Gaze Palsy as a Manifestation of Todd’s Phenomenon: Case Report and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
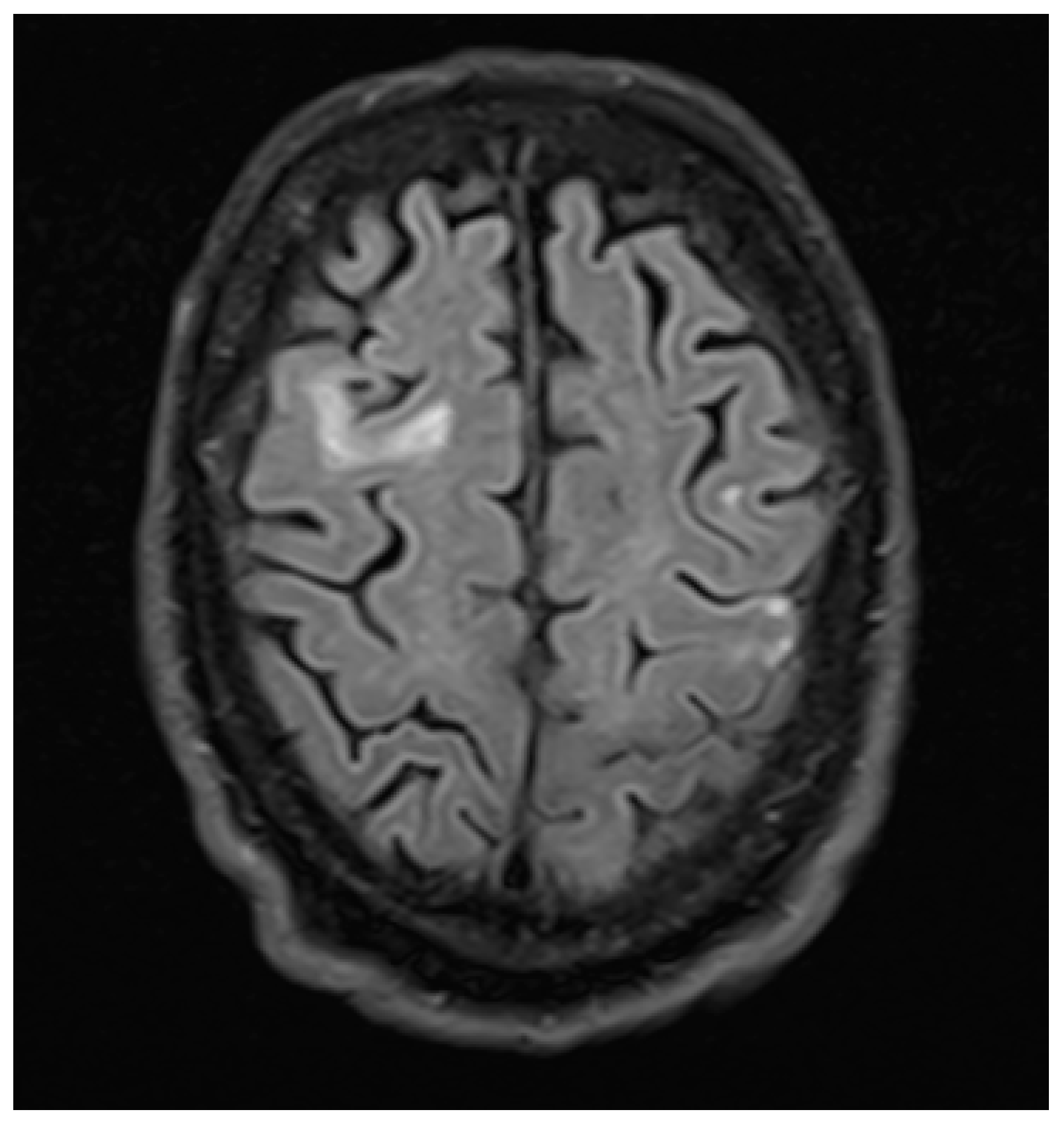
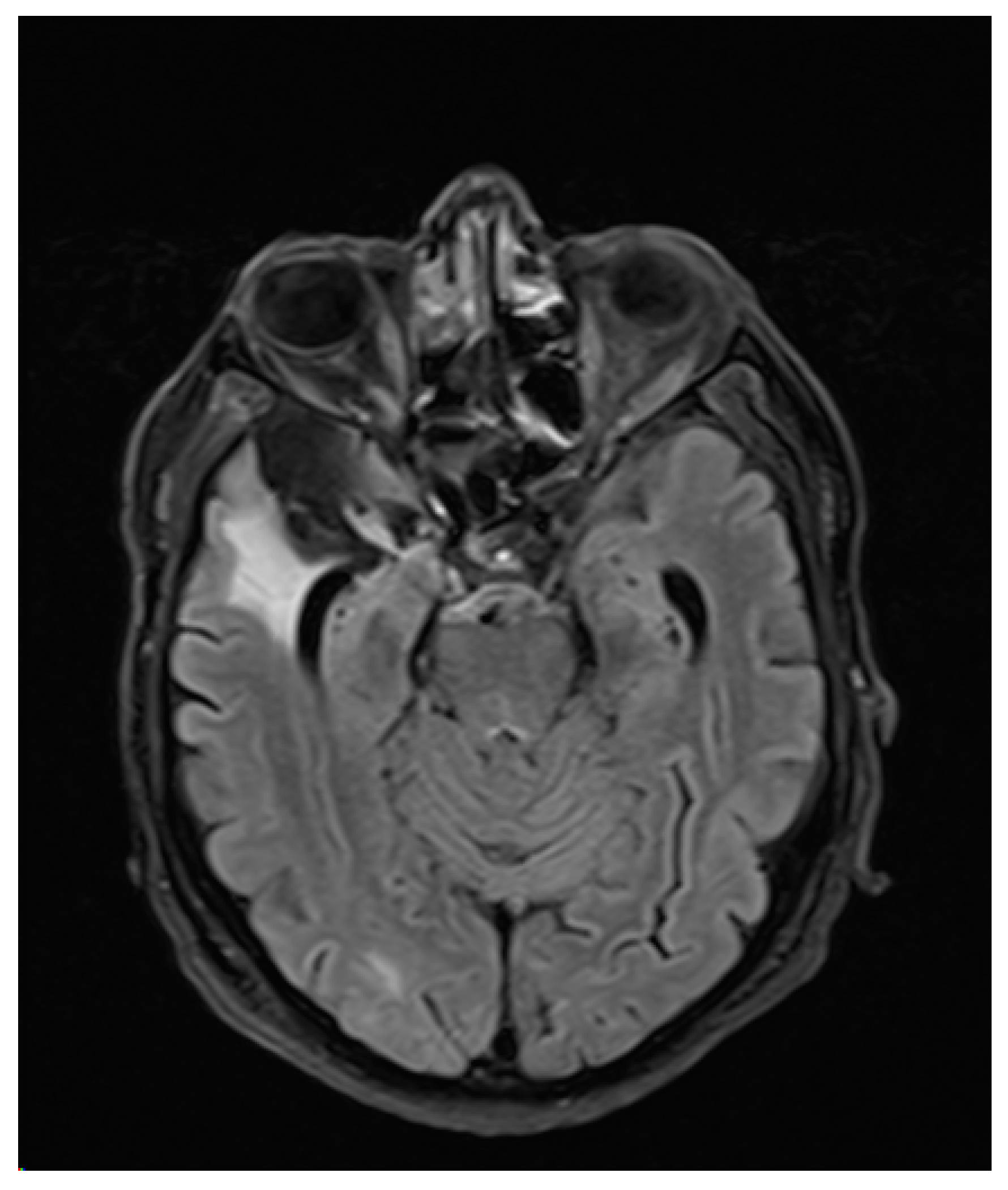
3. Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohno, T.; Oohira, A.; Hori, S. Near reflex substituting for acquired horizontal gaze palsy: A case report. Jpn. J. Ophthalmol. 2004, 48, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.; Lüders, H.; Dinner, D.S.; Morris, H.H.; Wyllie, E. Versive eye movements elicited by cortical stimulation of the human brain. Neurology 1990, 40, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.B.; Young, R.R. (Eds.) Movement Disorders in Neurology and Neuropsychiatry, 2nd ed.; Blackwell Science: Malden, MA, USA, 1999. [Google Scholar]
- Rasmussen, T.; Penfield, W. Movement of head and eyes from stimulation of human frontal cortex. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1948, 27, 346–361. [Google Scholar] [PubMed]
- Wyllie, E.; Luders, H.; Morris, H.H.; Lesser, R.P.; Dinner, D.S. The lateralizing significance of versive head and eye movements during epileptic seizures. Neurology 1986, 36, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Janszky, J.; Fogarasi, A.; Jokeit, H.; Ebner, A. Lateralizing value of unilateral motor and somatosensory manifestations in frontal lobe seizures. Epilepsy Res. 2001, 43, 125–133. [Google Scholar] [CrossRef]
- Lee, R.W.; Worrell, G.A. Dorsolateral Frontal Lobe Epilepsy. J. Clin. Neurophysiol. 2012, 29, 379–384. [Google Scholar] [CrossRef]
- Bravais, L.F. Recherches sur les Symptômes et le Traitement de L’épilepsie Hémiplégique; Faculté de Médecine: Paris, France, 1827. [Google Scholar]
- Todd, R.B. On the pathology and treatment of convulsive diseases. Lond. Med. Gaz. 1849, 8, 661–671. [Google Scholar]
- Binder, D.K. A history of Todd and his paralysis. Neurosurgery 2004, 54, 480–487. [Google Scholar] [CrossRef]
- Gowers, W.R. Epilepsy and Other Chronic Convulsive Diseases: Their Causes, Symptoms, and Treatment, 2nd ed.; Churchill: London, UK, 1901. [Google Scholar]
- Jackson, J.H. On temporary paralysis after epileptiform and epileptic seizures; a contribution to the study of dissolution of the nervous system. Brain 1881, 3, 433–451. [Google Scholar] [CrossRef]
- Efron, R. Post-epileptic paralysis: Theoretical critique and report of a case. Brain 1961, 84, 381–394. [Google Scholar] [CrossRef]
- Meyer, J.S.; Portnoy, H.D. Post-epileptic paralysis. A clinical and experimental study. Brain 1959, 82, 162–185. [Google Scholar] [CrossRef]
- Yarnell, P.R. Todd’s paralysis: A cerebrovascular phenomenon? Stroke 1975, 6, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Royter, V.; Paletz, L.; Waters, M.F. Stroke vs. status epilepticus. A case report utilizing CT perfusion. J. Neurol. Sci. 2007, 266, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, F.; Hufnagel, A.; Elger, C.E.; Biersack, H.J. Single-Photon-Emissions-Computertomographie (SPECT) in der Epilepsiediagnostik. Radiologe 1993, 33, 198–203. [Google Scholar]
- Newton, M.R.; Berkovic, S.F.; Austin, M.C.; Rowe, C.C.; McKay, W.J.; Bladin, P.F. Postictal switch in blood flow distribution and temporal lobe seizures. J. Neurol. Neurosurg. Psychiatry 1992, 55, 891–894. [Google Scholar] [CrossRef][Green Version]
- Mathews, M.S.; Smith, W.S.; Wintermark, M.; Dillon, W.P.; Binder, D.K. Local cortical hypoperfusion imaged with CT perfusion during postictal Todd’s paresis. Neuroradiology 2008, 50, 397–401. [Google Scholar] [CrossRef]
- Rupprecht, S.; Schwab, M.; Fitzek, C.; Witte, O.W.; Terborg, C.; Hagemann, G. Hemispheric hypoperfusion in postictal paresis mimics early brain ischemia. Epilepsy Res. 2010, 89, 355–359. [Google Scholar] [CrossRef]
- Hassan, A.E.; Cu, S.R.; Rodriguez, G.J.; Qureshi, A.I. Regional cerebral hyperperfusion associated with postictal paresis. J. Vasc. Interv. Neurol. 2012, 5, 40–42. [Google Scholar]
- Farrell, J.S.; Gaxiola-Valdez, I.; Wolff, M.D.; David, L.S.; Dika, H.I.; Geeraert, B.L.; Wang, X.R.; Singh, S.; Spanswick, S.C.; Dunn, J.F.; et al. Postictal Behavioural Impairments are Due to a Severe Prolonged Hypoperfusion/Hypoxia Event That is COX-2 Dependent. eLife 2016, 5, 499. [Google Scholar] [CrossRef]
- Rolak, L.A.; Rutecki, P.; Ashizawa, T.; Harati, Y. Clinical features of Todd’s post-epileptic paralysis. J. Neurol. Neurosurg. Psychiatry 1992, 55, 63–64. [Google Scholar] [CrossRef][Green Version]
- Gallmetzer, P.; Leutmezer, F.; Serles, W.; Assem-Hilger, E.; Spatt, J.; Baumgartner, C. Postictal paresis in focal epilepsies—Incidence, duration, and causes. A video-EEG monitoring study. Neurology 2004, 62, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, C.; Steinhoff, B.J.; Dichgans, M. Varianten der Todd’schen Parese: Postiktuale Apraxie und prolongierter postiktualer Hemineglekt. Nervenarzt 1994, 65, 700–703. [Google Scholar] [PubMed]
- Gadoth, N.; Margalith, D.; Bechar, M. Unilateral pupillary dilatation during focal seizures. J. Neurol. 1981, 225, 227–230. [Google Scholar] [CrossRef]
- Salmon, J.H. Transient postictal hemianopsia. Arch. Ophthalmol. 1968, 79, 523–525. [Google Scholar] [CrossRef]
- Kosnik, E.; Paulson, G.W.; Laguna, J.F. Postictal blindness. Neurology 1976, 26, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Badran, A.; Bartolini, L.; Ksendzovsky, A.; Ray-Chaudhury, A.; Abdennadher, M.; Zaghloul, K.; Inati, S.K. Transient postictal blindness after a focal posterior cingulate gyrus seizure. Seizure 2018, 54, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Skolik, S.A.; Mizen, T.R.; Burde, R.M. Transient postictal cortical blindness. J. Clin. Neuroophthalmol. 1987, 7, 151–154. [Google Scholar]
- LaCapra, S.; King, C. Mute postseizure patient: An unusual manifestation of Todd’s phenomenon. Ann. Emerg. Med. 1994, 23, 877–880. [Google Scholar] [CrossRef]
- Biton, V.; Gates, J.R.; Sussman, L.D. Prolonged postictal encephalopathy. Neurology 1990, 40, 963–966. [Google Scholar] [CrossRef]
- Remick, R.A.; Jones, M.W.; Campos, P.E. Postictal bulimia. J. Clin. Psychiatry 1980, 41, 256. [Google Scholar]
- Hanoglu, L.; Ertaş, N.K.; Altunhalka, A.; Kirbaş, D. Cognitive dysfunction of right hemisphere-like Todd’s paralysis after status epilepticus: A case report. Seizure 2001, 10, 125–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bansal, S.K.; Chopra, J.S. Reversible postictal ataxic hemiparesis. Neurol. Sci. 1991, 12, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.; Adam, C.; Rouleau, I.; Saint-Hilaire, J.-M. Postictal aphasia and paresis: A clinical and intracerebral EEG study. Can. J. Neurol. Sci. 2000, 27, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Morinaga, K.; Kohno, H.; Maruyama, Y.; Ninomiya, T.; Ishitobi, Y.; Tanaka, Y.; Tsuru, J.; Hanada, H.; Yoshikawa, T.; et al. An uncommon case of random fire-setting behavior associated with Todd paralysis: A case report. BMC Psychiatry 2012, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, B.-G.; Zeng, W.-Y.; Zhong, Y.; Cai, X.-S.; Zheng, L.-Q.; Wu, Z.-Y.; Wang, F. Clinical study of seven patients with special syndrome of post-epileptic dysfunction persisting over 24 hours. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3229–3233. [Google Scholar]
- Kanner, A.M.; Stagno, S.; Kotagal, P.; Morris, H.H. Postictal psychiatric events during prolonged video-electroencephalographic monitoring studies. Arch. Neurol. 1996, 53, 258–263. [Google Scholar] [CrossRef]
- Clancy, M.J.; Clarke, M.; Connor, D.; Cannon, M.; Cotter, D.R. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry 2014, 14, 75. [Google Scholar] [CrossRef]
- Down, J.F.; McLeod, A.D.M. Todd’s paralysis following sedation for sleep nasendoscopy. Anaesthesia 2003, 58, 183–203. [Google Scholar]
- Collier, H.W.; Engelking, K. Todd’s paralysis following an interscalene block. Anesthesiology 1984, 61, 342–343. [Google Scholar] [CrossRef]
- Bolumburu, E.U.; Franco, J.I.; Esteban, M.A.; Blázquez, D.L.; Schlumberger, E.; Granda, J.A.G.; Torres, C.V. Paresia poscrítica durante estudios de monitorización de vídeo-EEG. Rev. Neurol. 2002, 35, 404–407. [Google Scholar]
| Reference Number | Postictal Neurological Deficit | Duration | Type of Epilepsy | Seizure Type Prior to Onset of TP Symptoms | EEG Findings during TP | Imaging |
|---|---|---|---|---|---|---|
| 25 | Apraxia, Hemineglect | 72 h | TLE | BTCS | No | MRI: No epileptogenic lesions |
| 27 | Hemianopsia | 1 month | Symptomatic | BTCS | No | MRI: Glioblastoma multiforme |
| 28 | Blindness | Up to 3 days | N/A | N/A | N/A | N/A |
| 31 | Mutism, right hemiparesis | 48 h | N/A | BTCS series | No | CT: normal |
| 32 | Confusion | 4–10 days | N/A | Focal and generalized | Typical encephalopathic pattern | 9 of 11: Minimal structural abnormalities |
| 34 | Neglect, dyscalculia, and disturbed visuospatial perception | 1 month | Symptomatic parietal lobe epilepsy | Convulsive status epilepticus | Diffuse amplitude reduction in the right hemisphere | MRI: Pachygyria and polymicrogyria in the right parietal cortex |
| 35 | Ataxic hemiparesis | 7 days | N/A | N/A | N/A | N/A |
| 29 | Blindness | N/A | Focal epilepsy | Focal posterior cingulate gyrus seizure | N/A | N/A |
| 38 | Hemiplegia, global aphasia, gaze palsy | 2 days | Symptomatic epilepsy with focal and focal to bilateral tonic-clonic seizures | Convulsive status epilepticus | N/A | Postischemic defects, localization N/A |
| Lower limb paralysis, global aphasia, cognitive disorder | 2 months | First onset | Convulsive status epilepticus | N/A | Postischemic defects, meningeomas, localization N/A | |
| Hemiplegia, sensory aphasia | 2 months | Symptomatic epilepsy | Focal to bilateral tonic-clonic seizure | N/A | Postischemic defects, localization N/A | |
| Quadriplegia, motor aphasia, gaze palsy, cognitive disorder | 3 months | Symptomatic epilepsy | Convulsive status epilepticus | N/A | Postischemic and posthemorrhagic defects, localization N/A | |
| Lower limb paralysis, global aphasia, cognitive disorder | 3 months | First onset | Convulsive status epilepticus | N/A | MRI: Bilateral reversible white matter damage CT Perfusion imaging: prolonged bilateral cortical mean transit time, cortical cerebral blood volume reduced | |
| Hemiplegia, global aphasia, disorder of consciousness | 2 months | First onset | Convulsive status epilepticus | N/A | Postischemic defects, localization N/A | |
| Global aphasia, gaze palsy, hemianopsia | 2 days | N/A | Convulsive status epilepticus | N/A | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaciregui Dague, K.; Dafotakis, M.; Schulz, J.B.; Surges, R. Gaze Palsy as a Manifestation of Todd’s Phenomenon: Case Report and Review of the Literature. Brain Sci. 2020, 10, 298. https://doi.org/10.3390/brainsci10050298
Olaciregui Dague K, Dafotakis M, Schulz JB, Surges R. Gaze Palsy as a Manifestation of Todd’s Phenomenon: Case Report and Review of the Literature. Brain Sciences. 2020; 10(5):298. https://doi.org/10.3390/brainsci10050298
Chicago/Turabian StyleOlaciregui Dague, Karmele, Manuel Dafotakis, Jörg B. Schulz, and Rainer Surges. 2020. "Gaze Palsy as a Manifestation of Todd’s Phenomenon: Case Report and Review of the Literature" Brain Sciences 10, no. 5: 298. https://doi.org/10.3390/brainsci10050298
APA StyleOlaciregui Dague, K., Dafotakis, M., Schulz, J. B., & Surges, R. (2020). Gaze Palsy as a Manifestation of Todd’s Phenomenon: Case Report and Review of the Literature. Brain Sciences, 10(5), 298. https://doi.org/10.3390/brainsci10050298





