | 0.167 | −0.197 | 0.574 | 0.204 | 0.418 | 0.559 |
| Clinical Characteristics | ||||||||
| Male Gender | 0.090 | 0.034 | −0.124 | −0.200 | 0.680 | 0.848 | 0.355 | 0.204 |
| Non-Verbal Developmental Quotient (IQ) | 0.066 | −0.150 | 0.168 | 0.235 | 0.305 | 0.989 | 0.371 | 0.213 |
| Expressive Language Quotient (ELQ) | 0.097 | −0.172 | 0.462 *** | 0.186 | 0.249 | 0.158 | 0.006 | 0.284 |
| Receptive Language Quotient (RLQ) | 0.007 | −0.122 | 0.346 * | 0.332 | 0.662 | 0.659 | 0.037 | 0.097 |
| ADOS-Calibrated Severity Scores—Total CSS | ||||||||
| Social Affect—CSS | −0.111 | −0.128 | 0.144 | 0.023 | 0.916 | 0.623 | 0.294 | 0.779 |
| Repetitive Behaviour—CSS | 0.125 | 0.002 | −0.477 *** | −0.530 ** | 0.531 | 0.989 | 0.003 | 0.045 |
| Referred by paediatrician | −0.085 | −0.026 | −0.087 | −0.148 | 0.702 | 0.836 | 0.785 | 0.550 |
| b (SE) | β | 95%-CI | |
|---|---|---|---|
| Sociodemographic Characteristics | |||
| Family Residence in Urban Area | 1.139 (3.457) | 0.023 | (−0.113, 0.159) |
| Distance between Home and Hospital in km | −0.020 (0.038) | −0.033 | (−0.156, 0.089) |
| Parental Level of Education above High School | −3.719 (4.103) | −0.073 | (−0.231, 0.085) |
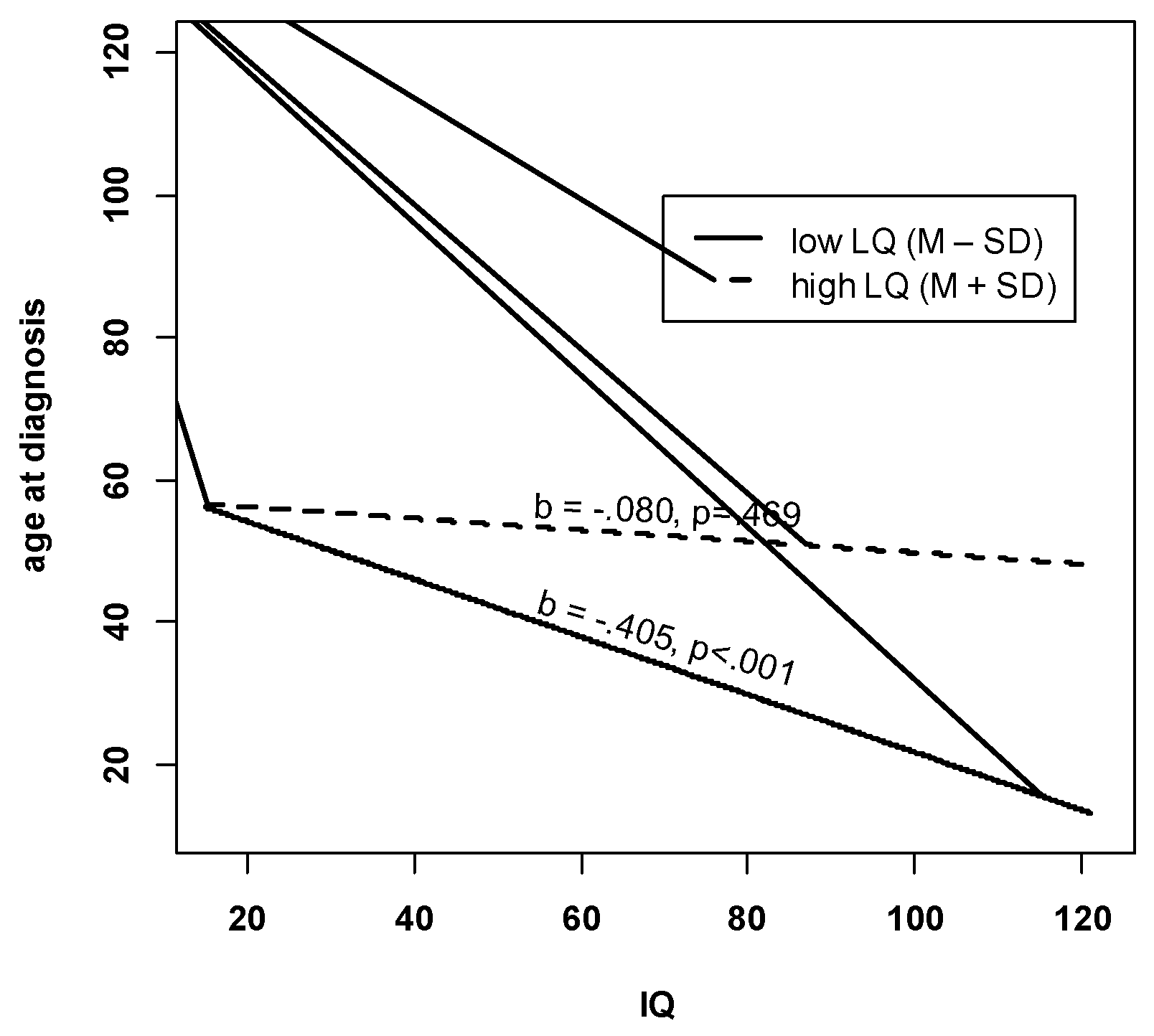
| Migration Status | −6.228 (3.305) | −0.136 | (−0.275, 0.004) |
| Clinical Characteristics | |||
| Male Gender | 1.539 (3.433) | 0.026 | (−0.087, 0.138) |
| Non-Verbal Developmental Quotient | −0.219 * (0.098) | −0.175 | (−0.336, −0.014) |
| Language Composite (Expressive and Receptive) | 0.363 *** (0.090) | 0.389 | (0.204, 0.574) |
| ADOS-Calibrated Severity Scores—Total CSS | |||
| Social Affect—CSS | −0.133 (0.739) | −0.013 | (−0.157, 0.131) |
| Repetitive Behaviour—CSS | −3.575 *** (0.941) | −0.301 | (−0.452, −0.150) |
| Referred by paediatrician | −9.370 ** (3.375) | −0.206 | (−0.349, −0.063) |
| R2 | 0.346 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia Primo, P.; Weber, C.; Posada de la Paz, M.; Fellinger, J.; Dirmhirn, A.; Holzinger, D. Explaining Age at Autism Spectrum Diagnosis in Children with Migrant and Non-Migrant Background in Austria. Brain Sci. 2020, 10, 448. https://doi.org/10.3390/brainsci10070448
Garcia Primo P, Weber C, Posada de la Paz M, Fellinger J, Dirmhirn A, Holzinger D. Explaining Age at Autism Spectrum Diagnosis in Children with Migrant and Non-Migrant Background in Austria. Brain Sciences. 2020; 10(7):448. https://doi.org/10.3390/brainsci10070448
Chicago/Turabian StyleGarcia Primo, Patricia, Christoph Weber, Manuel Posada de la Paz, Johannes Fellinger, Anna Dirmhirn, and Daniel Holzinger. 2020. "Explaining Age at Autism Spectrum Diagnosis in Children with Migrant and Non-Migrant Background in Austria" Brain Sciences 10, no. 7: 448. https://doi.org/10.3390/brainsci10070448
APA StyleGarcia Primo, P., Weber, C., Posada de la Paz, M., Fellinger, J., Dirmhirn, A., & Holzinger, D. (2020). Explaining Age at Autism Spectrum Diagnosis in Children with Migrant and Non-Migrant Background in Austria. Brain Sciences, 10(7), 448. https://doi.org/10.3390/brainsci10070448





