Cholinergic System and NGF Receptors: Insights from the Brain of the Short-Lived Fish Nothobranchius furzeri
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Sampling
2.2. RNA Isolation and cDNA Synthesis
2.3. Quantitative Real Time-PCR
2.4. Statistical Analysis
2.5. In Vitro Transcription and Probe Synthesis
2.6. Fluorescence In Situ Hybridization
2.7. Combined Fluorescence In Situ Hybridization with Immunofluorescence
2.8. Light Immunohistochemistry
2.9. Microscopy
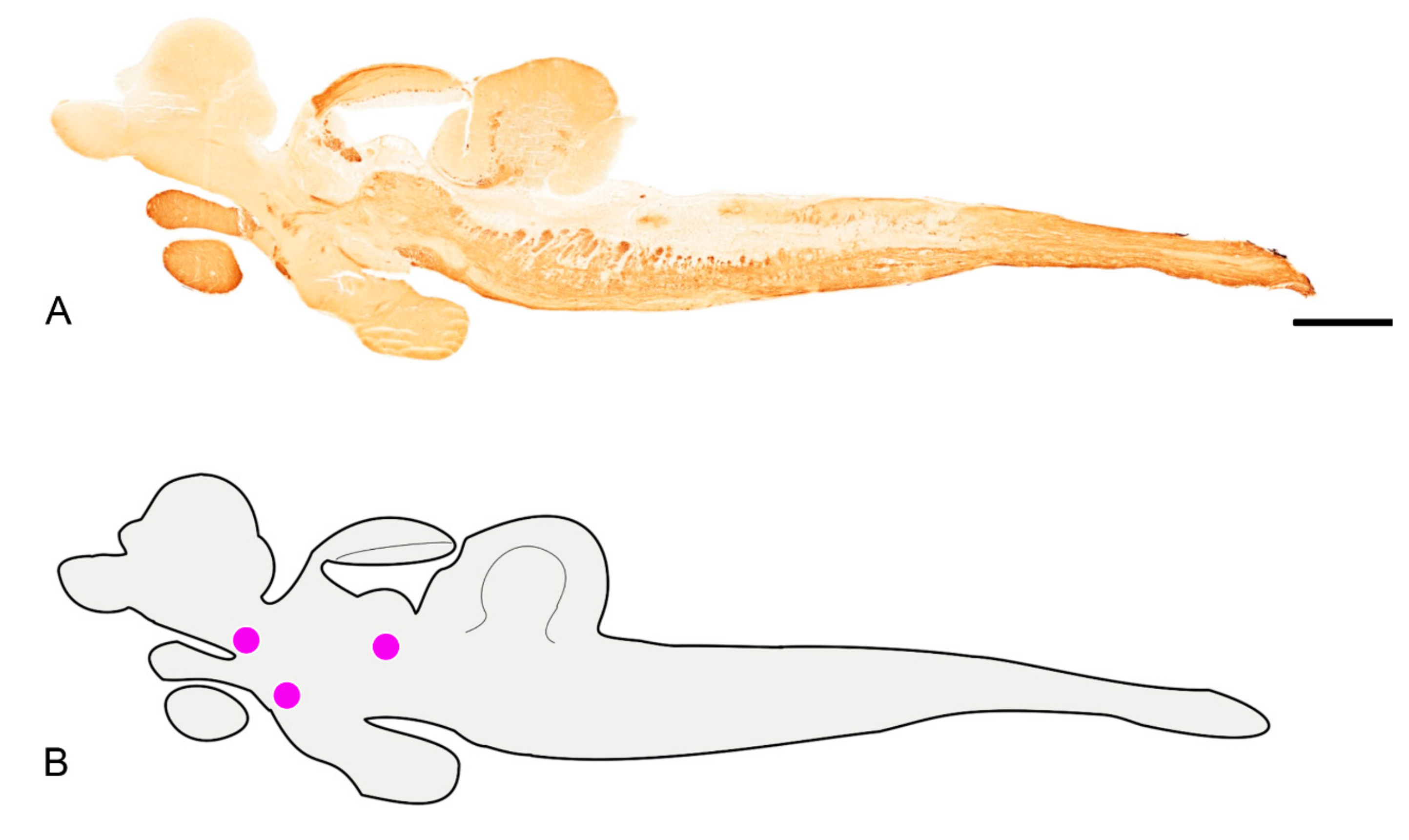
3. Results
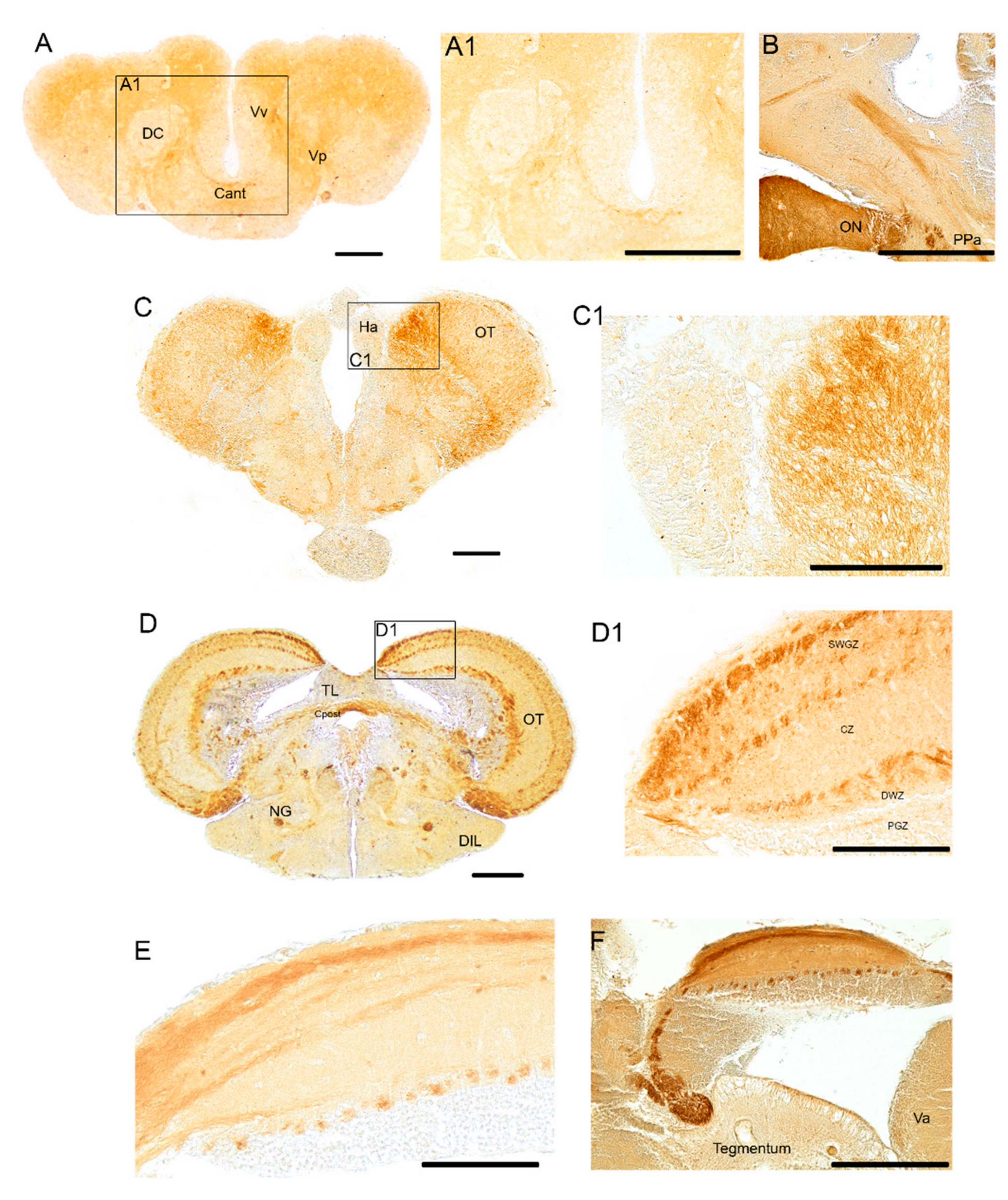
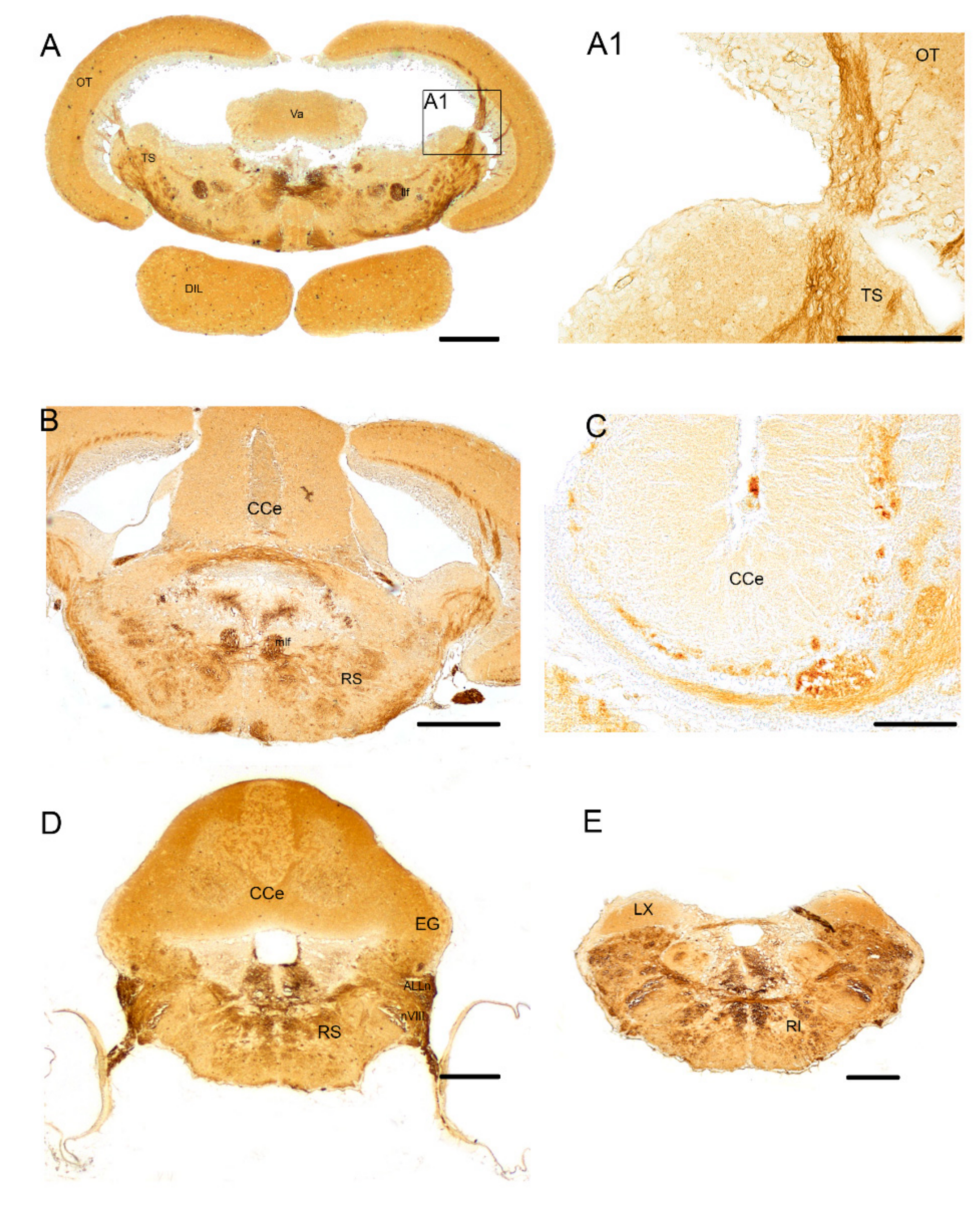
3.1. Pattern of Distribution of ChaT in the Adult Brain of N. furzeri
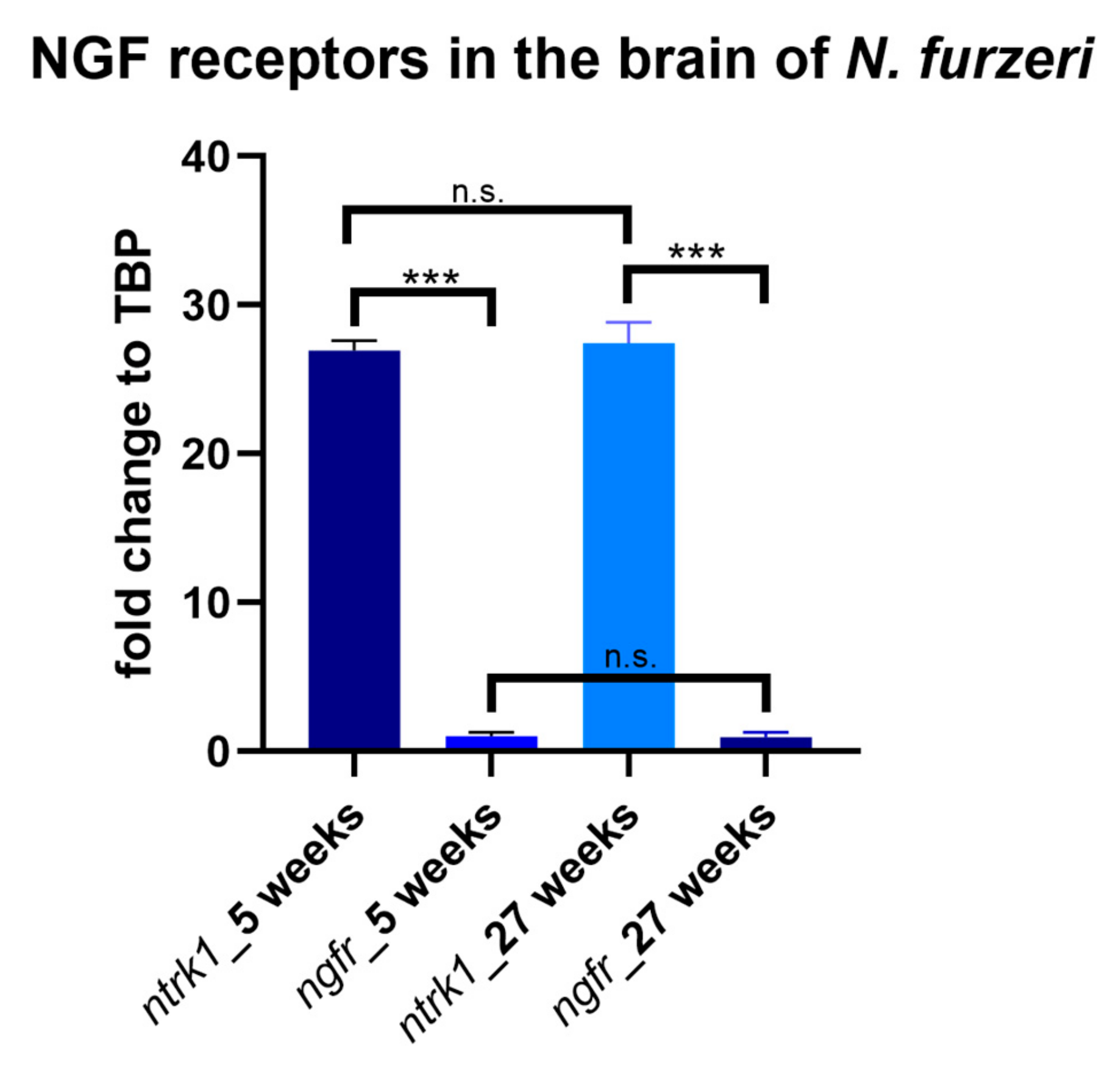
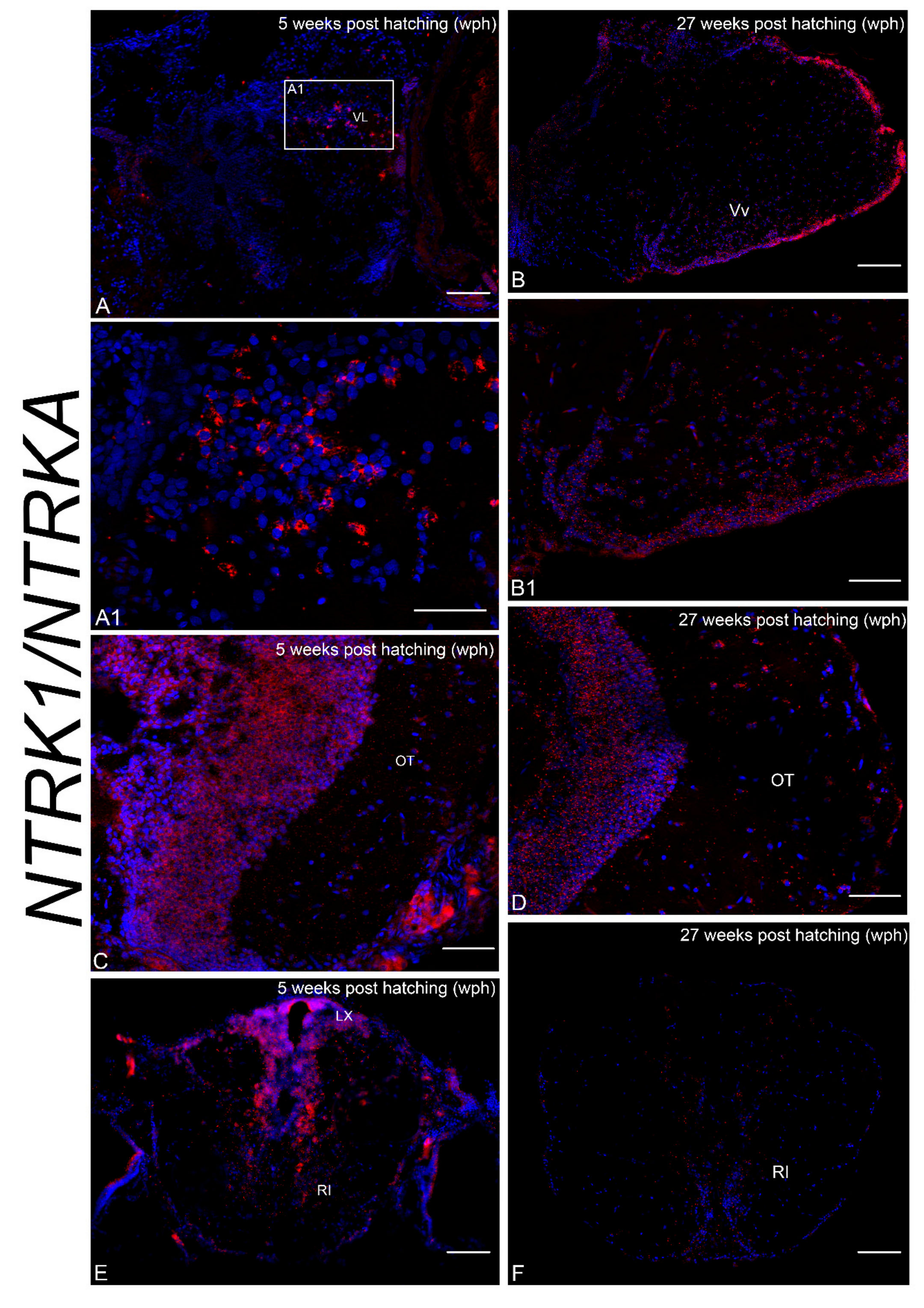
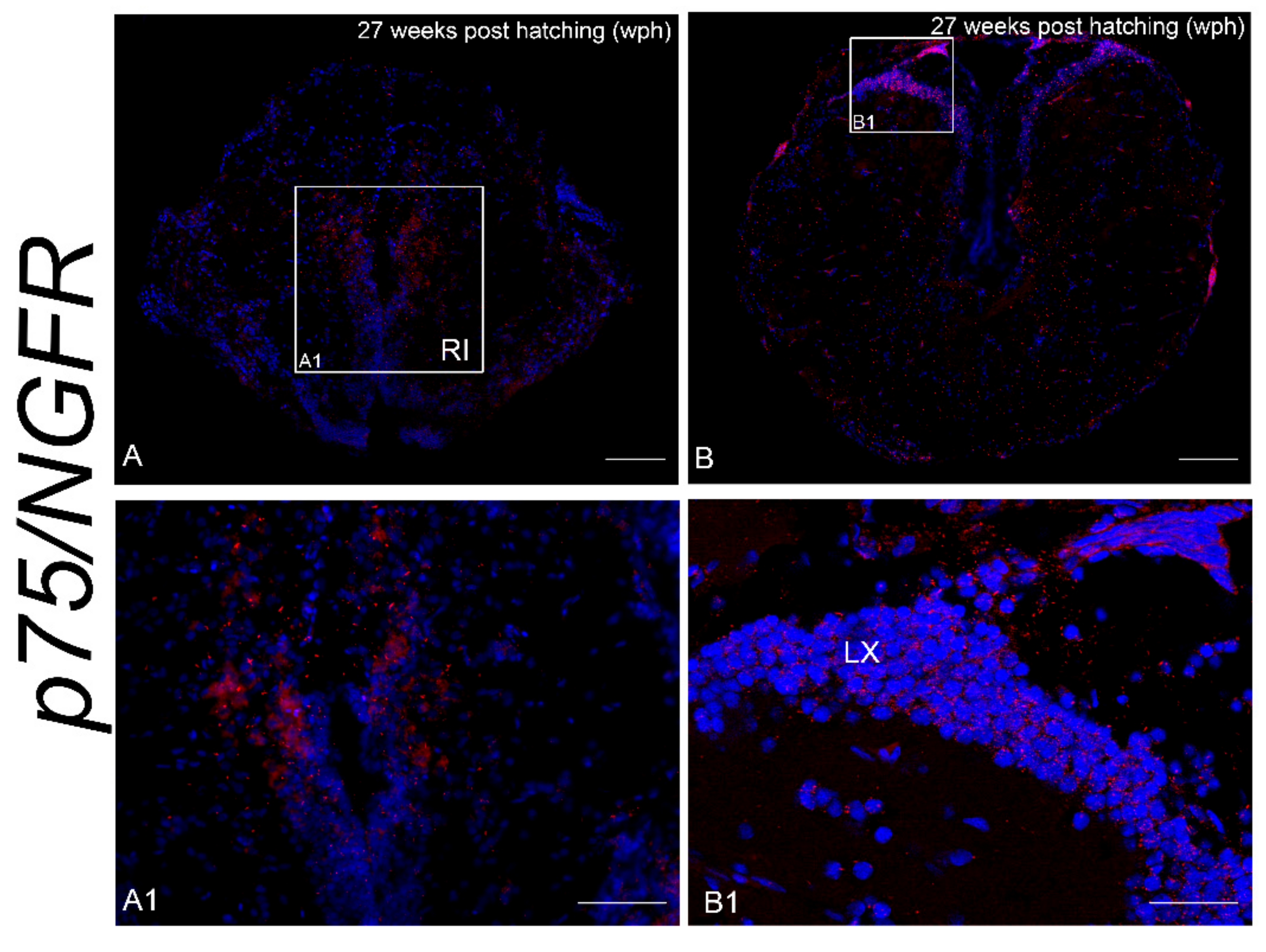
3.2. Age-Dependent Expression of NTRK1/NTRKA and p75/NGFR in the Brain of N. furzeri
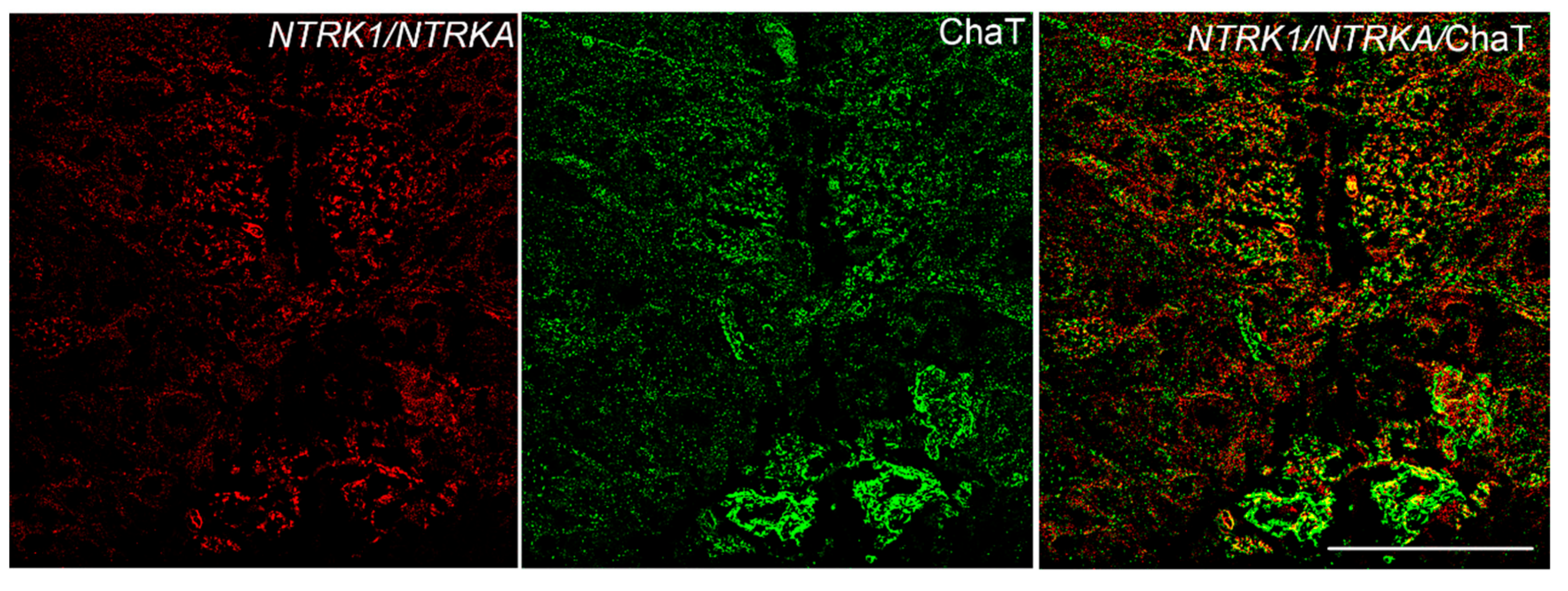
3.3. NTRK1/NTRKA and p75/NGFR Are Not Colocalized with ChaT
4. Discussion
4.1. Organization of the Cholinergic System in N. furzeri
4.2. Age-Associated Regulation of Nerve Growth Factor Receptors and Comparison of Their Neuroanatomical Expression
4.3. Nerve Growth Factor Receptors Are Not Expressed in Cholinergic Neurons of the Adult Brain of N. furzeri
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Angelo, L.; Lossi, L.; Merighi, A.; de Girolamo, P. Anatomical features for the adequate choice of experimental animal models in biomedicine: I. Fishes. Ann. Anat. 2016, 205, 75–84. [Google Scholar] [CrossRef]
- Fontana, B.D.; Mezzomo, N.J.; Kalueff, A.V.; Rosemberg, D.B. The developing utility of zebrafish models of neurological and neuropsychiatric disorders: A critical review. Exp. Neurol. 2018, 299, 157–171. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Tozzini, E.T.; Baumgart, M.; Battistoni, G.; Cellerino, A. Adult neurogenesis in the short-lived teleost Nothobranchius furzeri: Localization of neurogenic niches, molecular characterization and effects of aging. Aging Cell 2012, 11, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, K.; Glashauser, L.; Sprungala, S.; Hesl, B.; Fritschle, M.; Ninkovic, J.; Godinho, L.; Chapouton, P. Increased radial glia quiescence, decreased reactivation upon injury and unaltered neuroblast behavior underlie decreased neurogenesis in the aging zebrafish telencephalon. J Comp. Neurol. 2013, 521, 3099–3115. [Google Scholar] [CrossRef]
- Cellerino, A.; Valenzano, D.R.; Reichard, M. From the bush to the bench: The annual Nothobranchius fishes as a new model system in biology. Biol. Rev. Camb. Philos. Soc. 2016, 91, 511–533. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, H.G.; Valenzano, D.R. The short-lived African turquoise killifish: An emerging experimental model for ageing. Dis. Model. Mech. 2016, 9, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Groth, M.; Priebe, S.; Savino, A.; Testa, G.; Dix, A.; Ripa, R.; Spallotta, F.; Gaetano, C.; Ori, M.; et al. RNA-seq of the aging brain in the short-lived fish N. furzeri—Conserved pathways and novel genes associated with neurogenesis. Aging Cell 2014, 13, 965–974. [Google Scholar] [CrossRef]
- Terzibasi Tozzini, E.; Savino, A.; Ripa, R.; Battistoni, G.; Baumgart, M.; Cellerino, A. Regulation of microRNA expression in the neuronal stem cell niches during aging of the short-lived annual fish Nothobranchius furzeri. Front. Cell. Neurosci. 2014, 8, 51. [Google Scholar] [CrossRef]
- Leggieri, A.; Attanasio, C.; Palladino, A.; Cellerino, A.; Lucini, C.; Paolucci, M.; Terzibasi Tozzini, E.; de Girolamo, P.; D’Angelo, L. Identification and Expression of Neurotrophin-6 in the Brain of Nothobranchius furzeri: One More Piece in Neurotrophin Research. J. Clin. Med. 2019, 8, 595. [Google Scholar] [CrossRef]
- D’Angelo, L.; Avallone, L.; Cellerino, A.; de Girolamo, P.; Paolucci, M.; Varricchio, E.; Lucini, C. Neurotrophin-4 in the brain of adult Nothobranchius furzeri. Ann. Anat. 2016, 207, 47–54. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, L.; De Girolamo, P.; Lucini, C.; Terzibasi, E.T.; Baumgart, M.; Castaldo, L.; Cellerino, A. Brain-derived neurotrophic factor: mRNA expression and protein distribution in the brain of the teleost Nothobranchius furzeri. J. Comp. Neurol. 2014, 522, 1004–1030. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, L.; Castaldo, L.; Cellerino, A.; de Girolamo, P.; Lucini, C. Nerve growth factor in the adult brain of a teleostean model for aging research: Nothobranchius furzeri. Ann. Anat. 2014, 196, 183–191. [Google Scholar] [CrossRef]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. 2006, 110, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Underwood, C.K.; Coulson, E.J. The p75 neurotrophin receptor. Int. J. Biochem. Cell Biol. 2008, 40, 1664–1668. [Google Scholar] [CrossRef]
- Nykjaer, A.; Willnow, T.E.; Petersen, C.M. p75NTR--live or let die. Curr. Opin. Neurobiol. 2005, 15, 49–57. [Google Scholar] [CrossRef]
- Benito-Gutiérrez, E.; Garcia-Fernàndez, J.; Comella, J.X. Origin and evolution of the Trk family of neurotrophic receptors. Mol. Cell. Neurosci. 2006, 31, 179–192. [Google Scholar] [CrossRef]
- Martin, S.C.; Marazzi, G.; Sandell, J.H.; Heinrich, G. Five Trk receptors in the zebrafish. Dev. Biol. 1995, 169, 745–758. [Google Scholar] [CrossRef]
- D’Angelo, L.; de Girolamo, P.; Cellerino, A.; Tozzini, E.T.; Castaldo, L.; Lucini, C. Neurotrophin Trk receptors in the brain of a teleost fish, Nothobranchius furzeri. Microsc. Res. Tech. 2012, 75, 81–88. [Google Scholar] [CrossRef]
- Gatta, C.; Altamura, G.; Avallone, L.; Castaldo, L.; Corteggio, A.; D’Angelo, L.; de Girolamo, P.; Lucini, C. Neurotrophins and their Trk-receptors in the cerebellum of zebrafish. J. Morphol. 2016, 277, 725–736. [Google Scholar] [CrossRef]
- Ahuja, G.; Ivandic, I.; Saltürk, M.; Oka, Y.; Nadler, W.; Korsching, S.I. Zebrafish crypt neurons project to a single, identified mediodorsal glomerulus. Sci. Rep. 2013, 3, 2063. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Milani, L.; Lazzari, M.; Maurizii, M.G.; Franceschini, V. Crypt cell markers in the olfactory organ of Poecilia reticulata: Analysis and comparison with the fish model Danio rerio. Brain Struct. Funct. 2017, 222, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Sepahi, A.; Kraus, A.; Casadei, E.; Johnston, C.A.; Galindo-Villegas, J.; Kelly, C.; García-Moreno, D.; Muñoz, P.; Mulero, V.; Huertas, M.; et al. Olfactory sensory neurons mediate ultrarapid antiviral immune responses in a TrkA-dependent manner. Proc. Natl. Acad. Sci. USA 2019, 116, 12428–12436. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.; Diekmann, H.; Heinz, D.; Hirsch, C.; Hannbeck von Hanwehr, S.; Petrausch, B.; Oertle, T.; Schwab, M.E.; Stuermer, C.A. Identification of two nogo/rtn4 genes and analysis of Nogo-A expression in Xenopus laevis. Mol. Cell. Neurosci. 2004, 25, 205–216. [Google Scholar] [CrossRef][Green Version]
- Oertle, T.; Schwab, M.E. Nogo and its paRTNers. Trends Cell Biol. 2003, 13, 187–194. [Google Scholar] [CrossRef]
- Catchen, J.M.; Braasch, I.; Postlethwait, J.H. Conserved synteny and the zebrafish genome. Methods Cell Biol. 2011, 104, 259–285. [Google Scholar] [CrossRef]
- Huber, A.B.; Weinmann, O.; Brösamle, C.; Oertle, T.; Schwab, M.E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 2002, 22, 3553–3567. [Google Scholar] [CrossRef]
- O’Neill, P.; Whalley, K.; Ferretti, P. Nogo and Nogo-66 Receptor in Human and Chick: Implications for Development and Regeneration. Dev. Dyn. 2004, 231, 109–121. [Google Scholar] [CrossRef]
- Fournier, A.E.; GrandPre, T.; Strittmatter, S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001, 409, 341–346. [Google Scholar] [CrossRef]
- Josephson, A.; Widenfalk, J.; Widmer, H.W.; Olson, L.; Spenger, C. NOGO mRNA Expression in Adult and Fetal Human and Rat Nervous Tissue and in Weight Drop Injury. Exp. Neurol. 2001, 169, 319–328. [Google Scholar] [CrossRef]
- Segal, R.A. Selectivity in neurotrophin signaling: Theme and variations. Annu. Rev. Neurosci. 2003, 26, 299–330. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.O.; Casaccia-Bonnefil, P.; Carter, B.; Chao, M.V. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J. Neurosci. 1998, 18, 3273–3281. [Google Scholar] [CrossRef] [PubMed]
- Epa, W.R.; Markovska, K.; Barrett, G.L. The p75 Neurotrophin Receptor Enhances TrkA Signalling by Binding to Shc and Augmenting Its Phosphorylation. J. Neurochem. 2004, 89, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Pepeu, G.; Grazia Giovannini, M. The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 2017, 1670, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Merlio, J.P.; Ernfors, P.; Jaber, M.; Persson, H. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neuroscience 1992, 51, 513–532. [Google Scholar] [CrossRef]
- Yeo, T.T.; Chua-Couzens, J.; Butcher, L.L.; Bredesen, D.E.; Cooper, J.D.; Valletta, J.S.; Mobley, W.C.; Longo, F.M. Absence of p75NTR Causes Increased Basal Forebrain Cholinergic Neuron Size, Choline Acetyltransferase Activity, and Target Innervation. J. Neurosci. 1997, 17, 7594–7605. [Google Scholar] [CrossRef]
- Brösamle, C.; Halpern, M.E. Nogo-Nogo receptor signalling in PNS axon outgrowth and pathfinding. Mol. Cell. Neurosci. 2009, 40, 401–409. [Google Scholar] [CrossRef]
- Vilar, M.; Mira, H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef]
- Frade, J.M.; López-Sánchez, N. A Novel Hypothesis for Alzheimer Disease Based on Neuronal Tetraploidy Induced by p75 (NTR). Cell Cycle 2010, 9, 1934–1941. [Google Scholar] [CrossRef]
- Arancio, O.; Chao, M.V. Neurotrophins, Synaptic Plasticity and Dementia. Curr. Opin. Neurobiol. 2007, 17, 325–330. [Google Scholar] [CrossRef]
- Dawbarn, D.; Allen, S.J. Neurotrophins and Neurodegeneration. Neuropathol. Appl. Neurobiol. 2003, 29, 211–230. [Google Scholar] [CrossRef]
- Eckenstein, F.; Thoenen, H. Cholinergic neurons in the rat cerebral cortex demonstrated by immunohistochemical localization of choline acetyltransferase. Neurosci. Lett. 1983, 36, 211–215. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [PubMed]
- López, J.M.; Perlado, J.; Morona, R.; Northcutt, R.G.; González, A. Neuroanatomical organization of the cholinergic system in the central nervous system of a basal actinopterygian fish, the senegal bichir Polypterus senegalus. J. Comp. Neurol. 2013, 521, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Giraldez-Perez, R.M.; Gaytan, S.P.; Torres, B.; Pasaro, R. Co-localization of Nitric Oxide Synthase and Choline Acetyltransferase in the Brain of the Goldfish (Carassius Auratus). J. Chem. Neuroanat. 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Vernier, P.; Wullimann, M.F. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 2004, 1011, 156–169. [Google Scholar] [CrossRef]
- Kaslin, J.; Nystedt, J.M.; Ostergård, M.; Peitsaro, N.; Panula, P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J. Neurosci. 2004, 24, 2678–2689. [Google Scholar] [CrossRef]
- Clemente, D.; Porteros, A.; Weruaga, E.; Alonso, J.R.; Arenzana, F.J.; Aijón, J.; Arévalo, R. Cholinergic elements in the zebrafish central nervous system: Histochemical and immunohistochemical analysis. J. Comp. Neurol. 2004, 474, 75–107. [Google Scholar] [CrossRef]
- Pérez, S.E.; Yáñez, J.; Marín, O.; Anadón, R.; González, A.; Rodríguez-Moldes, I. Distribution of choline acetyltransferase (ChaT) immunoreactivity in the brain of the adult trout and tract-tracing observations on the connections of the nuclei of the isthmus. J. Comp. Neurol. 2000, 428, 450–474. [Google Scholar] [CrossRef]
- Molist, P.; Rodríguez-Moldes, I.; Anadón, R. Organization of catecholaminergic systems in the hypothalamus of two elasmobranch species, Raja undulata and Scyliorhinus canicula. A histofluorescence and immunohistochemical study. Brain Behav. Evol. 1993, 41, 290–302. [Google Scholar] [CrossRef]
- Brantley, R.K.; Bass, A.H. Cholinergic neurons in the brain of a teleost fish (Porichthys notatus) located with a monoclonal antibody to choline acetyltransferase. J. Comp. Neurol. 1988, 275, 87–105. [Google Scholar] [CrossRef]
- Ekström, P. Distribution of choline acetyltransferase-immunoreactive neurons in the brain of a cyprinid teleost (Phoxinus phoxinus L.). J. Comp. Neurol. 1987, 256, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Montesano, A.; Baumgart, M.; Avallone, L.; Castaldo, L.; Lucini, C.; Tozzini, E.T.; Cellerino, A.; D’Angelo, L.; de Girolamo, P. Age-related central regulation of orexin and NPY in the short-lived African killifish Nothobranchius furzeri. J. Comp. Neurol. 2019, 527, 1508–1526. [Google Scholar] [CrossRef]
- De Girolamo, P.; Lucini, C. Neuropeptide Localization in Nonmammalian Vertebrates. Methods Mol. Biol. 2011, 789, 37–56. [Google Scholar] [CrossRef]
- D’Angelo, L. Brain atlas of an emerging teleostean model: Nothobranchius furzeri. Anat. Rec. 2013, 296, 681–691. [Google Scholar] [CrossRef]
- Mesulam, M.M. Behavioral neuroanatomy of cholinergic innervation in the primate cerebral cortex. EXS 1989, 57, 1–11. [Google Scholar] [CrossRef]
- Panula, P.; Chen, Y.C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.J.; Bradburn, S.; Murgatroyd, C.; Toseeb, U.; Mir, P.; Kountouriotis, G.K.; Teipel, S.J.; Grothe, M.J. In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson’s disease. Brain 2018, 141, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Teipel, S.J.; Meindl, T.; Grinberg, L.; Grothe, M.; Cantero, J.L.; Reiser, M.F.; Möller, H.J.; Heinsen, H.; Hampel, H. The cholinergic system in mild cognitive impairment and Alzheimer’s disease: An in vivo MRI and DTI study. Hum. Brain Mapp. 2011, 32, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Allard, S.; Jacobs, M.L.; Do Carmo, S.; Cuello, A.C. Compromise of cortical proNGF maturation causes selective retrograde atrophy in cholinergic nucleus basalis neurons. Neurobiol. Aging 2018, 67, 10–20. [Google Scholar] [CrossRef]
- Motts, S.D.; Slusarczyk, A.S.; Sowick, C.S.; Schofield, B.R. Distribution of Cholinergic Cells in Guinea Pig Brainstem. Neuroscience 2008, 154, 186–195. [Google Scholar] [CrossRef]
- Gravett, N.; Bhagwandin, A.; Fuxe, K.; Manger, P.R. Nuclear Organization and Morphology of Cholinergic, Putative Catecholaminergic and Serotonergic Neurons in the Brain of the Rock Hyrax, Procavia Capensis. J. Chem. Neuroanat. 2009, 38, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Härtig, W.; Grosche, J.; Keijser, J.; Luiten, P.G.M.; Seeger, J.; Brauer, K.; Harkany, T. Rabbit Forebrain Cholinergic System: Morphological Characterization of Nuclei and Distribution of Cholinergic Terminals in the Cerebral Cortex and Hippocampus. J. Comp. Neurol. 2003, 460, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Manger, P.R.; Fahringer, H.M.; Pettigrew, J.D.; Siegel, J.M. The distribution and morphological characteristics of cholinergic cells in the brain of monotremes as revealed by ChaT immunohistochemistry. Brain Behav. Evol. 2002, 60, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Ajiki, K.; Matsuura, J.; Misawa, H. Localization of two cholinergic markers, choline acetyltransferase and vesicular acetylcholine transporter in the central nervous system of the rat: In situ hybridization histochemistry and immunohistochemistry. J. Chem. Neuroanat. 1997, 13, 23–39. [Google Scholar] [CrossRef]
- St-Jacques, R.; Gorczyca, W.; Mohr, G.; Schipper, H.M. Mapping of the Basal Forebrain Cholinergic System of the Dog: A Choline Acetyltransferase Immunohistochemical Study. J. Comp. Neurol. 1996, 366, 717–725. [Google Scholar] [CrossRef]
- Tago, H.; McGeer, P.L.; McGeer, E.G.; Akiyama, H.; Hersh, L.B. Distribution of choline acetyltransferase immunopositive structures in the rat brainstem. Brain Res. 1989, 495, 271–297. [Google Scholar] [CrossRef]
- Mufson, E.J.; Cunningham, M.G. Observations on Choline Acetyltransferase Containing Structures in the CD-1 Mouse Brain. Neurosci. Lett. 1988, 84, 7–12. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Mufson, E.J.; Levey, A.I.; Wainer, B.H. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience 1984, 12, 669–686. [Google Scholar] [CrossRef]
- Medina, L.; Reiner, A. Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. J. Comp. Neurol. 1994, 342, 497–537. [Google Scholar] [CrossRef]
- Sorenson, E.M.; Parkinson, D.; Dahl, J.L.; Chiappinelli, V.A. Immunohistochemical Localization of Choline Acetyltransferase in the Chicken Mesencephalon. J. Comp. Neurol. 1989, 281, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.S.; Reiner, A. The distribution of cholinergic neurons in the central nervous system of turtles. Brain Behav. Evol. 1993, 41, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Brauth, S.E.; Kitt, C.A.; Price, D.L.; Wainer, B.H. Cholinergic neurons in the telencephalon of the reptile Caiman crocodilus. Neurosci. Lett. 1985, 58, 235–240. [Google Scholar] [CrossRef]
- Mufson, E.J.; Desan, P.H.; Mesulam, M.M.; Wainer, B.H.; Levey, A.I. Choline acetyltransferase-like immunoreactivity in the forebrain of the red-eared pond turtle (Pseudemys scripta elegans). Brain Res. 1984, 323, 103–108. [Google Scholar] [CrossRef]
- González, A.; López, J.M.; Sánchez-Camacho, C.; Marín, O. Localization of choline acetyltransferase (ChaT) immunoreactivity in the brain of a caecilian amphibian, Dermophis mexicanus (Amphibia: Gymnophiona). J. Comp. Neurol. 2002, 448, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Marín, O.; Smeets, W.J.; González, A. Distribution of Choline Acetyltransferase Immunoreactivity in the Brain of Anuran (Rana Perezi, Xenopus Laevis) and Urodele (Pleurodeles Waltl) Amphibians. J. Comp. Neurol. 1997, 382, 499–534. [Google Scholar] [CrossRef]
- Maruska, K.P.; Butler, J.M.; Field, K.E.; Porter, D.T. Localization of Glutamatergic, GABAergic, and Cholinergic Neurons in the Brain of the African Cichlid Fish, Astatotilapia Burtoni. J. Comp. Neurol. 2017, 525, 610–638. [Google Scholar] [CrossRef]
- Giraldez-Perez, R.M.; Gaytan, S.P.; Pasaro, R. Cholinergic and Nitrergic Neuronal Networks in the Goldfish Telencephalon. Acta Neurobiol. Exp. 2013, 73, 338–353. [Google Scholar] [CrossRef]
- Rodríguez-Moldes, I.; Molist, P.; Adrio, F.; Pombal, M.A.; Yáñez, S.E.; Mandado, M.; Marín, O.; López, J.M.; González, A.; Anadón, R. Organization of cholinergic systems in the brain of different fish groups: A comparative analysis. Brain Res. Bull. 2002, 57, 331–334. [Google Scholar] [CrossRef]
- Edwards, J.G.; Greig, A.; Sakata, Y.; Elkin, D.; Michel, W.C. Cholinergic Innervation of the Zebrafish Olfactory Bulb. J. Comp. Neurol. 2007, 504, 631–645. [Google Scholar] [CrossRef]
- Casini, A.; Vaccaro, R.; Toni, M.; Cioni, C. Distribution of Choline Acetyltransferase (ChaT) Immunoreactivity in the Brain of the Teleost Cyprinus Carpio. Eur. J. Histochem. 2018, 62, 2932. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Reichwald, K.; Groth, M.; Taudien, S.; Hartmann, N.; Priebe, S.; Shagin, D.; Englert, C.; Platzer, M. The transcript catalogue of the short-lived fish Nothobranchius furzeri provides insights into age-dependent changes of mRNA levels. BMC Genom. 2013, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Scott-Solomon, E.; Kuruvilla, R. Mechanisms of Neurotrophin Trafficking via Trk Receptors Mol. Cell. Neurosci. 2018, 91, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Ma, S.Y.; Dills, J.; Cochran, E.J.; Leurgans, S.; Wuu, J.; Bennett, D.A.; Jaffar, S.; Gilmor, M.L.; Levey, A.I.; et al. Loss of basal forebrain P75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer’s disease. J. Comp. Neurol. 2002, 443, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Che, S.; Wuu, J.; Counts, S.E.; Mufson, E.J. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer’s disease. J. Neurochem. 2006, 97, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Counts, S.E.; Nadeem, M.; Wuu, J.; Ginsberg, S.D.; Saragovi, H.U.; Mufson, E.J. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer’s disease. Ann. Neurol. 2004, 56, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Josephy-Hernandez, S.; Jmaeff, S.; Pirvulescu, I.; Aboulkassim, T.; Saragovi, H.U. Neurotrophin receptor agonists and antagonists as therapeutic agents: An evolving paradigm. Neurobiol. Dis. 2017, 97, 139–155. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Girolamo, P.; Leggieri, A.; Palladino, A.; Lucini, C.; Attanasio, C.; D’Angelo, L. Cholinergic System and NGF Receptors: Insights from the Brain of the Short-Lived Fish Nothobranchius furzeri. Brain Sci. 2020, 10, 394. https://doi.org/10.3390/brainsci10060394
de Girolamo P, Leggieri A, Palladino A, Lucini C, Attanasio C, D’Angelo L. Cholinergic System and NGF Receptors: Insights from the Brain of the Short-Lived Fish Nothobranchius furzeri. Brain Sciences. 2020; 10(6):394. https://doi.org/10.3390/brainsci10060394
Chicago/Turabian Stylede Girolamo, Paolo, Adele Leggieri, Antonio Palladino, Carla Lucini, Chiara Attanasio, and Livia D’Angelo. 2020. "Cholinergic System and NGF Receptors: Insights from the Brain of the Short-Lived Fish Nothobranchius furzeri" Brain Sciences 10, no. 6: 394. https://doi.org/10.3390/brainsci10060394
APA Stylede Girolamo, P., Leggieri, A., Palladino, A., Lucini, C., Attanasio, C., & D’Angelo, L. (2020). Cholinergic System and NGF Receptors: Insights from the Brain of the Short-Lived Fish Nothobranchius furzeri. Brain Sciences, 10(6), 394. https://doi.org/10.3390/brainsci10060394








