Early Motor Development Predicts Clinical Outcomes of Siblings at High-Risk for Autism: Insight from an Innovative Motion-Tracking Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Kinematic Analysis through MOVIDEA Software
- Quantity of motion (Qmean), i.e., the mean number of pixels where movement occurred divided by the total number of pixels in the image (percentage);
- Centroid of motion (C), i.e., the central point (2D coordinates measured in pixels) of the infant’s movement computed for each motion image extracted from the video recording (Figure 1), with analysis of standard deviation in the X (Cxsd) and Y directions (Cysd), velocity (Vcmean), and acceleration (Acmean);
- Periodicity for the left and right hands (H-periodicity) and for the left and right feet (F-periodicity), which were nondimensional parameters aimed at measuring the presence of repetitive movements in the motion of the limbs.
2.3. Statistical Analysis
3. Results
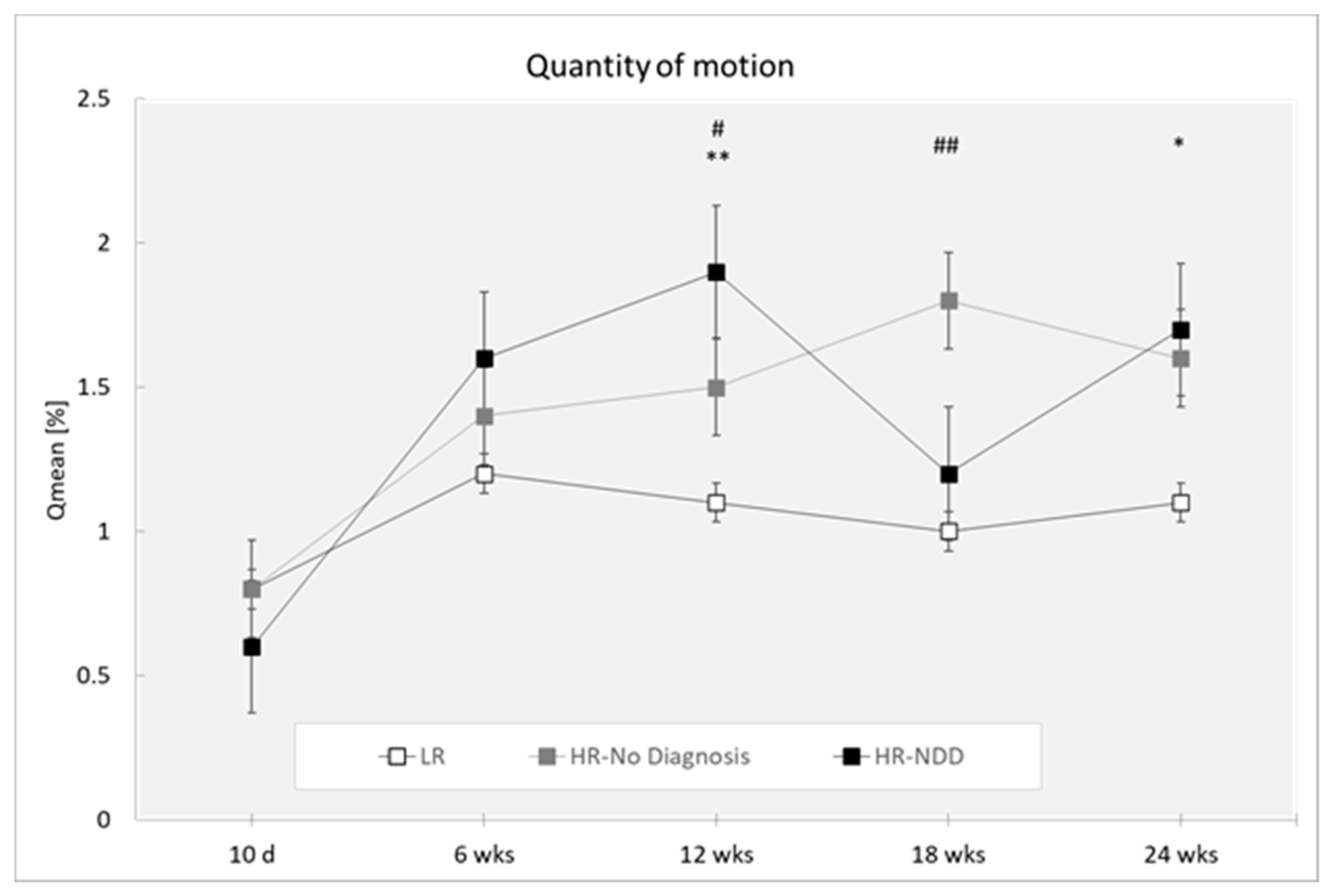
3.1. Quantity of Motion
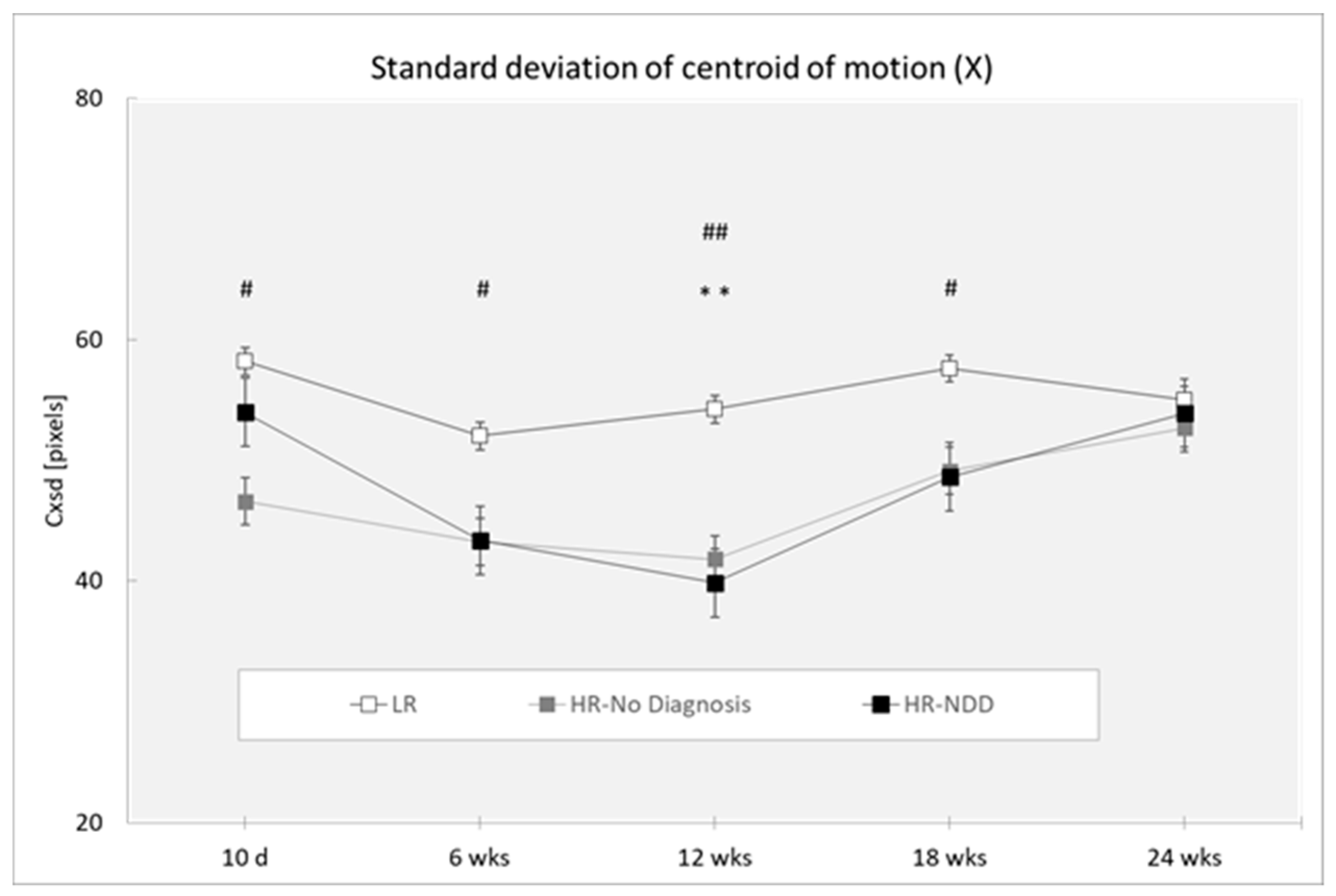
3.2. Centroid of Motion (Cxsd and Cysd)
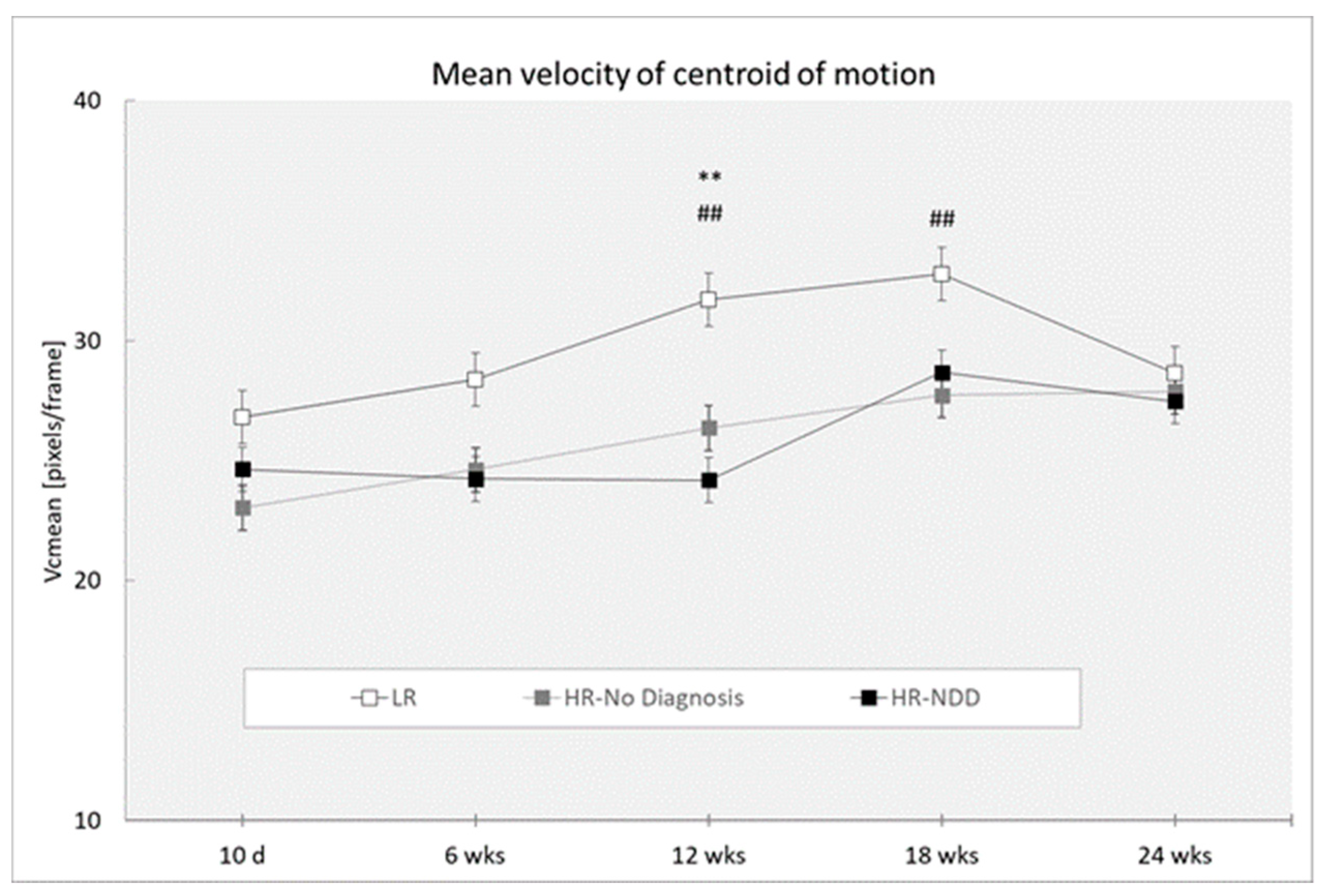
3.3. Velocity of Centroid of Motion
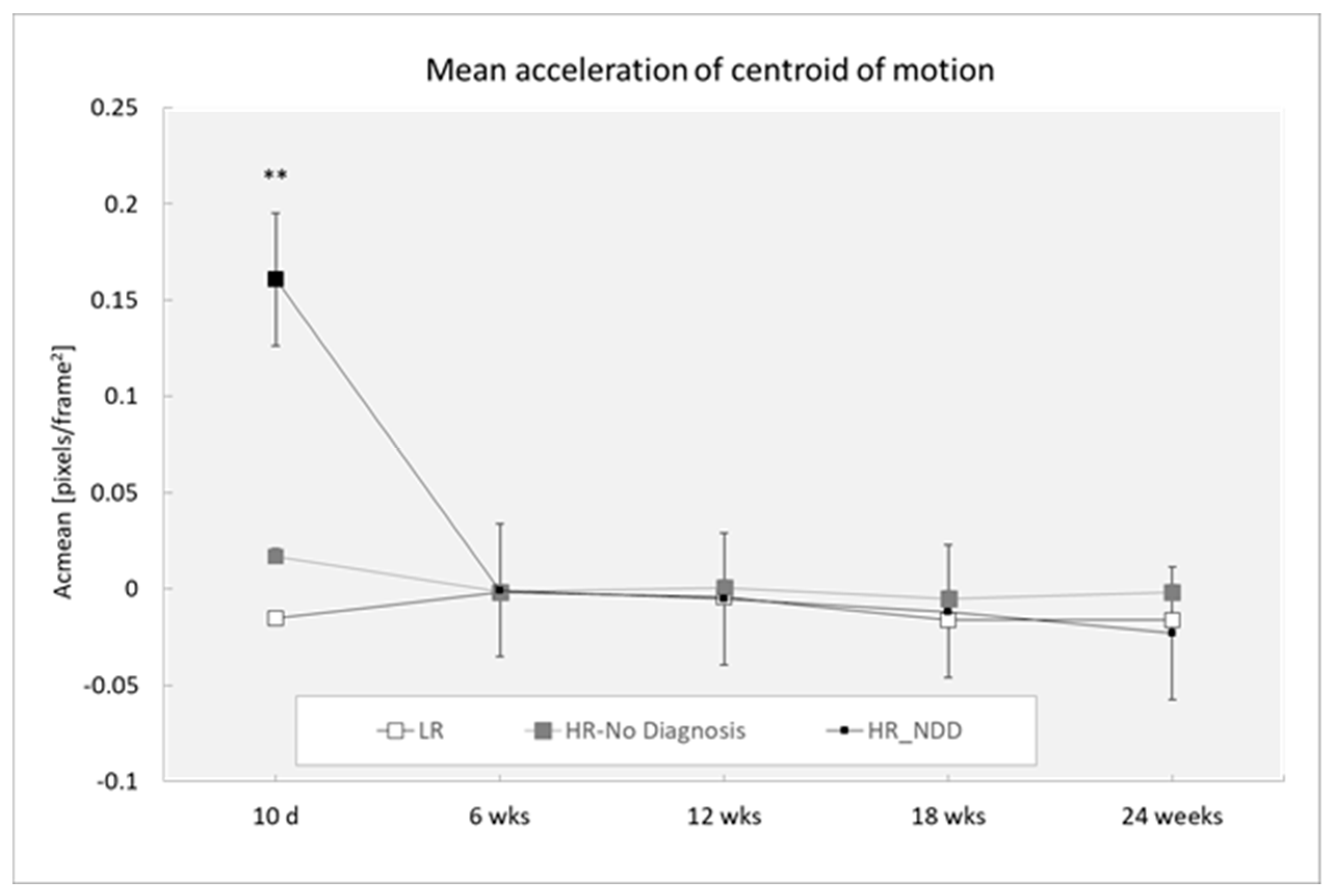
3.4. Acceleration of Centroid of Motion
3.5. Periodicity
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811. [Google Scholar] [CrossRef] [PubMed]
- Karmel, B.Z.; Gardner, J.M.; Meade, L.S.; Cohen, I.L.; London, E.; Flory, M.J.; Lennon, E.M.; Miroshnichenko, I.; Rabinowitz, S.; Parab, S.; et al. Early medical and behavioral characteristics of NICU infants later classified with ASD. Pediatrics 2010, 126, 457–467. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.C. Considerations in biomarker development for neurodevelopmental disorders. Curr. Opin. Neurol. 2016, 29, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Licari, M.K.; Alvares, G.A.; Varcin, K.; Evans, K.L.; Cleary, D.; Reid, S.L.; Glasson, E.J.; Bebbington, K.; Reynolds, J.E.; Wray, J.; et al. Prevalence of Motor Difficulties in Autism Spectrum Disorder: Analysis of a Population-Based Cohort. Autism Res. 2020, 13, 298–306. [Google Scholar] [CrossRef]
- Miller, M.; Iosif, A.M.; Young, G.S.; Hill, M.M.; Ozonoff, S. Early Detection of ADHD: Insights from Infant Siblings of Children With Autism. J. Clin. Child Adolesc. Psychol. 2018, 47, 737–744. [Google Scholar] [CrossRef]
- Sacrey, L.A.; Bennett, J.A.; Zwaigenbaum, L. Early Infant Development and Intervention for Autism Spectrum Disorder. J. Child Neurol. 2015, 30, 1921–1929. [Google Scholar] [CrossRef]
- Zwaigenbaum, L. Advances in the early detection of autism. Curr. Opin. Neurol. 2010, 23, 97–102. [Google Scholar] [CrossRef]
- Athanasiadou, A.; Buitelaar, J.K.; Brovedani, P.; Chorna, O.; Fulceri, F.; Guzzetta, A.; Scattoni, M.L. Early motor signs of attention-deficit hyperactivity disorder: A systematic review. Eur. Child Adolesc. Psychiatry 2019. [Google Scholar] [CrossRef]
- Canu, D.; Van der Paelt, S.; Canal-Bedia, R.; Posada, M.; Vanvuchelen, M.; Roeyers, H. Early non-social behavioural indicators of autism spectrum disorder (ASD) in siblings at elevated likelihood for ASD: A systematic review. Eur. Child Adolesc. Psychiatry 2020. [Google Scholar] [CrossRef]
- Garrido, D.; Petrova, D.; Watson, L.R.; Garcia-Retamero, R.; Carballo, G. Language and motor skills in siblings of children with autism spectrum disorder: A meta-analytic review. Autism Res. 2017, 10, 1737–1750. [Google Scholar] [CrossRef]
- Micai, M.; Fulceri, F.; Caruso, A.; Guzzetta, A.; Gila, L.; Scattoni, M.L. Early behavioral markers for neurodevelopmental disorders in the first 3 years of life: An overview of systematic reviews. manuscript submitted for publication.
- Fuentefria, R.D.N.; Silveira, R.C.; Procianoy, R.S. Motor development of preterm infants assessed by the Alberta Infant Motor Scale: Systematic review article. J. Pediatr. 2017, 93, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Palomo, R.; Belinchon, M.; Ozonoff, S. Autism and family home movies: A comprehensive review. J. Dev. Behav. Pediatr. 2006, 27, S59–S68. [Google Scholar] [CrossRef] [PubMed]
- Gurevitz, M.; Geva, R.; Varon, M.; Leitner, Y. Early markers in infants and toddlers for development of ADHD. J. Atten. Disord. 2014, 18, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, M.; de Winter, A.F.; Buitelaar, J.K.; Verhulst, F.C.; Reijneveld, S.A.; Hartman, C.A. Early childhood assessments of community pediatric professionals predict autism spectrum and attention deficit hyperactivity problems. J. Abnorm. Child Psychol. 2013, 41, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Jacobvitz, D.; Sroufe, L.A. The early caregiver-child relationship and attention-deficit disorder with hyperactivity in kindergarten: A prospective study. Child Dev. 1987, 58, 1496–1504. [Google Scholar] [CrossRef]
- Achermann, S.; Nystrom, P.; Bolte, S.; Falck-Ytter, T. Motor atypicalities in infancy are associated with general developmental level at 2 years, but not autistic symptoms. Autism 2020. [Google Scholar] [CrossRef]
- Focaroli, V.; Taffoni, F.; Parsons, S.M.; Keller, F.; Iverson, J.M. Performance of Motor Sequences in Children at Heightened vs. Low Risk for ASD: A Longitudinal Study from 18 to 36 Months of Age. Front. Psychol. 2016, 7, 724. [Google Scholar] [CrossRef]
- Jasmin, E.; Couture, M.; McKinley, P.; Reid, G.; Fombonne, E.; Gisel, E. Sensori-motor and daily living skills of preschool children with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 231–241. [Google Scholar] [CrossRef]
- Landa, R.; Garrett-Mayer, E. Development in infants with autism spectrum disorders: A prospective study. J. Child Psychol. Psychiatry 2006, 47, 629–638. [Google Scholar] [CrossRef]
- Landa, R.J.; Gross, A.L.; Stuart, E.A.; Faherty, A. Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child Dev. 2013, 84, 429–442. [Google Scholar] [CrossRef]
- LeBarton, E.S.; Landa, R.J. Infant motor skill predicts later expressive language and autism spectrum disorder diagnosis. Infant Behav. Dev. 2019, 54, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Libertus, K.; Landa, R.J. The Early Motor Questionnaire (EMQ): A parental report measure of early motor development. Infant Behav. Dev. 2013, 36, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Libertus, K.; Sheperd, K.A.; Ross, S.W.; Landa, R.J. Limited fine motor and grasping skills in 6-month-old infants at high risk for autism. Child Dev. 2014, 85, 2218–2231. [Google Scholar] [CrossRef] [PubMed]
- Phagava, H.; Muratori, F.; Einspieler, C.; Maestro, S.; Apicella, F.; Guzzetta, A.; Prechtl, H.F.; Cioni, G. General movements in infants with autism spectrum disorders. Georgian Med. News 2008, 156, 100–105. [Google Scholar]
- Harris, S.R. Early motor delays as diagnostic clues in autism spectrum disorder. Eur. J. Pediatr. 2017, 176, 1259–1262. [Google Scholar] [CrossRef]
- Iverson, J.M. Early Motor and Communicative Development in Infants With an Older Sibling With Autism Spectrum Disorder. J. Speech Lang Hear. Res. 2018, 61, 2673–2684. [Google Scholar] [CrossRef]
- Moseley, R.L.; Pulvermuller, F. What can autism teach us about the role of sensorimotor systems in higher cognition? New clues from studies on language, action semantics, and abstract emotional concept processing. Cortex 2018, 100, 149–190. [Google Scholar] [CrossRef]
- West, K.L. Infant Motor Development in Autism Spectrum Disorder: A Synthesis and Meta-analysis. Child Dev. 2019, 90, 2053–2070. [Google Scholar] [CrossRef]
- Einspieler, C.; Sigafoos, J.; Bolte, S.; Bratl-Pokorny, K.D.; Landa, R.; Marschik, P.B. Highlighting the first 5 months of life: General movements in infants later diagnosed with autism spectrum disorder or Rett Syndrome. Res. Autism Spectr. Disord. 2014, 8, 286–291. [Google Scholar] [CrossRef]
- Kim, S.H.; Lord, C. Restricted and repetitive behaviors in toddlers and preschoolers with autism spectrum disorders based on the Autism Diagnostic Observation Schedule (ADOS). Autism Res. 2010, 3, 162–173. [Google Scholar] [CrossRef]
- Morgan, L.; Wetherby, A.M.; Barber, A. Repetitive and stereotyped movements in children with autism spectrum disorders late in the second year of life. J. Child Psychol. Psychiatry 2008, 49, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Purpura, G.; Costanzo, V.; Chericoni, N.; Puopolo, M.; Scattoni, M.L.; Muratori, F.; Apicella, F. Bilateral Patterns of Repetitive Movements in 6- to 12-Month-Old Infants with Autism Spectrum Disorders. Front. Psychol. 2017, 8, 1168. [Google Scholar] [CrossRef]
- Ozonoff, S.; Young, G.S.; Carter, A.; Messinger, D.; Yirmiya, N.; Zwaigenbaum, L.; Bryson, S.; Carver, L.J.; Constantino, J.N.; Dobkins, K.; et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics 2011, 128, e488–e495. [Google Scholar] [CrossRef] [PubMed]
- Bryson, S.E.; Zwaigenbaum, L.; Brian, J.; Roberts, W.; Szatmari, P.; Rombough, V.; McDermott, C. A prospective case series of high-risk infants who developed autism. J. Autism Dev. Disord. 2007, 37, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.; Zwaigenbaum, L.; Gu, H.; St John, T.; Paterson, S.; Elison, J.T.; Hazlett, H.; Botteron, K.; Dager, S.R.; Schultz, R.T.; et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J. Neurodev. Disord. 2015, 7, 24. [Google Scholar] [CrossRef]
- Flanagan, J.E.; Landa, R.; Bhat, A.; Bauman, M. Head lag in infants at risk for autism: A preliminary study. Am. J. Occup. Ther. 2012, 66, 577–585. [Google Scholar] [CrossRef]
- Iverson, J.M.; Wozniak, R.H. Variation in vocal-motor development in infant siblings of children with autism. J. Autism Dev. Disord. 2007, 37, 158–170. [Google Scholar] [CrossRef]
- Iverson, J.M.; Shic, F.; Wall, C.A.; Chawarska, K.; Curtin, S.; Estes, A.; Gardner, J.M.; Hutman, T.; Landa, R.J.; Levin, A.R.; et al. Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. J. Abnorm. Psychol. 2019, 128, 69–80. [Google Scholar] [CrossRef]
- Nickel, L.R.; Thatcher, A.R.; Keller, F.; Wozniak, R.H.; Iverson, J.M. Posture Development in Infants at Heightened vs. Low Risk for Autism Spectrum Disorders. Infancy 2013, 18, 639–661. [Google Scholar] [CrossRef]
- Brian, J.; Bryson, S.E.; Garon, N.; Roberts, W.; Smith, I.M.; Szatmari, P.; Zwaigenbaum, L. Clinical assessment of autism in high-risk 18-month-olds. Autism 2008, 12, 433–456. [Google Scholar] [CrossRef]
- Elison, J.T.; Wolff, J.J.; Reznick, J.S.; Botteron, K.N.; Estes, A.M.; Gu, H.; Hazlett, H.C.; Meadows, A.J.; Paterson, S.J.; Zwaigenbaum, L.; et al. Repetitive behavior in 12-month-olds later classified with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Chawarska, K.; Shic, F.; Macari, S.; Campbell, D.J.; Brian, J.; Landa, R.; Hutman, T.; Nelson, C.A.; Ozonoff, S.; Tager-Flusberg, H.; et al. 18-month predictors of later outcomes in younger siblings of children with autism spectrum disorder: A baby siblings research consortium study. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Bartl-Pokorny, K.D.; Jonsson, U.; Berggren, S.; Zhang, D.; Kostrzewa, E.; Falck-Ytter, T.; Einspieler, C.; Pokorny, F.B.; Jones, E.J.; et al. How can clinicians detect and treat autism early? Methodological trends of technology use in research. Acta Paediatr. 2016, 105, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Adde, L.; Helbostad, J.L.; Jensenius, A.R.; Taraldsen, G.; Stoen, R. Using computer-based video analysis in the study of fidgety movements. Early Hum. Dev. 2009, 85, 541–547. [Google Scholar] [CrossRef]
- Baccinelli, W.; Bulgheroni, M.; Simonetti, V.; Fulceri, F.; Caruso, A.; Gila, L.; Scattoni, M.L. Movidea: A Software Package for Automatic Video Analysis of Movements in Infants at Risk for Neurodevelopmental Disorders. Brain Sci. 2020, 10, 203. [Google Scholar] [CrossRef]
- Karch, D.; Wochner, K.; Kim, K.; Philippi, H.; Hadders-Algra, M.; Pietz, J.; Dickhaus, H. Quantitative score for the evaluation of kinematic recordings in neuropediatric diagnostics. Detection of complex patterns in spontaneous limb movements. Methods Inf. Med. 2010, 49, 526–530. [Google Scholar] [CrossRef]
- Marchi, V.; Hakala, A.; Knight, A.; D’Acunto, F.; Scattoni, M.L.; Guzzetta, A.; Vanhatalo, S. Automated pose estimation captures key aspects of General Movements at eight to 17 weeks from conventional videos. Acta Paediatr. 2019, 108, 1817–1824. [Google Scholar] [CrossRef]
- Marcroft, C.; Khan, A.; Embleton, N.D.; Trenell, M.; Plotz, T. Movement recognition technology as a method of assessing spontaneous general movements in high risk infants. Front. Neurol. 2014, 5, 284. [Google Scholar] [CrossRef]
- Marchi, V.; Belmonti, V.; Cecchi, F.; Coluccini, M.; Ghirri, P.; Grassi, A.; Sabatini, A.M.; Guzzetta, A. Movement analysis in early infancy: Towards a motion biomarker of age. Early Hum. Dev. 2020, 142, 104942. [Google Scholar] [CrossRef]
- Marschik, P.B.; Pokorny, F.B.; Peharz, R.; Zhang, D.; O’Muircheartaigh, J.; Roeyers, H.; Bolte, S.; Spittle, A.J.; Urlesberger, B.; Schuller, B.; et al. A Novel Way to Measure and Predict Development: A Heuristic Approach to Facilitate the Early Detection of Neurodevelopmental Disorders. Curr. Neurol. Neurosci. Rep. 2017, 17, 43. [Google Scholar] [CrossRef]
- Tsuji, T.; Nakashima, S.; Hayashi, H.; Soh, Z.; Furui, A.; Shibanoki, T.; Shima, K.; Shimatani, K. Markerless Measurement and Evaluation of General Movements in Infants. Sci. Rep. 2020, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S.L. Autism Diagnostic Observation Schedule, 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Meinecke, L.; Breitbach-Faller, N.; Bartz, C.; Damen, R.; Rau, G.; Disselhorst-Klug, C. Movement analysis in the early detection of newborns at risk for developing spasticity due to infantile cerebral palsy. Hum. Mov. Sci. 2006, 25, 125–144. [Google Scholar] [CrossRef]
- Hsu, J.C. Multiple Comparisons: Theory and Methods; Hardback Published by Chapman & Hall in February 1996; The Ohio State University: Columbus, OH, USA, 1996. [Google Scholar]
- Maxwell, S.E.; Delaney, H.D.; Kelley, K. Designing Experiments and Analyzing Data: A Model Comparison Perspective, 3rd ed.; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Canals, J.; Hernandez-Martinez, C.; Esparo, G.; Fernandez-Ballart, J. Neonatal Behavioral Assessment Scale as a predictor of cognitive development and IQ in full-term infants: A 6-year longitudinal study. Acta Paediatr. 2011, 100, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, H.; Hirsch, O.; Albrecht, B.; Chavanon, M.L. Attention-Deficit/Hyperactivity Disorder (ADHD) and Emotion Regulation Over the Life Span. Curr. Psychiatry Rep. 2019, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R.; Baird, S. Identifying Early Indicators for Autism in Self-Regulation Difficulties. Focus Autism Dev. Disabil. J. 2005, 20, 106–116. [Google Scholar] [CrossRef]
- Bachevalier, J.; Loveland, K.A. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci. Biobehav. Rev. 2006, 30, 97–117. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Neural substrate and clinical significance of general movements: An update. Dev. Med. Child Neurol. 2018, 60, 39–46. [Google Scholar] [CrossRef]
- Dusing, S.C.; Brown, S.E.; Van Drew, C.M.; Thacker, L.R.; Hendricks-Munoz, K.D. Supporting Play Exploration and Early Development Intervention From NICU to Home: A Feasibility Study. Pediatr. Phys. Ther. 2015, 27, 267–274. [Google Scholar] [CrossRef]
- Dusing, S.C.; Harbourne, R.T. Variability in postural control during infancy: Implications for development, assessment, and intervention. Phys. Ther. 2010, 90, 1838–1849. [Google Scholar] [CrossRef]
- Adde, L.; Yang, H.; Saether, R.; Jensenius, A.R.; Ihlen, E.; Cao, J.Y.; Stoen, R. Characteristics of general movements in preterm infants assessed by computer-based video analysis. Physiother. Theory Pract. 2018, 34, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M. Variation and variability: Key words in human motor development. Phys. Ther. 2010, 90, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Kyvelidou, A.; Harbourne, R.T.; Stergiou, N. Severity and characteristics of developmental delay can be assessed using variability measures of sitting posture. Pediatr. Phys. Ther. 2010, 22, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hamer, E.G.; Bos, A.F.; Hadders-Algra, M. Specific characteristics of abnormal general movements are associated with functional outcome at school age. Early Hum. Dev. 2016, 95, 9–13. [Google Scholar] [CrossRef]
- Zappella, M.; Einspieler, C.; Bartl-Pokorny, K.D.; Krieber, M.; Coleman, M.; Bolte, S.; Marschik, P.B. What do home videos tell us about early motor and socio-communicative behaviours in children with autistic features during the second year of life—An exploratory study. Early Hum. Dev. 2015, 91, 569–575. [Google Scholar] [CrossRef]
- Abrishami, M.S.; Nocera, L.; Mert, M.; Trujillo-Priego, I.A.; Purushotham, S.; Shahabi, C.; Smith, B.A. Identification of Developmental Delay in Infants Using Wearable Sensors: Full-Day Leg Movement Statistical Feature Analysis. IEEE J. Transl. Eng. Health Med. 2019, 7, 2800207. [Google Scholar] [CrossRef]
- Thelen, E. Rhythmical stereotypies in normal human infants. Anim. Behav. 1979, 27, 699–715. [Google Scholar] [CrossRef]
- Leekam, S.; Tandos, J.; McConachie, H.; Meins, E.; Parkinson, K.; Wright, C.; Turner, M.; Arnott, B.; Vittorini, L.; Le Couteur, A. Repetitive behaviours in typically developing 2-year-olds. J. Child Psychol. Psychiatry 2007, 48, 1131–1138. [Google Scholar] [CrossRef]
- Einspieler, C.; Prechtl, H.F. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 61–67. [Google Scholar] [CrossRef]
- Prechtl, H.F.; Einspieler, C.; Cioni, G.; Bos, A.F.; Ferrari, F.; Sontheimer, D. An early marker for neurological deficits after perinatal brain lesions. Lancet 1997, 349, 1361–1363. [Google Scholar] [CrossRef]


| Age of Assessment | LR (n = 53) | HR-No Diagnosis (n = 18) | HR-NDD (n = 32) | |||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| n | n | n | n | n | n | |
| 10 days | 16 | 31 | 7 | 15 | 5 | 7 |
| 6 weeks | 21 | 43 | 12 | 21 | 4 | 12 |
| 12 weeks | 24 | 46 | 15 | 24 | 5 | 13 |
| 18 weeks | 16 | 36 | 15 | 23 | 5 | 8 |
| 24 weeks | 12 | 28 | 8 | 13 | 7 | 14 |
| Parameter | LR | HR-No Diagnosis | HR-NDD | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Qmean | ||||||
| 10 days | 31 | 0.008 (0.006) | 15 | 0.008 (0.003) | 7 | 0.006 (0.005) |
| 6 weeks | 43 | 0.012 (0.008) | 21 | 0.014 (0.005) | 12 | 0.016 (0.009) |
| 12 weeks | 46 | 0.011 (0.006) | 24 | 0.015 (0.009) | 13 | 0.019 (0.005) |
| 18 weeks | 36 | 0.010 (0.007) | 23 | 0.018 (0.011) | 8 | 0.012 (0.005) |
| 24 weeks | 28 | 0.011 (0.009) | 13 | 0.016 (0.009) | 14 | 0.017 (0.009) |
| Cxsd | ||||||
| 10 days | 31 | 58.210 (21.422) | 15 | 46.589 (13.116) | 7 | 53.976 (13.967) |
| 6 weeks | 43 | 51.999 (13.722) | 21 | 43.243 (10.278) | 12 | 43.372 (9.371) |
| 12 weeks | 46 | 54.223 (14.314) | 24 | 41.800 (10.001) | 13 | 39.843 (9.384) |
| 18 weeks | 36 | 57.612 (16.725) | 23 | 49.113 (23.310) | 8 | 48.621 (15.325) |
| 24 weeks | 28 | 55.010 (15.419) | 13 | 52.645 (19.299) | 14 | 53.890 (16.267) |
| Cysd | ||||||
| 10 days | 31 | 72.398 (31.810) | 15 | 78.819 (34.812) | 7 | 78.012 (23.567) |
| 6 weeks | 43 | 57.223 (19.110) | 21 | 68.222 (23.111) | 12 | 65.830 (21.765) |
| 12 weeks | 46 | 55.230 (20.111) | 24 | 68.450 (20.701) | 13 | 62.692 (18.991) |
| 18 weeks | 36 | 58.716 (25.416) | 23 | 68.412 (24.411) | 8 | 71.768 (26.802) |
| 24 weeks | 28 | 62.320 (18.799) | 13 | 63.899 (23.789) | 14 | 68.175 (16.267) |
| Acmean | ||||||
| 10 days | 31 | −0.015 (0.071) | 15 | 0.017 (0.052) | 7 | 0.161 (0.307) |
| 6 weeks | 43 | −0.002 (0.024) | 21 | −0.002 (0.028) | 12 | −0.001 (0.043) |
| 12 weeks | 46 | −0.004 (0.030) | 24 | 0.001 (0.027) | 13 | −0.005 (0.015) |
| 18 weeks | 36 | 0.016 (0.052) | 23 | −0.005 (0.033) | 8 | −0.011 (0.034) |
| 24 weeks | 28 | −0.017 (0.139) | 13 | −0.002 (0.041) | 14 | −0.023 (0.030) |
| Vcmean | ||||||
| 10 days | 31 | 26.835 (7.432) | 15 | 23.040 (5.128) | 7 | 24.640 (5.665) |
| 6 weeks | 43 | 28.396 (6.225) | 21 | 24.624 (5.356) | 12 | 24.250 (4.036) |
| 12 weeks | 46 | 31.726 (7.301) | 24 | 26.374 (5.170) | 13 | 24.194 (4.570) |
| 18 weeks | 36 | 32.792 (8.828) | 23 | 27.730 (9.116) | 8 | 28.687 (6.638) |
| 24 weeks | 28 | 28.652 (7.402) | 13 | 27.884 (10.032) | 14 | 27.466 (8.335) |
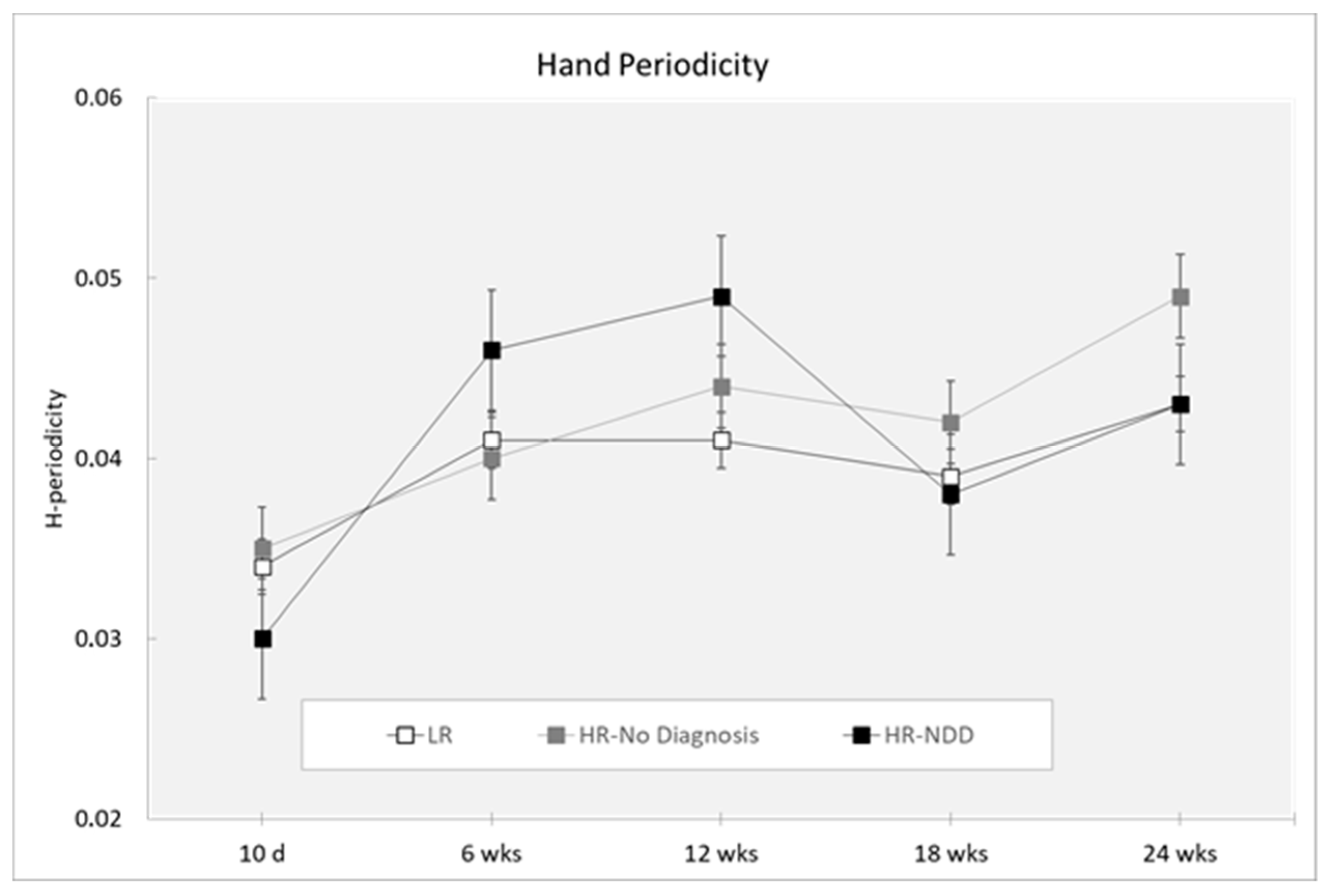
| H-periodicity | ||||||
| 10 days | 31 | 0.034 (0.012) | 15 | 0.035 (0.006) | 7 | 0.030 (0.004) |
| 6 weeks | 43 | 0.041 (0.011) | 21 | 0.040 (0.013) | 12 | 0.046 (0.014) |
| 12 weeks | 46 | 0.041 (0.010) | 24 | 0.044 (0.018) | 13 | 0.049 (0.013) |
| 18 weeks | 36 | 0.039 (0.010) | 23 | 0.042 (0.018) | 8 | 0.038 (0.010) |
| 24 weeks | 28 | 0.043 (0.013) | 13 | 0.049 (0.023) | 14 | 0.043 (0.011) |
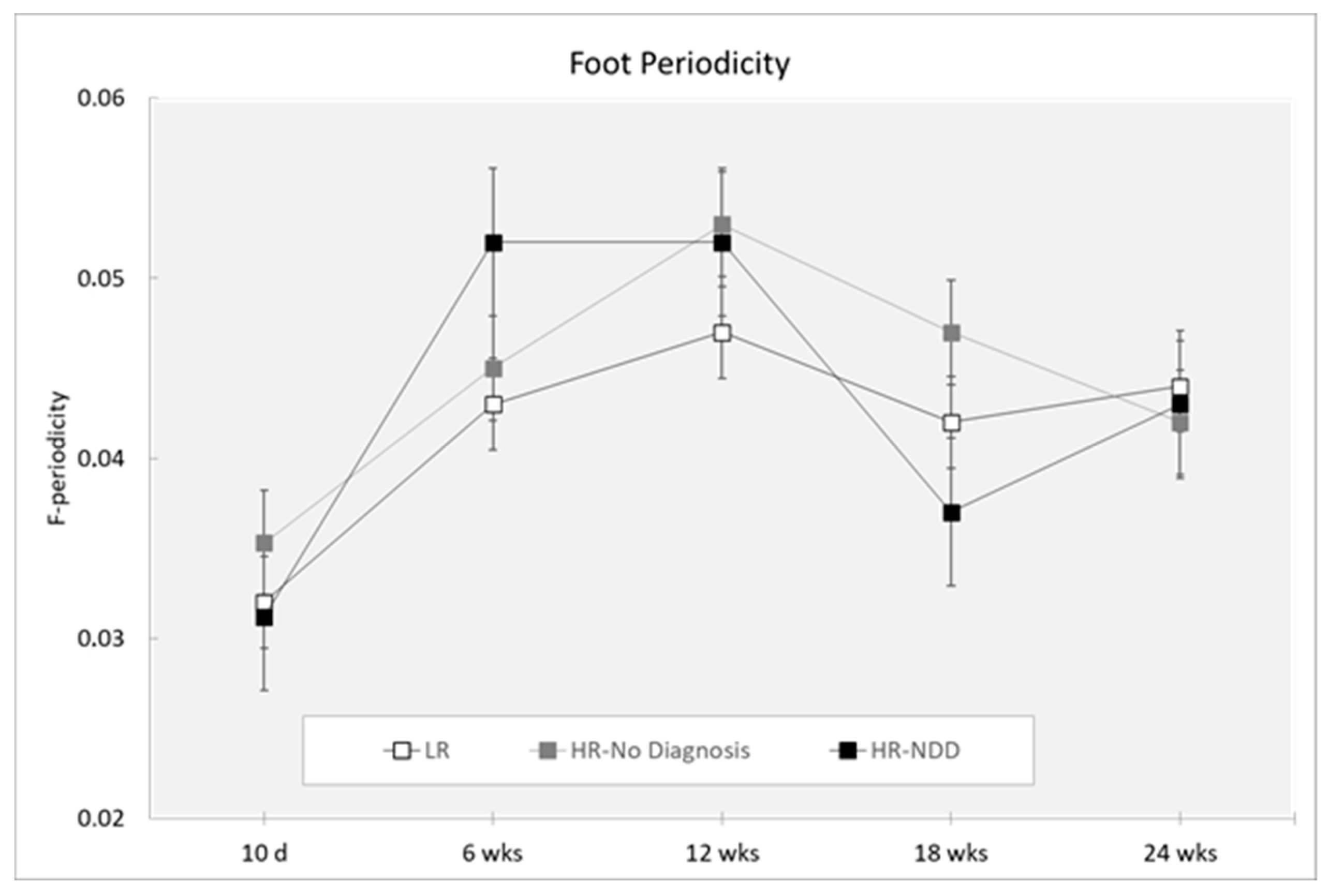
| F-periodicity | ||||||
| 10 days | 31 | 0.032 (0.007) | 15 | 0.035 (0.007) | 7 | 0.031 (0.003) |
| 6 weeks | 43 | 0.043 (0.011) | 21 | 0.045 (0.014) | 12 | 0.052 (0.018) |
| 12 weeks | 46 | 0.047 (0.015) | 24 | 0.053 (0.022) | 13 | 0.052 (0.017) |
| 18 weeks | 36 | 0.042 (0.015) | 23 | 0.047 (0.016) | 8 | 0.037 (0.010) |
| 24 weeks | 28 | 0.044 (0.014) | 13 | 0.042 (0.012) | 14 | 0.043 (0.010) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, A.; Gila, L.; Fulceri, F.; Salvitti, T.; Micai, M.; Baccinelli, W.; Bulgheroni, M.; Scattoni, M.L., on behalf of the NIDA Network Group. Early Motor Development Predicts Clinical Outcomes of Siblings at High-Risk for Autism: Insight from an Innovative Motion-Tracking Technology. Brain Sci. 2020, 10, 379. https://doi.org/10.3390/brainsci10060379
Caruso A, Gila L, Fulceri F, Salvitti T, Micai M, Baccinelli W, Bulgheroni M, Scattoni ML on behalf of the NIDA Network Group. Early Motor Development Predicts Clinical Outcomes of Siblings at High-Risk for Autism: Insight from an Innovative Motion-Tracking Technology. Brain Sciences. 2020; 10(6):379. https://doi.org/10.3390/brainsci10060379
Chicago/Turabian StyleCaruso, Angela, Letizia Gila, Francesca Fulceri, Tommaso Salvitti, Martina Micai, Walter Baccinelli, Maria Bulgheroni, and Maria Luisa Scattoni on behalf of the NIDA Network Group. 2020. "Early Motor Development Predicts Clinical Outcomes of Siblings at High-Risk for Autism: Insight from an Innovative Motion-Tracking Technology" Brain Sciences 10, no. 6: 379. https://doi.org/10.3390/brainsci10060379
APA StyleCaruso, A., Gila, L., Fulceri, F., Salvitti, T., Micai, M., Baccinelli, W., Bulgheroni, M., & Scattoni, M. L., on behalf of the NIDA Network Group. (2020). Early Motor Development Predicts Clinical Outcomes of Siblings at High-Risk for Autism: Insight from an Innovative Motion-Tracking Technology. Brain Sciences, 10(6), 379. https://doi.org/10.3390/brainsci10060379





