Cholesterol and Alzheimer’s Disease Risk: A Meta-Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Studies of LDL-C Levels
3.2. Studies on HDL-C Levels
3.3. Studies on TC Levels
3.4. Studies of TG Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Zhong, X.; Shen, J.; Jiao, L.; Tong, J.; Zhao, W.; Du, K.; Gong, S.; Liu, M.; Wei, M. Elevated serum TC and LDL-C levels in Alzheimer’s disease and mild cognitive impairment: A meta-analysis study. Brain Res. 2020, 1727, 146554. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Anders, W.; Maëlenn, G.; Ali, G.C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia; Alzheimer’s Disease International (ADI): London, UK, 2015. [Google Scholar]
- Kapogiannis, D.; Mustapic, M.; Shardell, M.D.; Berkowitz, S.T.; Diehl, T.C.; Spangler, R.D.; Tran, J.; Lazaropoulos, M.P.; Chawla, S.; Gulyani, S. Association of extracellular vesicle biomarkers with Alzheimer disease in the Baltimore longitudinal study of aging. JAMA Neurol. 2019, 76, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shou, Y.; Pan, J.; Du, Y.; Liu, C.; Wang, H. The relationship between cholesterol level and Alzheimer’s disease-associated APP proteolysis/Aβ metabolism. Nutr. Neurosci. 2019, 22, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Khachaturian, Z.S.; Khachaturian, A.S. Politics of science: Progress toward prevention of the dementia–Alzheimer’s syndrome. Mol. Aspects Med. 2015, 43, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Álvarez-Álvarez, I.; Guillén-Grima, F.; Aguinaga-Ontoso, I. Prevalencia e incidencia de la enfermedad de Alzheimer en Europa: Metaanálisis. Neurología 2017, 32, 523–532. [Google Scholar] [CrossRef]
- Cao, Q.; Tan, C.-C.; Xu, W.; Hu, H.; Cao, X.-P.; Dong, Q.; Tan, L.; Yu, J.T. The Prevalence of Dementia: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2020, 73, 1157–1166. [Google Scholar] [CrossRef]
- Pedro-Cuesta, J.; Virués-Ortega, J.; Vega, S.; Seijo-Martínez, M.; Saz, P.; Rodríguez, F.; Rodríguez-Laso, A.; Reñé, R.; de las Heras, S.P.; Mateos, R.; et al. Prevalence of dementia and major dementia subtypes in Spanish populations: A reanalysis of dementia prevalence surveys, 1990–2008. BMC Neurol. 2009, 9, 55. [Google Scholar] [CrossRef]
- Dement, A. Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 459–509. [Google Scholar] [CrossRef]
- Lesser, G.T. Association of Alzheimer Disease Pathology with Abnormal Lipid Metabolism: The Hisayama Study. Neurology 2012, 78, 1280. [Google Scholar] [CrossRef]
- Agirbasli, M.; Tanrikulu, A.; Sevim, B.A.; Azizy, M.; Bekiroglu, N. Total cholesterol-to-high-density lipoprotein cholesterol ratio predicts high-sensitivity C-reactive protein levels in Turkish children. J. Clin. Lipidol. 2015, 9, 195–200. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Jia, X.; Zhang, H.; Zhang, H.; Li, J.; Zhang, K. Prediction of Alzheimer’s disease with serum lipid levels in Asian individuals: A meta-analysis. Biomarkers 2019, 24, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Zhao, Q.-F.; Li, J.-Q.; Wang, J.; Yu, J.-T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Sidney, S.; Selby, J.; Johnston, S.C.; Yaffe, K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005, 64, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Versmissen, J.; Oosterveer, D.M.; Hoekstra, M.; Out, R.; Berbée, J.F.; Blommesteijn-Touw, A.C.; van Vark-van der Zee, L.; Vongpromek, R.; Vanmierlo, T.; Defesche, J.C.; et al. Apolipoprotein Isoform E4 Does Not Increase Coronary Heart Disease Risk in Carriers of Low-Density Lipoprotein Receptor Mutations. Circ. Cardiovasc. Genet. 2011, 4, 655–660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nägga, K.; Gustavsson, A.-M.; Stomrud, E.; Lindqvist, D.; van Westen, D.; Blennow, K.; Zetterberg, H.; Melander, O.; Hansson, O. Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology 2018, 90, e73–e81. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. Alzheimer’s disease: The amyloid cascade hypothesis: An update and reappraisal. J. Alzheimers Dis. 2006, 9, 151–153. [Google Scholar] [CrossRef]
- Wang, H.-L.; Wang, Y.-Y.; Liu, X.-G.; Kuo, S.-H.; Liu, N.; Song, Q.-Y.; Wang, M.-W. Cholesterol, 24-Hydroxycholesterol, and 27-Hydroxycholesterol as Surrogate Biomarkers in Cerebrospinal Fluid in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2016, 51, 45–55. [Google Scholar] [CrossRef]
- Shibata, N.; Ohnuma, T.; Higashi, S.; Higashi, M.; Usui, C.; Ohkubo, T.; Watanabe, T.; Kawashima, R.; Kitajima, A.; Ueki, A.; et al. No genetic association between PCSK9 polymorphisms and Alzheimer’s disease and plasma cholesterol level in Japanese patients. Psychiatr. Genet. 2005, 15, 239. [Google Scholar] [CrossRef]
- Tynkkynen, J.; Hernesniemi, J.A.; Laatikainen, T.; Havulinna, A.S.; Sundvall, J.; Leiviskä, J.; Salo, P.; Salomaa, V. Apolipoproteins and HDL cholesterol do not associate with the risk of future dementia and Alzheimer’s disease: The National Finnish population study (FINRISK). AGE 2016, 38, 465–473. [Google Scholar] [CrossRef]
- Michikawa, M. Cholesterol paradox: Is high total or low HDL cholesterol level a risk for Alzheimer’s disease? J. Neurosci. Res. 2003, 72, 141–146. [Google Scholar] [CrossRef]
- Wolf, H.; Hensel, A.; Arendt, T.; Kivipelto, M.; Winblad, B.; Gertz, H.J. Association between serum HDL cholesterol and hippocampal volume: A link to Alzheimer’s disease? Neurobiol. Aging 2004, 25, 52. [Google Scholar] [CrossRef]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Hamel, C.; Wells, G.A.; Bouter, L.M.; Kristjansson, E.; Grimshaw, J.; Henry, D.A.; Boers, M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009, 62, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Cleophas, T.J.; Zwinderman, A.H. Meta-Meta-analysis. In Modern Meta-Analysis. Review and Update of Methodologies; Springer: Berlin/Heidelberg, Germany, 2017; pp. 135–143. [Google Scholar] [CrossRef]
- Reis, H.T.; Judd, C.M. Handbook of Research Methods in Social and Personality Psychology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Shrier, I.; Steele, R. Understanding the Relationship between Risks and Odds Ratios. Clin. J. Sport Med. 2006, 16, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Hussain, S.; Najmi, A.K. Number of studies, heterogeneity, generalisability, and the choice of method for meta-analysis. J. Neurol. Sci. 2017, 381, 347. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Borenstein, M.; Hedges, L.; Higgins, J.P.T.; Rothstein, H. Comprehensive meta-analysis version 3, Biostat: Englewood, NJ, USA, 2013.
- Cohen, J. Statistical Power Analysis For The Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, Y.; Zhang, X.; Xu, J.; Lin, J.; Zhang, R.; Kang, K.; Liu, C.; Zhao, C.; Zhao, M. Low-Density Lipoprotein Cholesterol and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 5. [Google Scholar] [CrossRef]
- Hall, J.R.; Wiechmann, A.R.; Johnson, L.A.; Edwards, M.; Barber, R.C.; Cunningham, R.; Singh, M.; O’bryant, S.E. Total cholesterol and neuropsychiatric symptoms in Alzheimer’s disease: The impact of total cholesterol level and gender. Dement. Geriatr. Cogn. Disord. 2014, 38, 300–309. [Google Scholar] [CrossRef]
- Tan, Z.S.; Seshadri, S.; Beiser, A.; Wilson, P.W.; Kiel, D.P.; Tocco, M.; D’Agostino, R.B.; Wolf, P.A. Plasma total cholesterol level as a risk factor for Alzheimer disease: The Framingham Study. Arch. Intern. Med. 2003, 163, 1053–1057. [Google Scholar] [CrossRef]
- Launer, L.J.; White, L.R.; Petrovitch, H.; Ross, G.W.; Curb, J.D. Cholesterol and neuropathologic markers of AD: A population-based autopsy study. Neurology 2001, 57, 1447–1452. [Google Scholar] [CrossRef]
- Reitz, C.; Tang, M.-X.; Luchsinger, J.; Mayeux, R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 2004, 61, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Praticò, D. Alzheimer’s disease: Phenotypic approaches using disease models and the targeting of tau protein. Expert Opin. Ther. Targets 2020. [Google Scholar] [CrossRef] [PubMed]
- Theofilas, P.; Ehrenberg, A.J.; Nguy, A.; Thackrey, J.M.; Dunlop, S.; Mejia, M.B.; Alho, A.T.; Leite, R.E.P.; Rodriguez, R.D.; Suemoto, C.K. Probing the correlation of neuronal loss, neurofibrillary tangles, and cell death markers across the Alzheimer’s disease Braak stages: A quantitative study in humans. Neurobiol. Aging 2018, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carleton, R.A.; Dwyer, J.; Finberg, L.; Flora, J.; Goodman, D.S.; Grundy, S.M.; Havas, S.; Hunter, G.T.; Kritchevsky, D.; Lauer, R.M. Report of the Expert Panel on Population Strategies for Blood Cholesterol Reduction. A statement from the National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health. Circulation 1991, 83, 2154–2232. [Google Scholar] [CrossRef]
- Sharrett, A.R.; Patsch, W.; Sorlie, P.D.; Heiss, G.; Bond, M.G.; Davis, C.E. Associations of lipoprotein cholesterols, apolipoproteins AI and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 1098–1104. [Google Scholar] [CrossRef]
- Breteler, M.M.; Claus, J.J.; Grobbee, D.E.; Hofman, A. Cardiovascular disease and distribution of cognitive function in elderly people: The Rotterdam Study. BMJ 1994, 308, 1604–1608. [Google Scholar] [CrossRef]
- Tatemichi, T.K.; Desmond, D.W.; Prohovnik, I.; Eidelberg, D. Dementia associated with bilateral carotid occlusions: Neuropsychological and haemodynamic course after extracranial to intracranial bypass surgery. J. Neurol. Neurosurg. Psychiatry 1995, 58, 633–636. [Google Scholar] [CrossRef][Green Version]
- Moroney, J.T.; Tang, M.-X.; Berglund, L.; Small, S.; Merchant, C.; Bell, K.; Stern, Y.; Mayeux, R. Low-density lipoprotein cholesterol and the risk of dementia with stroke. JAMA 1999, 282, 254–260. [Google Scholar] [CrossRef]
- Dias, I.H.; Polidori, M.C.; Li, L.; Weber, D.; Stahl, W.; Nelles, G.; Grune, T.; Griffiths, H.R. Plasma levels of HDL and carotenoids are lower in dementia patients with vascular comorbidities. J. Alzheimers Dis. 2014, 40, 399–408. [Google Scholar] [CrossRef]
- Button, E.B.; Robert, J.; Caffrey, T.M.; Fan, J.; Zhao, W.; Wellington, C.L. HDL from an Alzheimer’s disease perspective. Curr. Opin. Lipidol. 2019, 30, 224. [Google Scholar] [CrossRef]
- Lepara, O.; Valjevac, A.; Alajbegović, A.; Zaćiragić, A.; Nakaš-Ićindić, E. Decreased serum lipids in patients with probable Alzheimer’s disease. Bosn. J. Basic Med. Sci. 2009, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Tang, M.-X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010, 67, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B.; Kellman, W.; Ruosseau, P.; Celesia, G.G.; Siegel, G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 2000, 57, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Jick, H.; Zornberg, G.L.; Jick, S.S.; Seshadri, S.; Drachman, D.A. Statins and the risk of dementia. Lancet 2000, 356, 1627–1631. [Google Scholar] [CrossRef]
- Reitz, C.; Luchsinger, J.; Tang, M.-X.; Manly, J.; Mayeux, R. Impact of plasma lipids and time on memory performance in healthy elderly without dementia. Neurology 2005, 64, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Helkala, E.-L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissinen, A. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ 2001, 322, 1447–1451. [Google Scholar] [CrossRef]
- Skoog, I.; Kalaria, R.N.; Breteler, M.M. Vascular factors and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1999, 13, S106–s114. [Google Scholar] [CrossRef]
- An, Y.; Zhang, X.; Wang, Y.; Wang, Y.; Liu, W.; Wang, T.; Qin, Z.; Xiao, R. Longitudinal and nonlinear relations of dietary and Serum cholesterol in midlife with cognitive decline: Results from EMCOA study. Mol. Neurodegener. 2019, 14, 1–19. [Google Scholar] [CrossRef]
- Clark, L.R.; Norton, D.; Berman, S.E.; Johnson, S.C.; Bendlin, B.B.; Wieben, O.; Turski, P.; Carlsson, C.; Asthana, S.; Gleason, C.E. Association of Cardiovascular and Alzheimer’s Disease Risk Factors with Intracranial Arterial Blood Flow in Whites and African Americans. J. Alzheimers Dis. 2019, 1–11. [Google Scholar] [CrossRef]
- Yanagisawa, K. Cholesterol and pathological processes in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 361–366. [Google Scholar] [CrossRef]

| Study | Variable | Total n | Design | K | Country (N) | Sample | % F | Age | Result | Effect Size | AMSTAR Scores | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size | 95% CI LL~UL | p | |||||||||||
| Zhou et al. [33] | LDL-C | AD n = 2266 HC n = 4767 | C | 20 | EU (7), USA (6), AS (4), AF (2), OC (1) | AD n = 2266 HC n = 4767 | 69.50 | 50-87 | > LDL-C > AD | SMD = 0.35 | 0.12~0.58 | <0.01 | 10 |
| Liu et al. [1] | LDL-C | AD n = 891 HC n = 2399 | C | 9 | EU (3), USA (4), AS (2) | AD n = 584 HC n = 2130 | 70 | 59-92 | > LDL-C > AD | SMD = 1.40 | 0.70~2.10 | 0.000 | 11 |
| HDL-C | 11 | EU (4), USA (4), AS (3) | AD n = 727 HC n = 2233 | HDL-C = AD | SMD = −0.53 | −1.12~0.07 | 0.082 | ||||||
| TC | 13 | EU (6), USA (4), AS (3) | AD n = 809 HC n = 2303 | > TC > AD | SMD = 0.76 | 0.13~1.40 | 0.019 | ||||||
| TG | 6 | EU (4), USA (2) | AD n = 273 HC n = 239 | > TG = AD ns. | SMD = −0.02 | −0.25~0.21 | 0.859 | ||||||
| Wu et al. [12] | LDL-C | AD n = 3037 HC n = 5375 | C | 33 | AS (33) | AD n = 2843 HC n = 5174 | 53.87 | 56–84 | > LDL-C > AD | OR = 1.64 | 1.07~2.51 | 10 | |
| HDL-C | 33 | AD n = 2921 HC n = 5271 | < HDL = AD ns. | OR = 0.81 | 0.55~1.19 | ||||||||
| TC | 33 | AD n = 2661 HC n = 5189 | > TC > AD | OR = 1.58 | 1.10~2.92 | ||||||||
| TG | 28 | AD n = 2556 HC n = 4903 | > TG = AD ns. | OR = 1.33 | 0.99~1.79 | ||||||||
| Wang et al. [18] | TC | AD n = 959 HC n = 694 | C | 16 | - | AD n = 959 HC n = 694 | 60.21 | 60–94, M = 71.38 | > TC = AD | SMD = −0.23 | 0.65~0.19 | 0.29 | 10 |
| Xu et al. [13] | HDL-C | AD n = 12604 HC n = 2,256,519 | L(2–9) | 6 | USA (2), EU (4) | AD n = 499 HC n = 11,991 | 56.3 | M = 71.21 | > HDL = AD | RR = 1.00 | 0.86~1.14 | 0.942 | 11 |
| TC | L (3.2–32) | 16 | USA (8), EU (4), AS (4) | AD n = 12275 HC n = 2,246,750 | 49.5 | M = 68.5 | > TC = AD | RR = 0.96 | 0.81–1.11 | 0.000 | |||
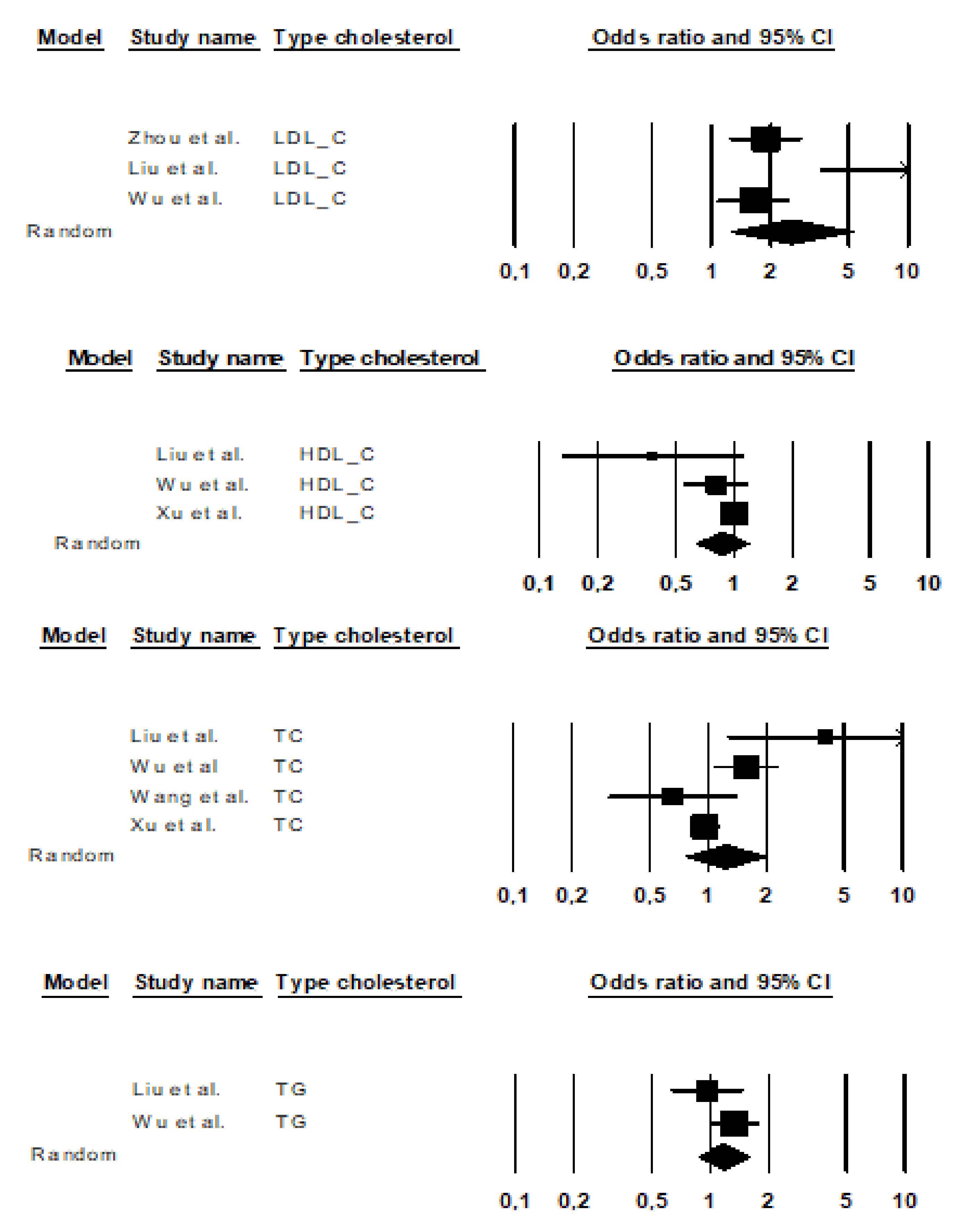
| Model | Study | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower Limit | Upper Limit | Z | p | Weight (Random) | Std Residual | ||
| Zhou et al. [33] | 1.89 | 1.24 | 2.86 | 2.98 | 0.003 | 40.64 | −0.69 | |
| Liu et al. [1] | 12.67 | 3.56 | 45.08 | 3.92 | 0.000 | 18.96 | 2.13 | |
| Wu et al. [12] | 1.64 | 1.07 | 2.50 | 2.25 | 0.024 | 40.39 | −1.01 | |
| Random effect | 2.55 | 1.25 | 5.22 | 2.57 | 0.010 | |||
| Model | Study | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower Limit | Upper Limit | Z | p | Weight (Random) | Std Residual | ||
| Liu et al. [1] | 0.38 | 0.13 | 1.13 | −1.75 | 0.081 | 7.35 | −1.46 | |
| Wu et al. [12] | 0.81 | 0.55 | 1.19 | −1.07 | 0.285 | 33.30 | −0.31 | |
| Xu et al. [13] | 1.00 | 0.87 | 1.16 | 0.00 | 1.000 | 59.35 | 1.08 | |
| Random effect | 0.87 | 0.64 | 1.18 | −0.89 | 0.374 | |||
| Model | Study | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower Limit | Upper Limit | Z | p | Weight (Random) | Std Residual | ||
| Liu et al. [1] | 3.97 | 1.25 | 12.55 | 2.35 | 0.019 | 11.41 | 1.55 | |
| Wu et al. [12] | 1.57 | 1.09 | 2.28 | 2.39 | 0.017 | 31.52 | 0.26 | |
| Wang et al. [18] | 1.52 | 0.70 | 3.25 | 1.07 | 0.283 | 19.04 | 0.11 | |
| Xu et al. [13] | 0.96 | 0.83 | 1.12 | −0.4 −0.53 | 0.597 | 37.25 | −1.35 | |
| Random effect | 1.44 | 0.91 | 2.28 | 1.55 | 0.121 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáiz-Vazquez, O.; Puente-Martínez, A.; Ubillos-Landa, S.; Pacheco-Bonrostro, J.; Santabárbara, J. Cholesterol and Alzheimer’s Disease Risk: A Meta-Meta-Analysis. Brain Sci. 2020, 10, 386. https://doi.org/10.3390/brainsci10060386
Sáiz-Vazquez O, Puente-Martínez A, Ubillos-Landa S, Pacheco-Bonrostro J, Santabárbara J. Cholesterol and Alzheimer’s Disease Risk: A Meta-Meta-Analysis. Brain Sciences. 2020; 10(6):386. https://doi.org/10.3390/brainsci10060386
Chicago/Turabian StyleSáiz-Vazquez, Olalla, Alicia Puente-Martínez, Silvia Ubillos-Landa, Joaquín Pacheco-Bonrostro, and Javier Santabárbara. 2020. "Cholesterol and Alzheimer’s Disease Risk: A Meta-Meta-Analysis" Brain Sciences 10, no. 6: 386. https://doi.org/10.3390/brainsci10060386
APA StyleSáiz-Vazquez, O., Puente-Martínez, A., Ubillos-Landa, S., Pacheco-Bonrostro, J., & Santabárbara, J. (2020). Cholesterol and Alzheimer’s Disease Risk: A Meta-Meta-Analysis. Brain Sciences, 10(6), 386. https://doi.org/10.3390/brainsci10060386






