Selected Ionotropic Receptors and Voltage-Gated Ion Channels: More Functional Competence for Human Induced Pluripotent Stem Cell (iPSC)-Derived Nociceptors
Abstract
1. Introduction
2. Materials and Methods
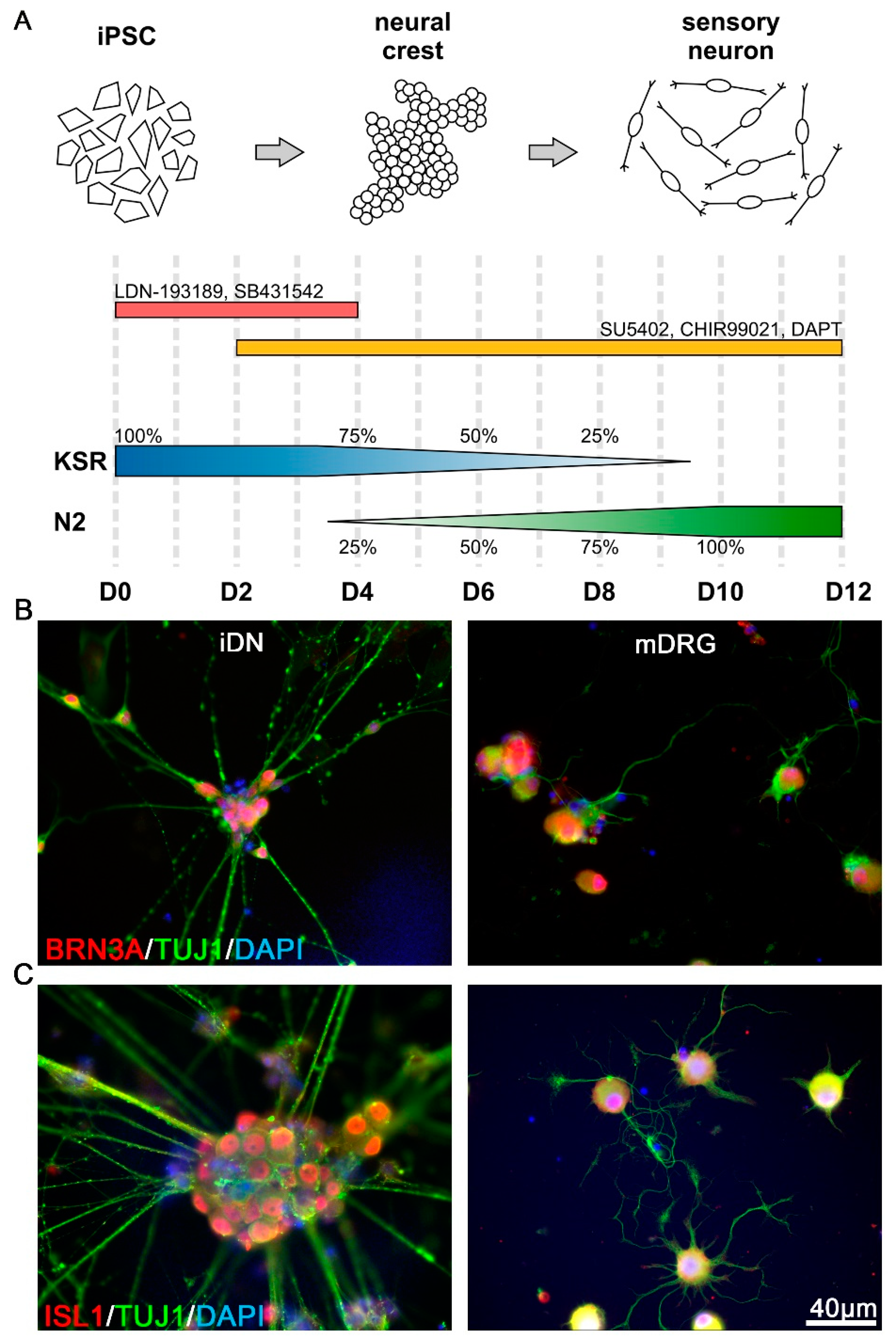
2.1. Differentiation of Human iPSCs into Nociceptors by Small Molecule Inhibition
2.2. Primary Sensory Neuron Culture
2.3. Immunofluorescence Microscopy
2.4. Microfluorimetric Ca2+ Measurements
2.5. Statistical Analyses
3. Results
3.1. Expression of Early Transcription Factors Regulating Sensory Differentiation
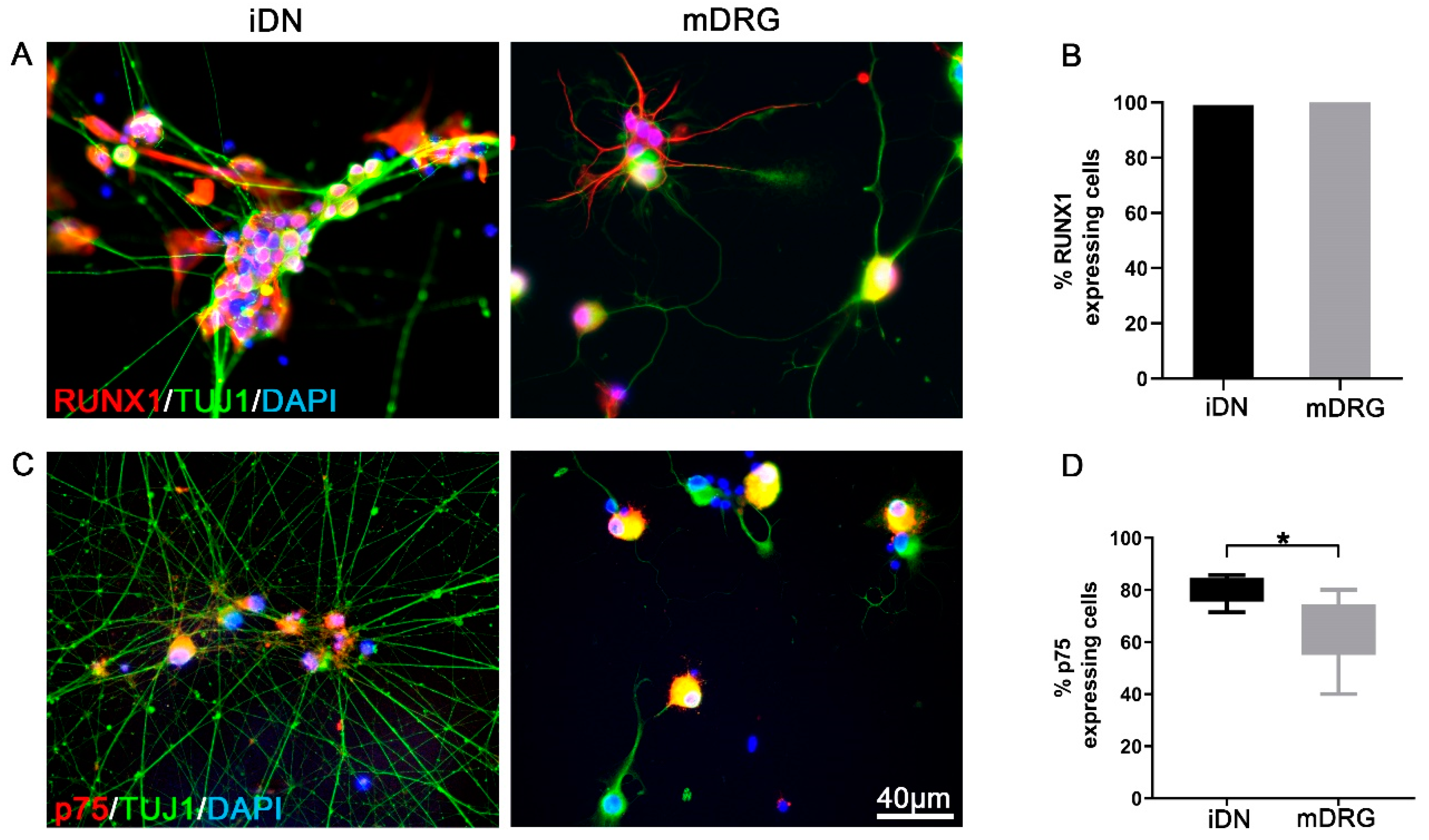
3.2. RUNX1 and p75 Expression Reveal a Nociceptor Neuron Phenotype
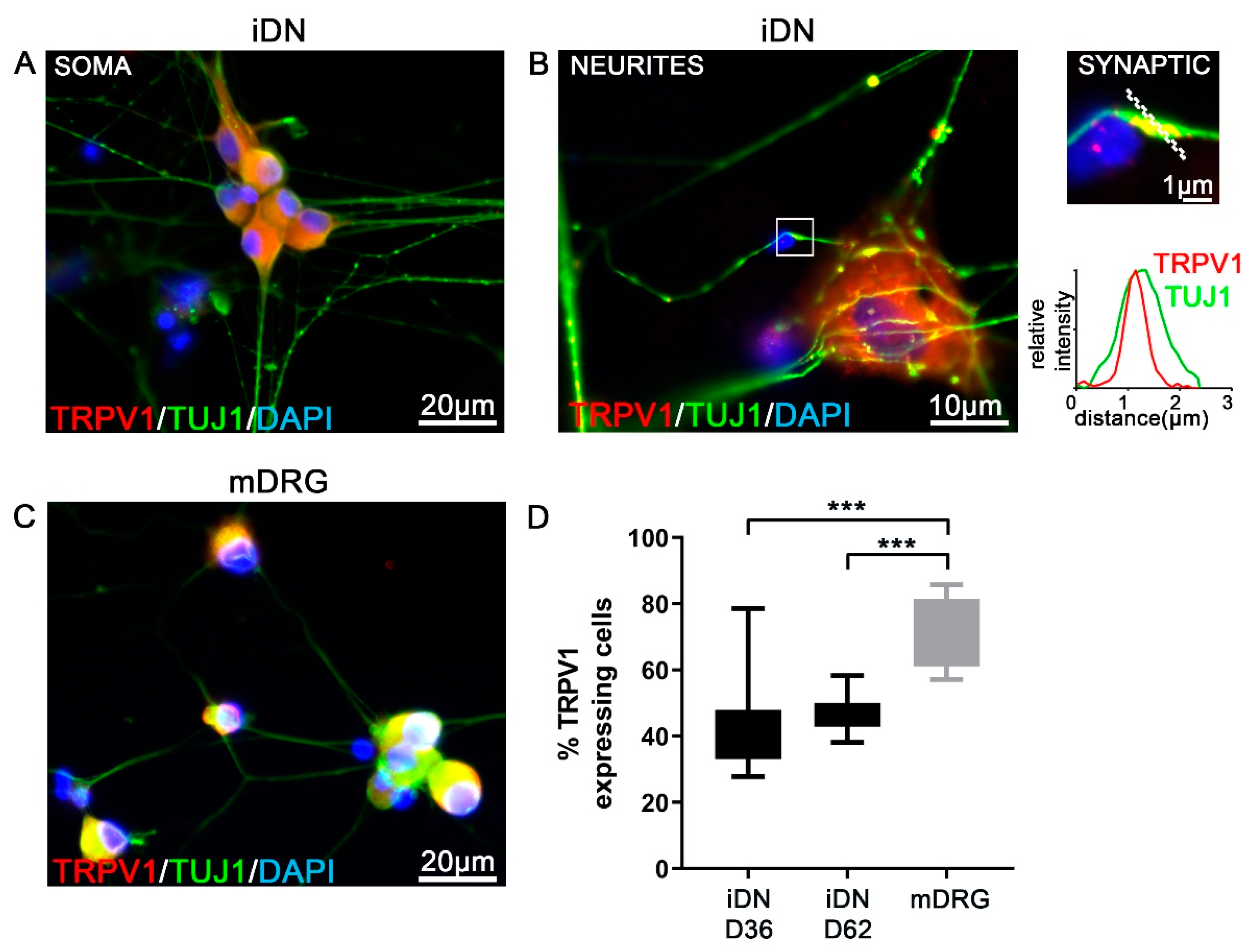
3.3. TRPV1 Experession in iDNs
3.4. Expression of Pacemaker Channel HCN1–3 but Not HCN4 in iDNs and mDRG Neurons
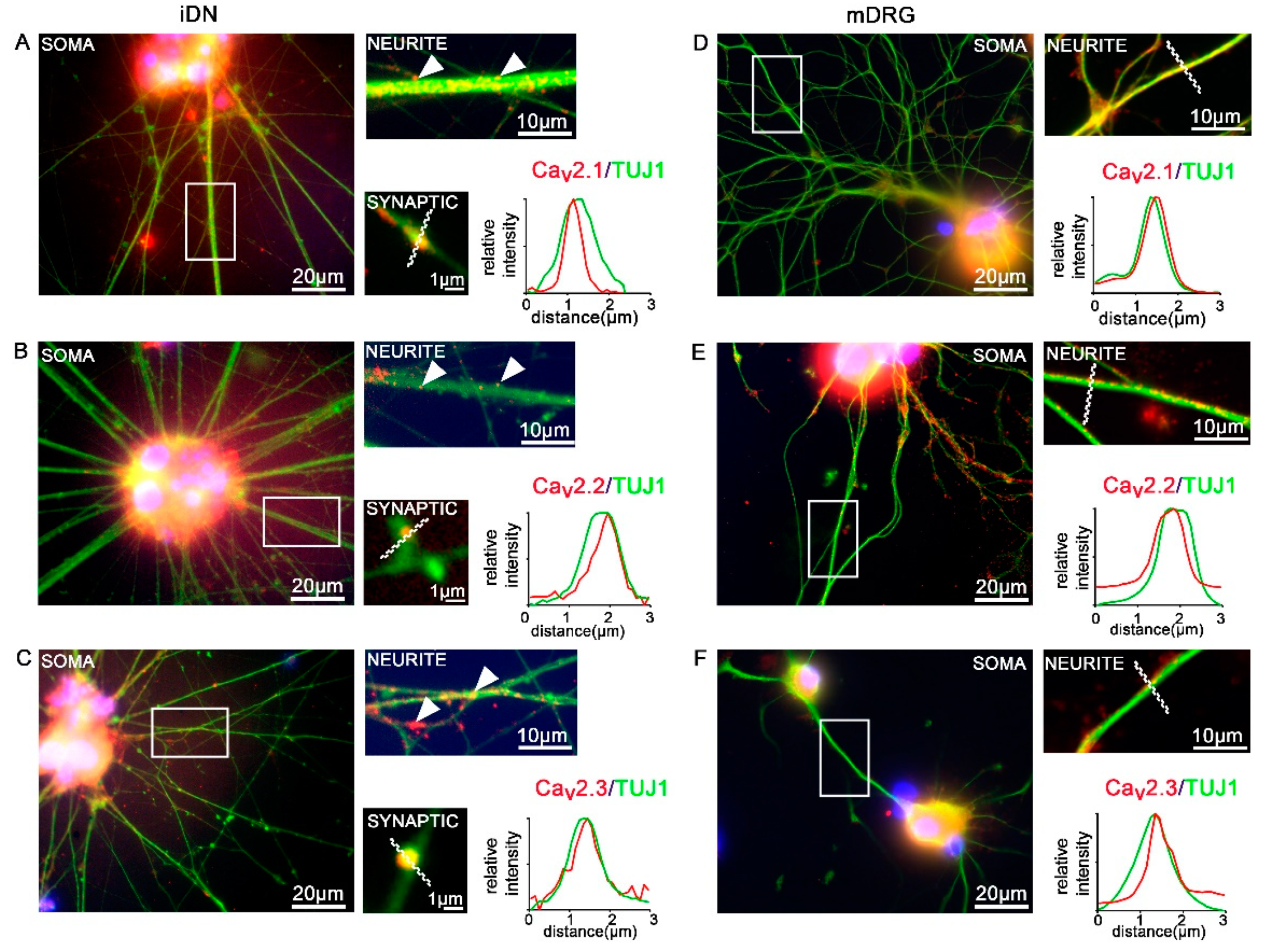
3.5. Expression of CaV2 High Voltage-Activated Calcium Channels
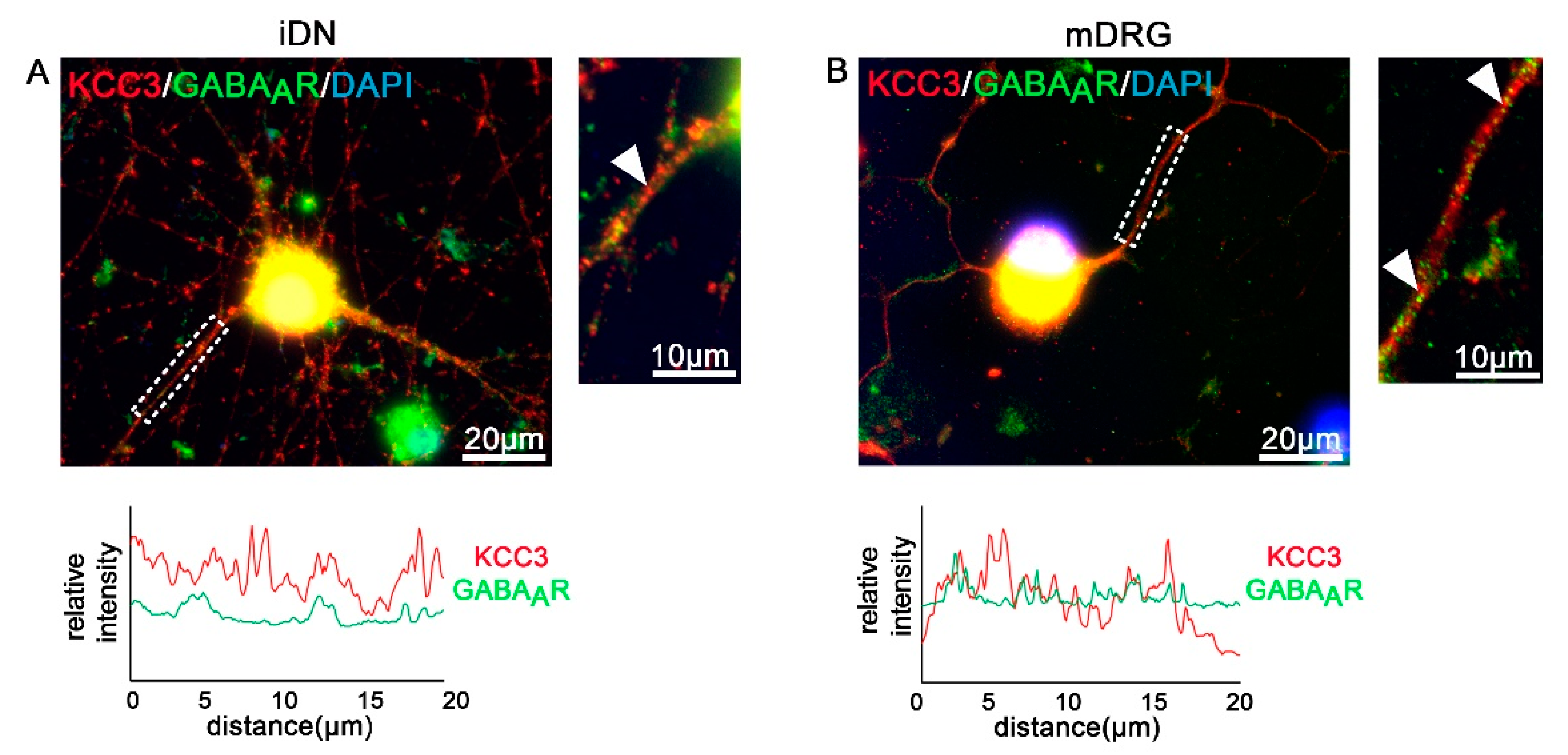
3.6. GABAA Receptors and Transporters in iDNs
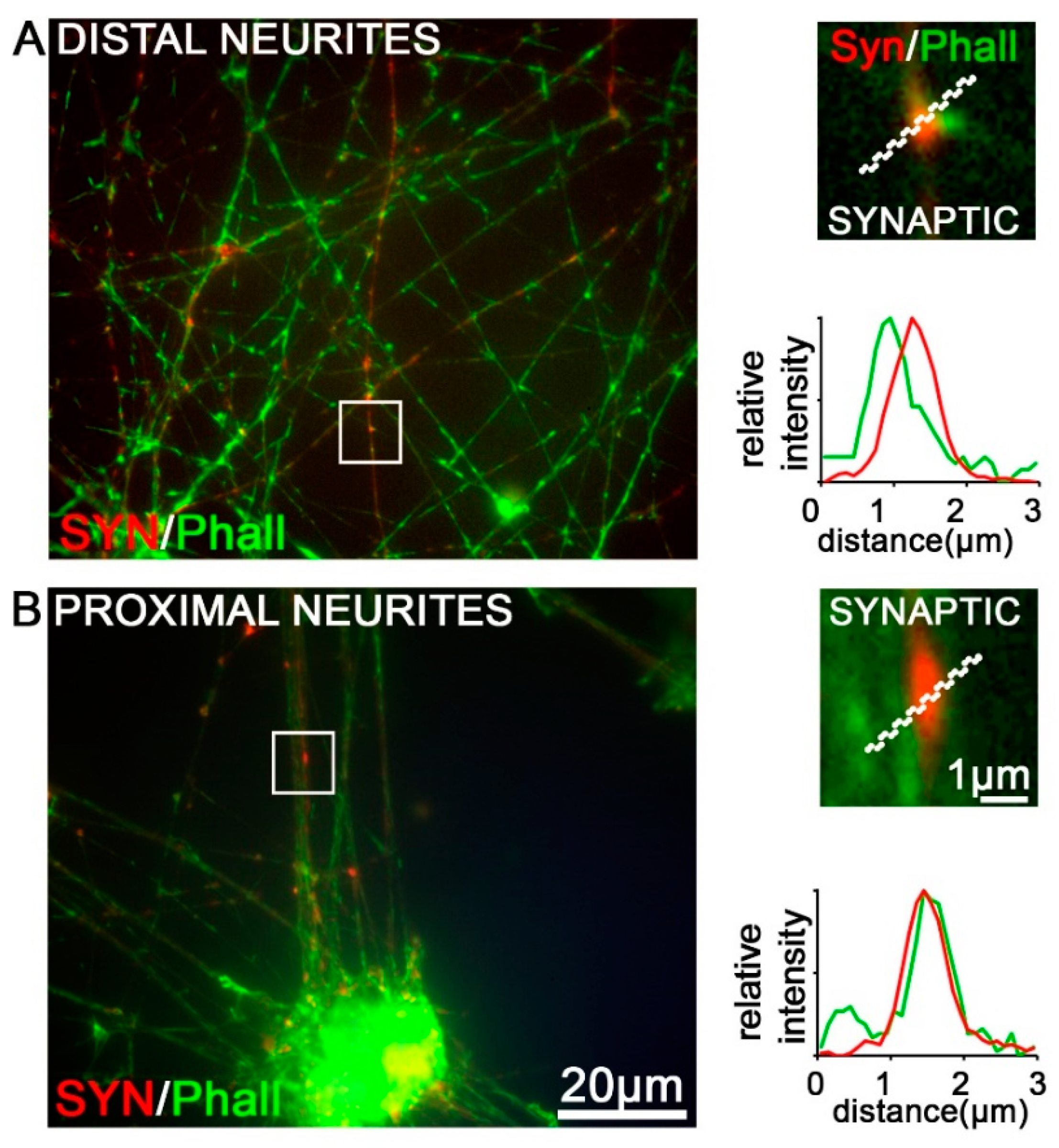
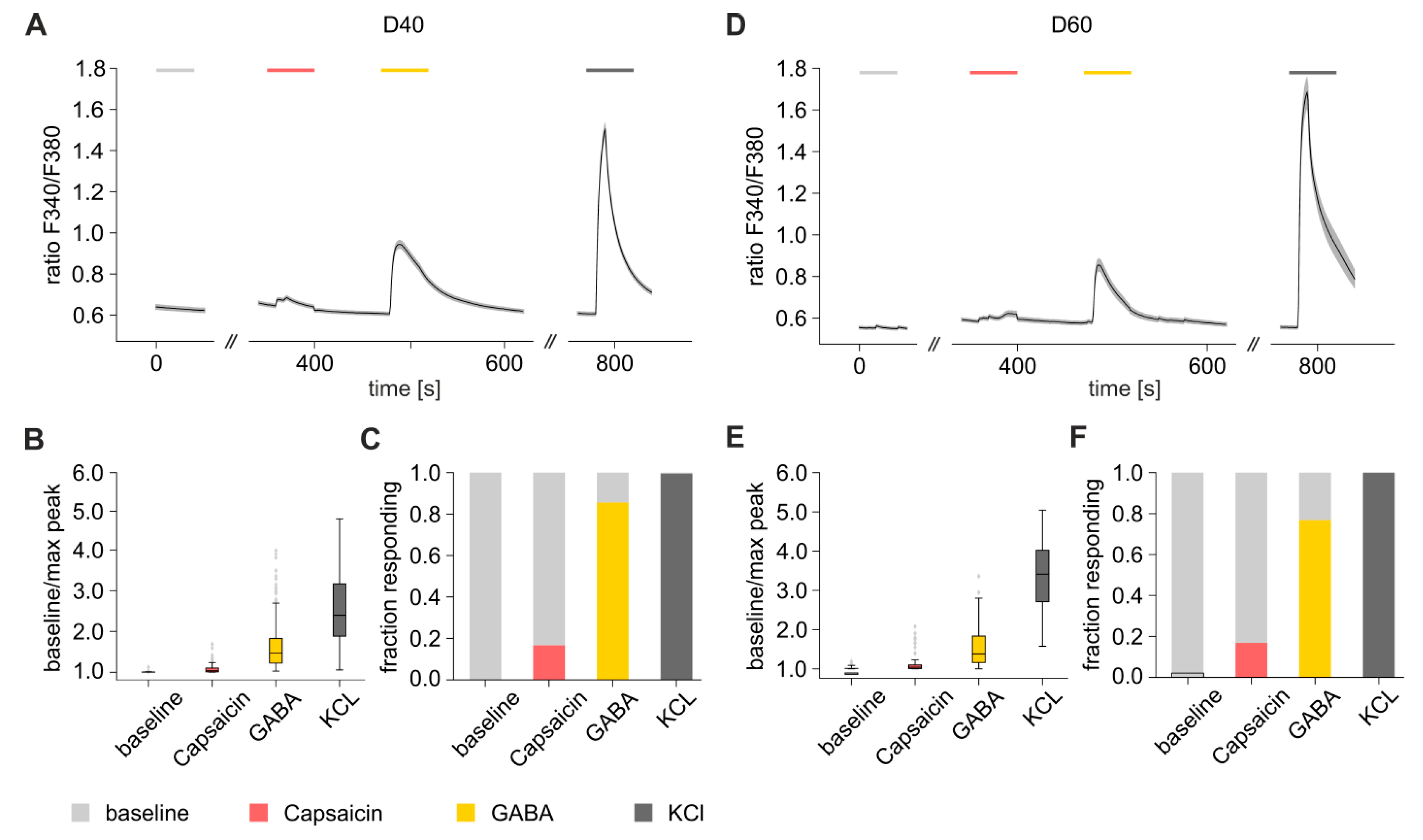
3.7. Functional Characterization of iDNs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rostock, C.; Schrenk-Siemens, K.; Pohle, J.; Siemens, J. Human vs. Mouse Nociceptors-Similarities and Differences. Neuroscience 2018, 387, 13–27. [Google Scholar] [CrossRef]
- Schwaid, A.G.; Krasowka-Zoladek, A.; Chi, A.; Cornella-Taracido, I. Comparison of the Rat and Human Dorsal Root Ganglion Proteome. Sci. Rep. 2018, 8, 13469. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.A.; Jin, S.W.; Nam, M.H.; Kim, S.D. Human Induced Pluripotent Stem Cells: Clinical Significance and Applications in Neurologic Diseases. J. Korean Neurosurg. Soc. 2019, 62, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Qi, Y.; Mica, Y.; Lee, G.; Zhang, X.J.; Niu, L.; Bilsland, J.; Cao, L.; Stevens, E.; Whiting, P.; et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 2012, 30, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, E.; Havlicek, S.; Schmidt, D.; Link, A.S.; Neacsu, C.; Kohl, Z.; Hampl, M.; Kist, A.M.; Klinger, A.; Nau, C.; et al. Pattern of Functional TTX-Resistant Sodium Channels Reveals a Developmental Stage of Human iPSC- and ESC-Derived Nociceptors. Stem Cell Rep. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Nahorski, M.S.; Al-Gazali, L.; Hertecant, J.; Owen, D.J.; Borner, G.H.; Chen, Y.C.; Benn, C.L.; Carvalho, O.P.; Shaikh, S.S.; Phelan, A.; et al. A novel disorder reveals clathrin heavy chain-22 is essential for human pain and touch development. Brain 2015, 138, 2147–2160. [Google Scholar] [CrossRef]
- Namer, B.; Schmidt, D.; Eberhardt, E.; Maroni, M.; Dorfmeister, E.; Kleggetveit, I.P.; Kaluza, L.; Meents, J.; Gerlach, A.; Lin, Z.; et al. Pain relief in a neuropathy patient by lacosamide: Proof of principle of clinical translation from patient-specific iPS cell-derived nociceptors. EBioMedicine 2019, 39, 401–408. [Google Scholar] [CrossRef]
- Meents, J.E.; Bressan, E.; Sontag, S.; Foerster, A.; Hautvast, P.; Rosseler, C.; Hampl, M.; Schuler, H.; Goetzke, R.; Le, T.K.C.; et al. The role of Nav1.7 in human nociceptors: Insights from human induced pluripotent stem cell-derived sensory neurons of erythromelalgia patients. Pain 2019, 160, 1327–1341. [Google Scholar] [CrossRef]
- McDermott, L.A.; Weir, G.A.; Themistocleous, A.C.; Segerdahl, A.R.; Blesneac, I.; Baskozos, G.; Clark, A.J.; Millar, V.; Peck, L.J.; Ebner, D.; et al. Defining the Functional Role of NaV1.7 in Human Nociception. Neuron 2019, 101, 905–919. [Google Scholar] [CrossRef]
- Pettingill, P.; Weir, G.A.; Wei, T.; Wu, Y.; Flower, G.; Lalic, T.; Handel, A.; Duggal, G.; Chintawar, S.; Cheung, J.; et al. A causal role for TRESK loss of function in migraine mechanisms. Brain 2019, 142, 3852–3867. [Google Scholar] [CrossRef]
- Chen, Y.C.; Auer-Grumbach, M.; Matsukawa, S.; Zitzelsberger, M.; Themistocleous, A.C.; Strom, T.M.; Samara, C.; Moore, A.W.; Cho, L.T.; Young, G.T.; et al. Transcriptional regulator PRDM12 is essential for human pain perception. Nat. Genet. 2015, 47, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; McDonnell, A.; Nitzsche, A.; Alexandrou, A.; Saintot, P.P.; Loucif, A.J.; Brown, A.R.; Young, G.; Mis, M.; Randall, A.; et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci. Transl. Med. 2016, 8, 335ra356. [Google Scholar] [CrossRef] [PubMed]
- Wainger, B.J.; Buttermore, E.D.; Oliveira, J.T.; Mellin, C.; Lee, S.; Saber, W.A.; Wang, A.J.; Ichida, J.K.; Chiu, I.M.; Barrett, L.; et al. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat. Neurosci. 2015, 18, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Young, G.T.; Berrocoso, E.M.; Chen, L.; McNaughton, P.A. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011, 333, 1462–1466. [Google Scholar] [CrossRef]
- Ricoy, U.M.; Frerking, M.E. Distinct roles for Cav2.1-2.3 in activity-dependent synaptic dynamics. J. Neurophysiol. 2014, 111, 2404–2413. [Google Scholar] [CrossRef]
- Mochida, S. Presynaptic calcium channels. Neurosci. Res. 2018, 127, 33–44. [Google Scholar] [CrossRef]
- Mochida, S. Presynaptic Calcium Channels. Int. J. Mol. Sci. 2019, 20, 2217. [Google Scholar] [CrossRef]
- Chen, J.T.; Guo, D.; Campanelli, D.; Frattini, F.; Mayer, F.; Zhou, L.; Kuner, R.; Heppenstall, P.A.; Knipper, M.; Hu, J. Presynaptic GABAergic inhibition regulated by BDNF contributes to neuropathic pain induction. Nat. Commun. 2014, 5, 5331. [Google Scholar] [CrossRef]
- Morrison, M.; Klein, C.; Clemann, N.; Collier, D.A.; Hardy, J.; Heisserer, B.; Cader, M.Z.; Graf, M.; Kaye, J. StemBANCC: Governing Access to Material and Data in a Large Stem Cell Research Consortium. Stem Cell Rev. Rep. 2015, 11, 681–687. [Google Scholar] [CrossRef]
- Quarta, S.; Mitric, M.; Kalpachidou, T.; Mair, N.; Schiefermeier-Mach, N.; Andratsch, M.; Qi, Y.; Langeslag, M.; Malsch, P.; Rose-John, S.; et al. Impaired mechanical, heat, and cold nociception in a murine model of genetic TACE/ADAM17 knockdown. FASEB J. 2019, 33, 4418–4431. [Google Scholar] [CrossRef]
- Geisler, S.; Schopf, C.L.; Stanika, R.; Kalb, M.; Campiglio, M.; Repetto, D.; Traxler, L.; Missler, M.; Obermair, G.J. Presynaptic alpha2delta-2 Calcium Channel Subunits Regulate Postsynaptic GABAA Receptor Abundance and Axonal Wiring. J. Neurosci. 2019, 39, 2581–2605. [Google Scholar] [CrossRef] [PubMed]
- Ghinia, M.G.; Novelli, E.; Sajgo, S.; Badea, T.C.; Strettoi, E. Brn3a and Brn3b knockout mice display unvaried retinal fine structure despite major morphological and numerical alterations of ganglion cells. J. Comp. Neurol. 2019, 527, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Khattar, P.; Friedrich, F.W.; Bonne, G.; Carrier, L.; Eschenhagen, T.; Evans, S.M.; Schwartz, K.; Fiszman, M.Y.; Vilquin, J.T. Distinction between two populations of islet-1-positive cells in hearts of different murine strains. Stem Cells Dev. 2011, 20, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Lenkey, N.; Kirizs, T.; Holderith, N.; Mate, Z.; Szabo, G.; Vizi, E.S.; Hajos, N.; Nusser, Z. Tonic endocannabinoid-mediated modulation of GABA release is independent of the CB1 content of axon terminals. Nat. Commun. 2015, 6, 6557. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, L.K.; Nakajima, C.; Kulik, A.; Matsui, K.; Schneider, T.; Shigemoto, R.; Fukazawa, Y. Quantitative regional and ultrastructural localization of the Ca(v)2.3 subunit of R-type calcium channel in mouse brain. J. Neurosci. 2012, 32, 13555–13567. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, C.; Kojo, S.; Yamashita, M.; Moro, K.; Lacaud, G.; Shiroguchi, K.; Taniuchi, I.; Ebihara, T. Runx/Cbfbeta complexes protect group 2 innate lymphoid cells from exhausted-like hyporesponsiveness during allergic airway inflammation. Nat. Commun. 2019, 10, 447. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Liu, G.; Yin, S.; Ma, J.; Liu, J.; Zhang, M.; Wang, Y. p75 neurotrophin receptor regulates NGF-induced myofibroblast differentiation and collagen synthesis through MRTF-A. Exp. Cell Res. 2019, 383, 111504. [Google Scholar] [CrossRef]
- Ono, K.; Ye, Y.; Viet, C.T.; Dang, D.; Schmidt, B.L. TRPV1 expression level in isolectin B(4)-positive neurons contributes to mouse strain difference in cutaneous thermal nociceptive sensitivity. J. Neurophysiol. 2015, 113, 3345–3355. [Google Scholar] [CrossRef]
- Quarta, S.; Baeumer, B.E.; Scherbakov, N.; Andratsch, M.; Rose-John, S.; Dechant, G.; Bandtlow, C.E.; Kress, M. Peripheral nerve regeneration and NGF-dependent neurite outgrowth of adult sensory neurons converge on STAT3 phosphorylation downstream of neuropoietic cytokine receptor gp130. J. Neurosci. 2014, 34, 13222–13233. [Google Scholar] [CrossRef]
- Hughes, D.I.; Sikander, S.; Kinnon, C.M.; Boyle, K.A.; Watanabe, M.; Callister, R.J.; Graham, B.A. Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: A likely source of axo-axonic inputs in the mouse spinal dorsal horn. J. Physiol. 2012, 590, 3927–3951. [Google Scholar] [CrossRef]
- Camprubi-Robles, M.; Mair, N.; Andratsch, M.; Benetti, C.; Beroukas, D.; Rukwied, R.; Langeslag, M.; Proia, R.L.; Schmelz, M.; Ferrer Montiel, A.V.; et al. Sphingosine-1-phosphate-induced nociceptor excitation and ongoing pain behavior in mice and humans is largely mediated by S1P3 receptor. J. Neurosci. 2013, 33, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Tempest, L.; Lee, S.I.; Turner, E.E. Brn3a and Islet1 act epistatically to regulate the gene expression program of sensory differentiation. J. Neurosci. 2011, 31, 9789–9799. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.H.; Yarmishyn, A.A.; Hwang, D.K.; Hsu, C.C.; Wang, M.L.; Yang, Y.P.; Chien, K.H.; Chiou, S.H.; Peng, C.H.; Chen, S.J. Expression profiling of cell-intrinsic regulators in the process of differentiation of human iPSCs into retinal lineages. Stem Cell Res. Ther. 2018, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Broom, D.C.; Liu, Y.; de Nooij, J.C.; Li, Z.; Cen, C.; Samad, O.A.; Jessell, T.M.; Woolf, C.J.; Ma, Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 2006, 49, 365–377. [Google Scholar] [CrossRef]
- Wang, J.W.; Stifani, S. Roles of Runx Genes in Nervous System Development. Adv. Exp. Med. Biol. 2017, 962, 103–116. [Google Scholar] [CrossRef]
- Marmigere, F.; Montelius, A.; Wegner, M.; Groner, Y.; Reichardt, L.F.; Ernfors, P. The Runx1/AML1 transcription factor selectively regulates development and survival of TrkA nociceptive sensory neurons. Nat. Neurosci. 2006, 9, 180–187. [Google Scholar] [CrossRef]
- Lopes, C.; Liu, Z.; Xu, Y.; Ma, Q. Tlx3 and Runx1 act in combination to coordinate the development of a cohort of nociceptors, thermoceptors, and pruriceptors. J. Neurosci. 2012, 32, 9706–9715. [Google Scholar] [CrossRef]
- Lou, S.; Pan, X.; Huang, T.; Duan, B.; Yang, F.C.; Yang, J.; Xiong, M.; Liu, Y.; Ma, Q. Incoherent feed-forward regulatory loops control segregation of C-mechanoreceptors, nociceptors, and pruriceptors. J. Neurosci. 2015, 35, 5317–5329. [Google Scholar] [CrossRef]
- Obata, K.; Katsura, H.; Sakurai, J.; Kobayashi, K.; Yamanaka, H.; Dai, Y.; Fukuoka, T.; Noguchi, K. Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury. J. Neurosci. 2006, 26, 11974–11986. [Google Scholar] [CrossRef]
- O’Leary, V.B.; O’Connell, M.; Antyborzec, I.; Ntziachristos, V.; Oliver Dolly, J.; Ovsepian, S.V. Alleviation of Trigeminal Nociception Using p75 Neurotrophin Receptor Targeted Lentiviral Interference Therapy. Neurotherapeutics 2018, 15, 489–499. [Google Scholar] [CrossRef]
- Nocchi, L.; Portulano, C.; Franciosa, F.; Doleschall, B.; Panea, M.; Roy, N.; Maffei, M.; Gargano, A.; Perlas, E.; Heppenstall, P.A. Nerve growth factor-mediated photoablation of nociceptors reduces pain behavior in mice. Pain 2019, 160, 2305–2315. [Google Scholar] [CrossRef]
- Chen, Z.; Donnelly, C.R.; Dominguez, B.; Harada, Y.; Lin, W.; Halim, A.S.; Bengoechea, T.G.; Pierchala, B.A.; Lee, K.F. p75 Is Required for the Establishment of Postnatal Sensory Neuron Diversity by Potentiating Ret Signaling. Cell Rep. 2017, 21, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Valente, J.; Andreou, A.P.; Urban, L.; Nagy, I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br. J. Pharmacol. 2014, 171, 2508–2527. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dean, C.; Isacoff, E. Alternative splicing of neuroligin regulates the rate of presynaptic differentiation. J. Neurosci. 2010, 30, 11435–11446. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Zuniga, R.; Concha, G.; Zuniga, L. HCN Channels: New Therapeutic Targets for Pain Treatment. Molecules 2018, 23, 2094. [Google Scholar] [CrossRef] [PubMed]
- Israel, M.R.; Tanaka, B.S.; Castro, J.; Thongyoo, P.; Robinson, S.D.; Zhao, P.; Deuis, J.R.; Craik, D.J.; Durek, T.; Brierley, S.M.; et al. NaV 1.6 regulates excitability of mechanosensitive sensory neurons. J. Physiol. 2019, 597, 3751–3768. [Google Scholar] [CrossRef]
- Erickson, A.; Deiteren, A.; Harrington, A.M.; Garcia-Caraballo, S.; Castro, J.; Caldwell, A.; Grundy, L.; Brierley, S.M. Voltage-gated sodium channels: (NaV)igating the field to determine their contribution to visceral nociception. J. Physiol. 2018, 596, 785–807. [Google Scholar] [CrossRef]
- Eijkelkamp, N.; Linley, J.E.; Baker, M.D.; Minett, M.S.; Cregg, R.; Werdehausen, R.; Rugiero, F.; Wood, J.N. Neurological perspectives on voltage-gated sodium channels. Brain 2012, 135, 2585–2612. [Google Scholar] [CrossRef]
- Mis, M.A.; Yang, Y.; Tanaka, B.S.; Gomis-Perez, C.; Liu, S.; Dib-Hajj, F.; Adi, T.; Garcia-Milian, R.; Schulman, B.R.; Dib-Hajj, S.D.; et al. Resilience to Pain: A Peripheral Component Identified Using Induced Pluripotent Stem Cells and Dynamic Clamp. J. Neurosci. 2019, 39, 382–392. [Google Scholar] [CrossRef]
- Bourinet, E.; Altier, C.; Hildebrand, M.E.; Trang, T.; Salter, M.W.; Zamponi, G.W. Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014, 94, 81–140. [Google Scholar] [CrossRef]
- Wilke, B.U.; Kummer, K.K.; Leitner, M.G.; Kress, M. Chloride-The Underrated Ion in Nociceptors. Front. Neurosci. 2020, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Cervero, F.; Gold, M.S.; Hammond, D.L.; Prescott, S.A. Chloride regulation in the pain pathway. Brain Res. Rev. 2009, 60, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Burma, N.E.; Leduc-Pessah, H.; Fan, C.Y.; Trang, T. Animal models of chronic pain: Advances and challenges for clinical translation. J. Neurosci. Res. 2017, 95, 1242–1256. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, J.; Foskolou, S.; Kilpinen, H.; Rodrigues, J.; Alasoo, K.; Knights, A.J.; Patel, M.; Goncalves, A.; Ferreira, R.; Benn, C.L.; et al. Molecular and functional variation in iPSC-derived sensory neurons. Nat. Genet. 2018, 50, 54–61. [Google Scholar] [CrossRef]
- Volpato, V.; Smith, J.; Sandor, C.; Ried, J.S.; Baud, A.; Handel, A.; Newey, S.E.; Wessely, F.; Attar, M.; Whiteley, E.; et al. Reproducibility of Molecular Phenotypes after Long-Term Differentiation to Human iPSC-Derived Neurons: A Multi-Site Omics Study. Stem Cell Rep. 2018, 11, 897–911. [Google Scholar] [CrossRef]
- Schrenk-Siemens, K.; Rosseler, C.; Lampert, A. Translational Model Systems for Complex Sodium Channel Pathophysiology in Pain. Handb. Exp. Pharmacol. 2018, 246, 355–369. [Google Scholar] [CrossRef]
- Alsaloum, M.; Estacion, M.; Almomani, R.; Gerrits, M.M.; Bonhof, G.J.; Ziegler, D.; Malik, R.; Ferdousi, M.; Lauria, G.; Merkies, I.S.; et al. A gain-of-function sodium channel beta2-subunit mutation in painful diabetic neuropathy. Mol. Pain 2019, 15, 1744806919849802. [Google Scholar] [CrossRef]
- Klein, T.; Klug, K.; Henkel, L.; Kwok, C.K.; Edenhofer, F.; Klopocki, E.; Kurth, I.; Uceyler, N. Generation of two induced pluripotent stem cell lines from skin fibroblasts of sisters carrying a c.1094C>A variation in the SCN10A gene potentially associated with small fiber neuropathy. Stem Cell Res. 2019, 35, 101396. [Google Scholar] [CrossRef]
- Nebe, J.; Vanegas, H.; Neugebauer, V.; Schaible, H.G. Omega-agatoxin IVA, a P-type calcium channel antagonist, reduces nociceptive processing in spinal cord neurons with input from the inflamed but not from the normal knee joint--an electrophysiological study in the rat in vivo. Eur. J. Neurosci. 1997, 9, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Dickenson, A.H. Blockade of spinal N- and P-type, but not L-type, calcium channels inhibits the excitability of rat dorsal horn neurones produced by subcutaneous formalin inflammation. Pain 1997, 69, 93–100. [Google Scholar] [CrossRef]
- Matthews, E.A.; Bee, L.A.; Stephens, G.J.; Dickenson, A.H. The Cav2.3 calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur. J. Neurosci. 2007, 25, 3561–3569. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Schopf, C.L.; Obermair, G.J. Emerging evidence for specific neuronal functions of auxiliary calcium channel alpha(2)delta subunits. Gen. Physiol. Biophys. 2015, 34, 105–118. [Google Scholar] [CrossRef]
- Skubatz, H. Neuropeptide FF (FLFQPQRF-NH2) and its Fragments Bind to alpha2delta Subunit of Voltage-Gated Calcium Channels. J. Pharm. Pharm. Sci. 2019, 22, 292–300. [Google Scholar] [CrossRef]
- Field, M.J.; Cox, P.J.; Stott, E.; Melrose, H.; Offord, J.; Su, T.Z.; Bramwell, S.; Corradini, L.; England, S.; Winks, J.; et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl. Acad. Sci. USA 2006, 103, 17537–17542. [Google Scholar] [CrossRef]
- Sondermann, J.R.; Barry, A.M.; Jahn, O.; Michel, N.; Abdelaziz, R.; Kugler, S.; Gomez-Varela, D.; Schmidt, M. Vti1b promotes TRPV1 sensitization during inflammatory pain. Pain 2019, 160, 508–527. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Steinhoff, M.; Dolly, J.O. TNFalpha induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Sci. Rep. 2016, 6, 21226. [Google Scholar] [CrossRef]
- Goswami, C.; Rademacher, N.; Smalla, K.H.; Kalscheuer, V.; Ropers, H.H.; Gundelfinger, E.D.; Hucho, T. TRPV1 acts as a synaptic protein and regulates vesicle recycling. J. Cell Sci. 2010, 123, 2045–2057. [Google Scholar] [CrossRef]
- Raingo, J.; Castiglioni, A.J.; Lipscombe, D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat. Neurosci. 2007, 10, 285–292. [Google Scholar] [CrossRef]
- Kouranova, E.V.; Strassle, B.W.; Ring, R.H.; Bowlby, M.R.; Vasilyev, D.V. Hyperpolarization-activated cyclic nucleotide-gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons: Correlation with hyperpolarization-activated current gating. Neuroscience 2008, 153, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.; McMullan, S.; Djouhri, L.; Gao, L.; Watkins, R.; Berry, C.; Dempsey, K.; Lawson, S.N. HCN1 and HCN2 in Rat DRG neurons: Levels in nociceptors and non-nociceptors, NT3-dependence and influence of CFA-induced skin inflammation on HCN2 and NT3 expression. PLoS ONE 2012, 7, e50442. [Google Scholar] [CrossRef] [PubMed]
- Lainez, S.; Tsantoulas, C.; Biel, M.; McNaughton, P.A. HCN3 ion channels: Roles in sensory neuronal excitability and pain. J. Physiol. 2019, 597, 4661–4675. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Jin, L.; Tan, Y.; Li, W.; Tang, J. HCN2 contributes to oxaliplatin-induced neuropathic pain through activation of the CaMKII/CREB cascade in spinal neurons. Mol. Pain 2018, 14, 1744806918778490. [Google Scholar] [CrossRef] [PubMed]
- Djouhri, L.; Smith, T.; Ahmeda, A.; Alotaibi, M.; Weng, X. Hyperpolarization-activated cyclic nucleotide-gated channels contribute to spontaneous activity in L4 C-fiber nociceptors, but not Abeta-non-nociceptors, after axotomy of L5-spinal nerve in the rat in vivo. Pain 2018, 159, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Al Otaibi, M.; Sathish, J.; Djouhri, L. Increased expression of HCN2 channel protein in L4 dorsal root ganglion neurons following axotomy of L5- and inflammation of L4-spinal nerves in rats. Neuroscience 2015, 295, 90–102. [Google Scholar] [CrossRef]
- Young, G.T.; Emery, E.C.; Mooney, E.R.; Tsantoulas, C.; McNaughton, P.A. Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels. Pain 2014, 155, 1708–1719. [Google Scholar] [CrossRef]
- Emery, E.C.; Young, G.T.; McNaughton, P.A. HCN2 ion channels: An emerging role as the pacemakers of pain. Trends Pharmacol. Sci. 2012, 33, 456–463. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Xing, G.G.; Wang, S.L.; Tu, H.Y.; Chi, Y.N.; Li, J.; Liu, F.Y.; Han, J.S.; Wan, Y. Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain 2008, 137, 495–506. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Guo, H.Q.; Lee, D.H.; Luo, L.; Liu, C.; Kuei, C.; Velumian, A.A.; Butler, M.P.; Brown, S.M.; Dubin, A.E. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J. Neurosci. 2003, 23, 1169–1178. [Google Scholar] [CrossRef]
- Schnorr, S.; Eberhardt, M.; Kistner, K.; Rajab, H.; Kasser, J.; Hess, A.; Reeh, P.; Ludwig, A.; Herrmann, S. HCN2 channels account for mechanical (but not heat) hyperalgesia during long-standing inflammation. Pain 2014, 155, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Labrakakis, C.; Tong, C.K.; Weissman, T.; Torsney, C.; MacDermott, A.B. Localization and function of ATP and GABAA receptors expressed by nociceptors and other postnatal sensory neurons in rat. J. Physiol. 2003, 549, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Tanaka, K.; Nishibe, Y.; Hasegawa, J.; Ueno, H. GABA-synthesizing enzyme, GAD67, from dermal fibroblasts: Evidence for a new skin function. Biochim. Biophys. Acta 2007, 1770, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ault, B.; Hildebrand, L.M. GABAA receptor-mediated excitation of nociceptive afferents in the rat isolated spinal cord-tail preparation. Neuropharmacology 1994, 33, 109–114. [Google Scholar] [CrossRef]
- Zimmerman, A.L.; Kovatsis, E.M.; Pozsgai, R.Y.; Tasnim, A.; Zhang, Q.; Ginty, D.D. Distinct Modes of Presynaptic Inhibition of Cutaneous Afferents and Their Functions in Behavior. Neuron 2019, 102, 420–434.e428. [Google Scholar] [CrossRef]

| Marker | iDN | mDRG |
|---|---|---|
| BRN3A | 100% (high and low expressing cells) | 100% |
| ISL1 | 100% | 100% |
| RUNX1 | 100% (high and low expressing cells) | 100% |
| p75 | ~79% | ~64% |
| TRPV1 | ~45% | ~74% |
| SYN | positive varicosities/boutons | no varicosities/boutons |
| HCN1–3 | 100% | 100% |
| HCN4 | not expressed | not expressed |
| CaV2.1 CaV2.2 CaV2.3 | expressed, synaptic localization patterns | expressed on neurites, no synaptic localization patterns |
| KCC3/GABAAR | expressed/lower levels | expressed/higher levels higher degree of colocalization |
| TUJ1/Phall | neurites and varicosities/boutons | only on neurites |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoepf, C.L.; Zeidler, M.; Spiecker, L.; Kern, G.; Lechner, J.; Kummer, K.K.; Kress, M. Selected Ionotropic Receptors and Voltage-Gated Ion Channels: More Functional Competence for Human Induced Pluripotent Stem Cell (iPSC)-Derived Nociceptors. Brain Sci. 2020, 10, 344. https://doi.org/10.3390/brainsci10060344
Schoepf CL, Zeidler M, Spiecker L, Kern G, Lechner J, Kummer KK, Kress M. Selected Ionotropic Receptors and Voltage-Gated Ion Channels: More Functional Competence for Human Induced Pluripotent Stem Cell (iPSC)-Derived Nociceptors. Brain Sciences. 2020; 10(6):344. https://doi.org/10.3390/brainsci10060344
Chicago/Turabian StyleSchoepf, Clemens L., Maximilian Zeidler, Lisa Spiecker, Georg Kern, Judith Lechner, Kai K. Kummer, and Michaela Kress. 2020. "Selected Ionotropic Receptors and Voltage-Gated Ion Channels: More Functional Competence for Human Induced Pluripotent Stem Cell (iPSC)-Derived Nociceptors" Brain Sciences 10, no. 6: 344. https://doi.org/10.3390/brainsci10060344
APA StyleSchoepf, C. L., Zeidler, M., Spiecker, L., Kern, G., Lechner, J., Kummer, K. K., & Kress, M. (2020). Selected Ionotropic Receptors and Voltage-Gated Ion Channels: More Functional Competence for Human Induced Pluripotent Stem Cell (iPSC)-Derived Nociceptors. Brain Sciences, 10(6), 344. https://doi.org/10.3390/brainsci10060344






