Differential Changes in Early Somatosensory Evoked Potentials between the Dominant and Non-Dominant Hand, Following a Novel Motor Tracing Task
Abstract
1. Introduction
2. Methods
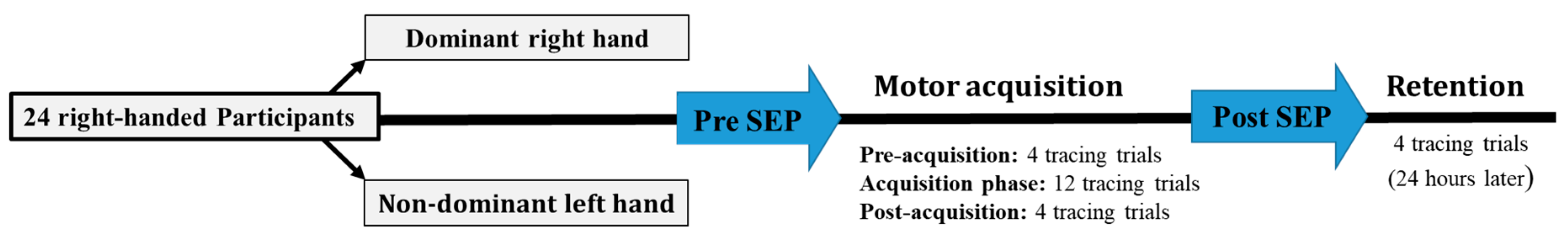
2.1. Participants
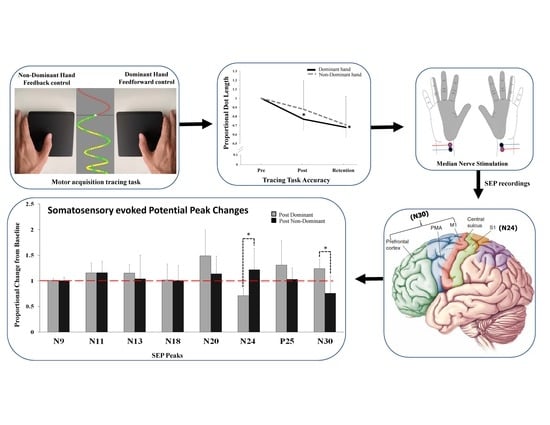
2.2. Experimental Protocol
2.3. Stimulation Parameters
2.4. Motor Acquisition Tracing Task Parameters
3. Data Analysis
4. Statistical Analysis
4.1. Neurophysiological Data
4.2. Behavioral Data
5. Results
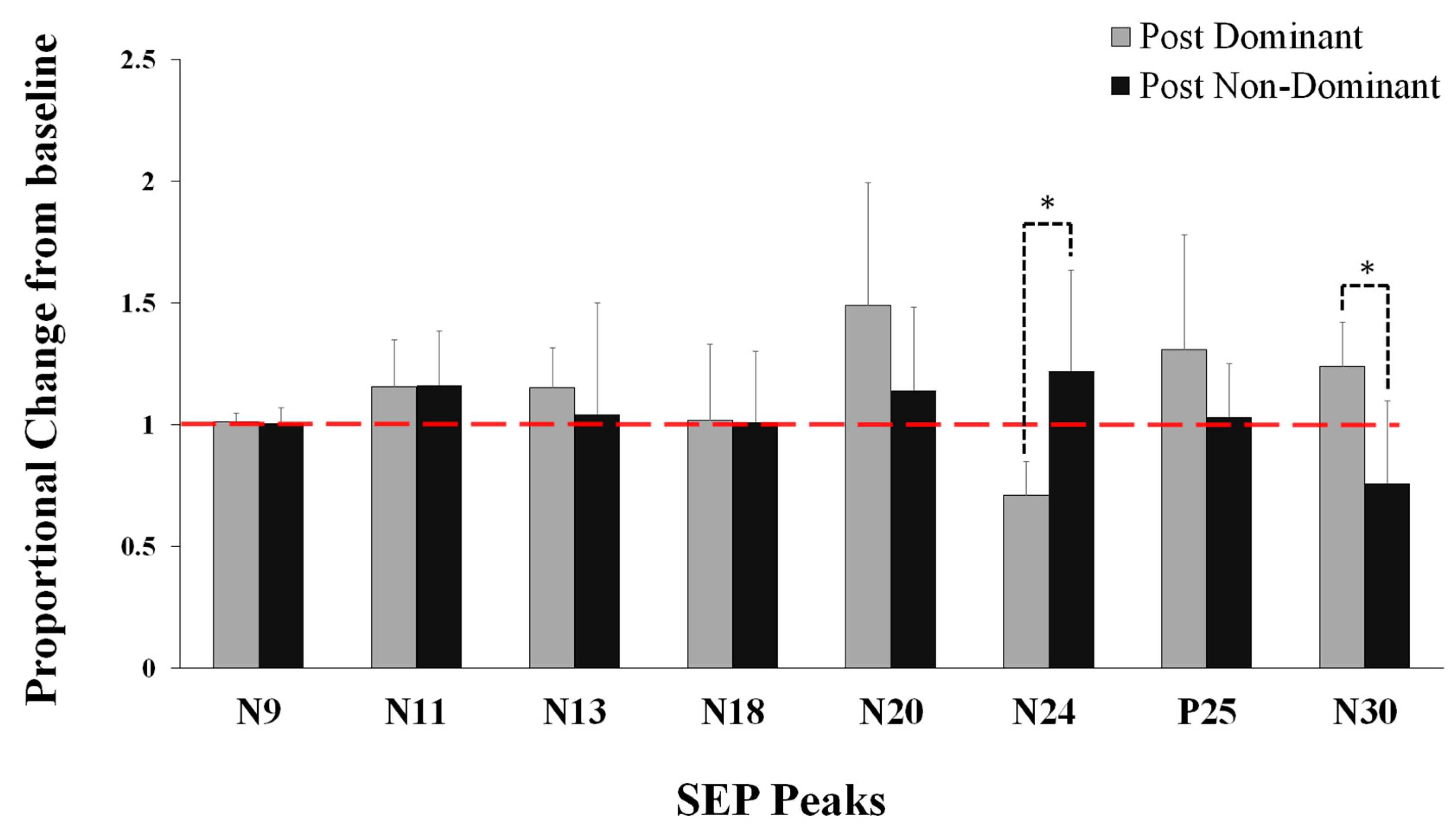
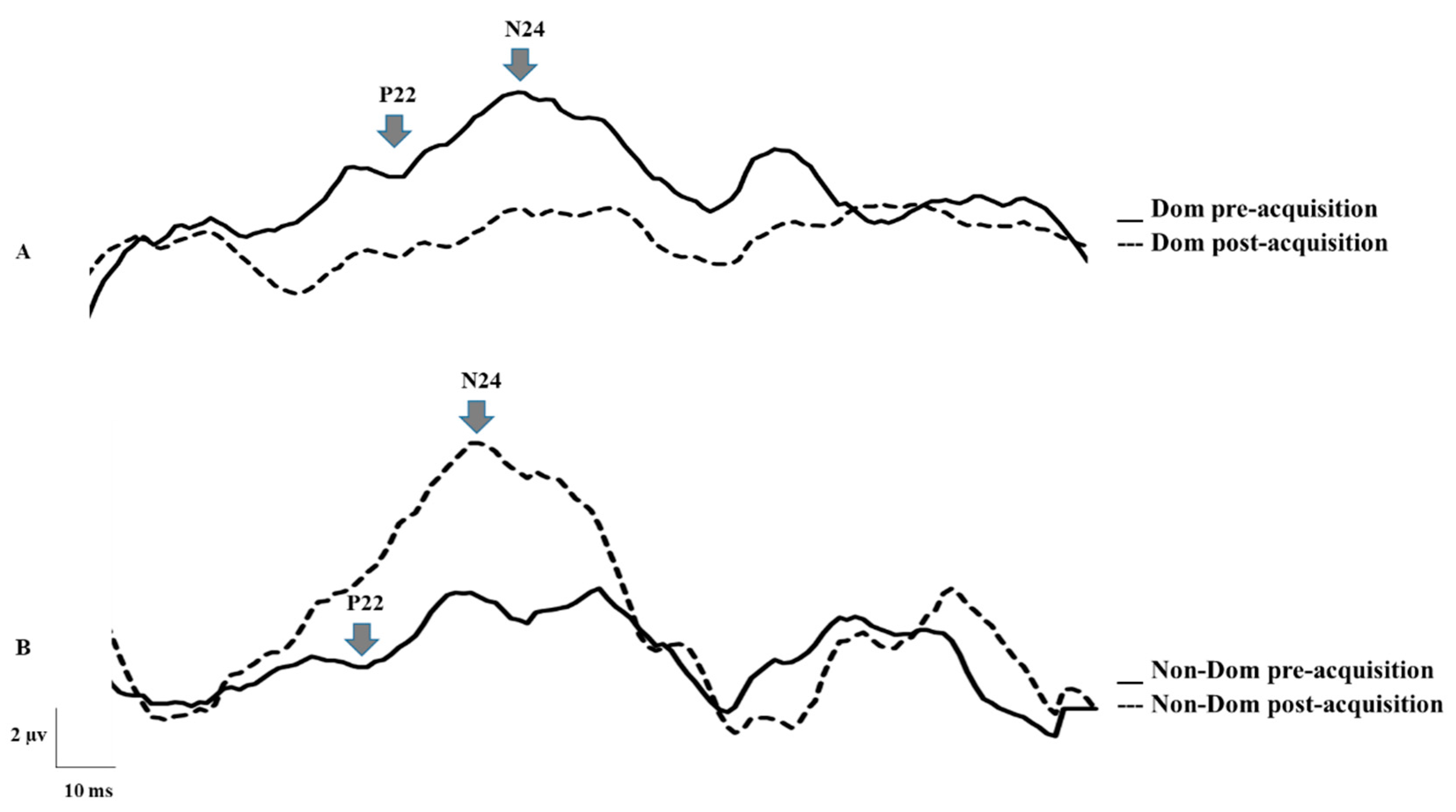
5.1. Neurophysiological Data
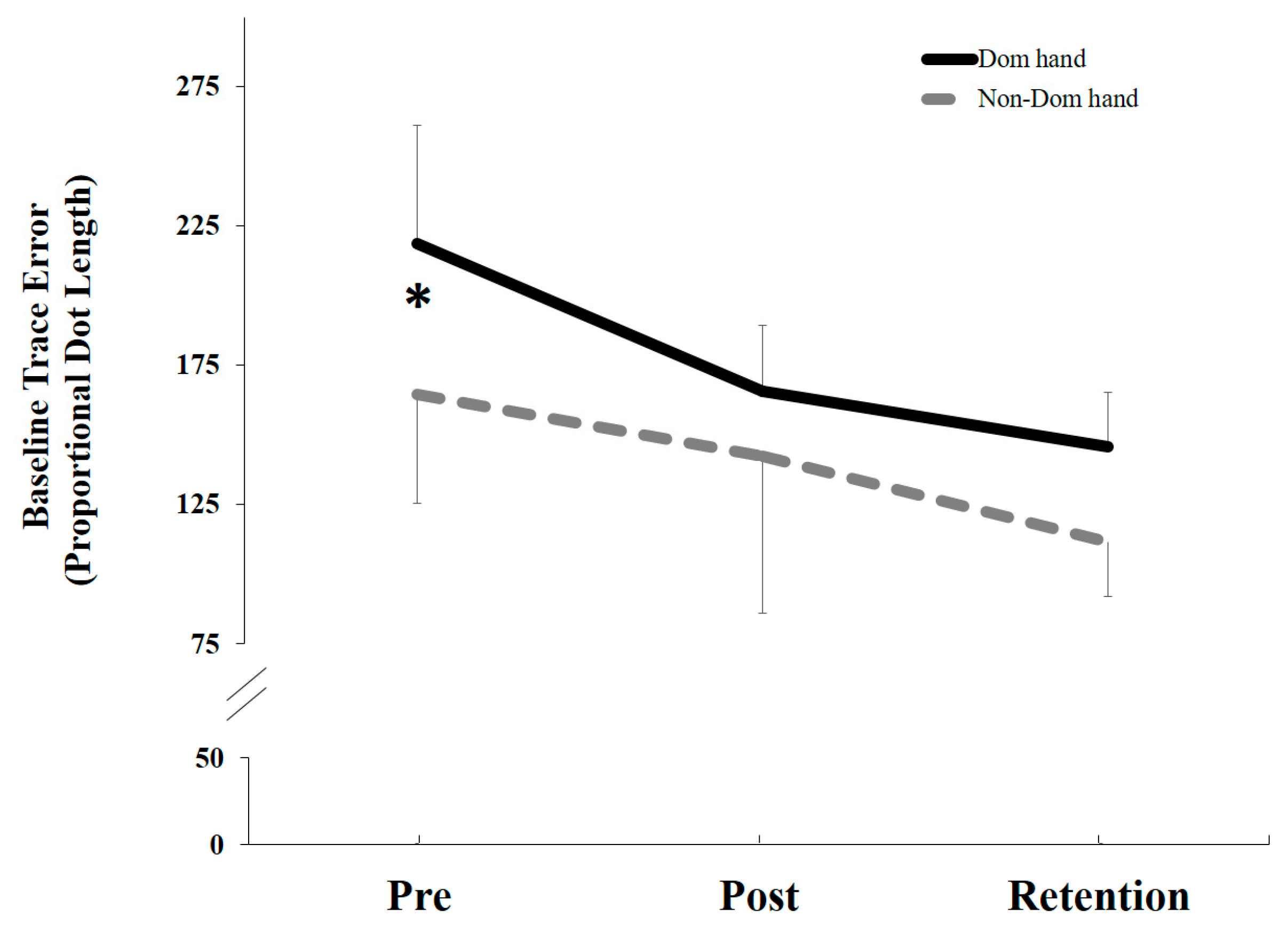
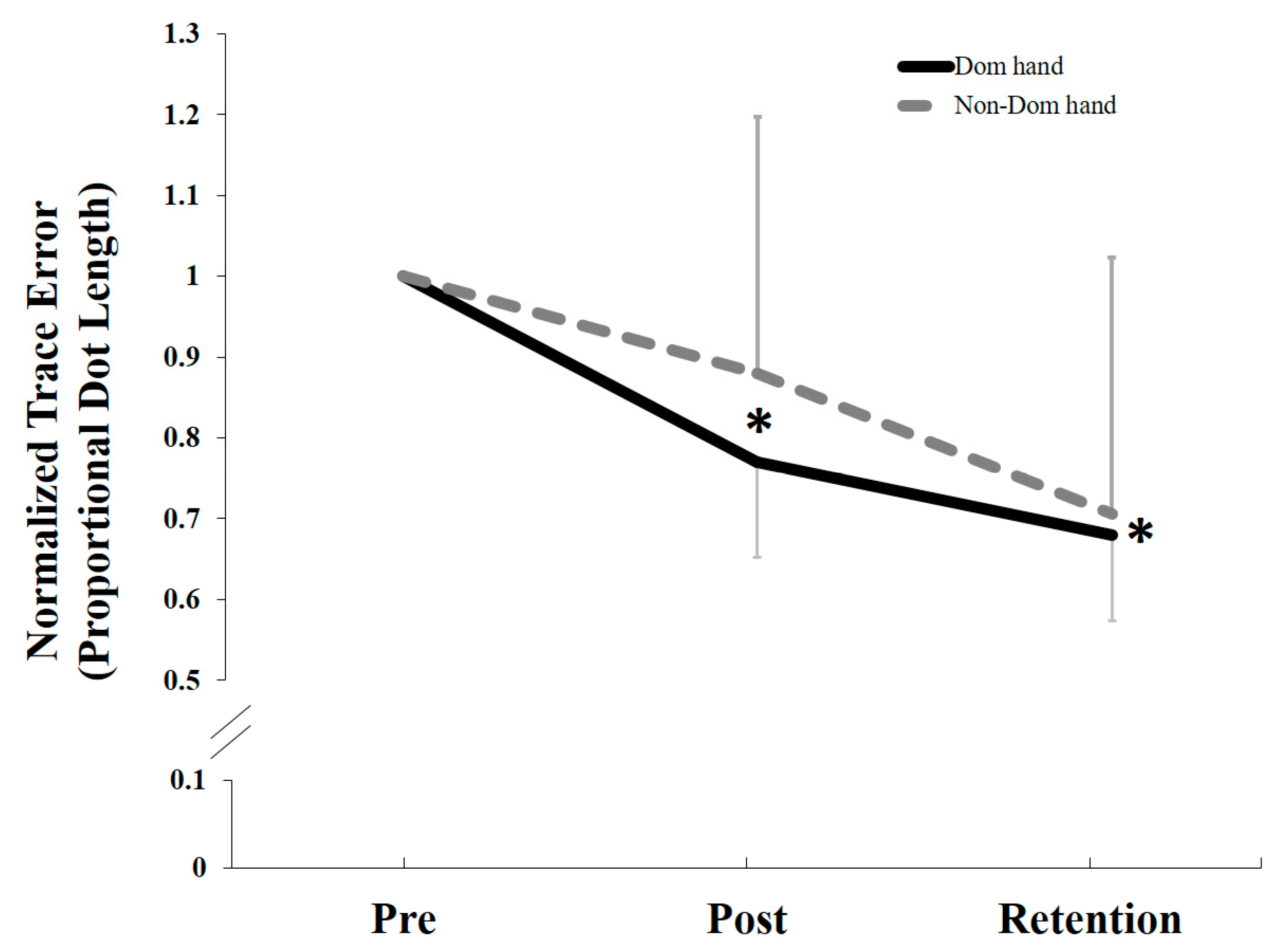
5.2. Behavioral Data
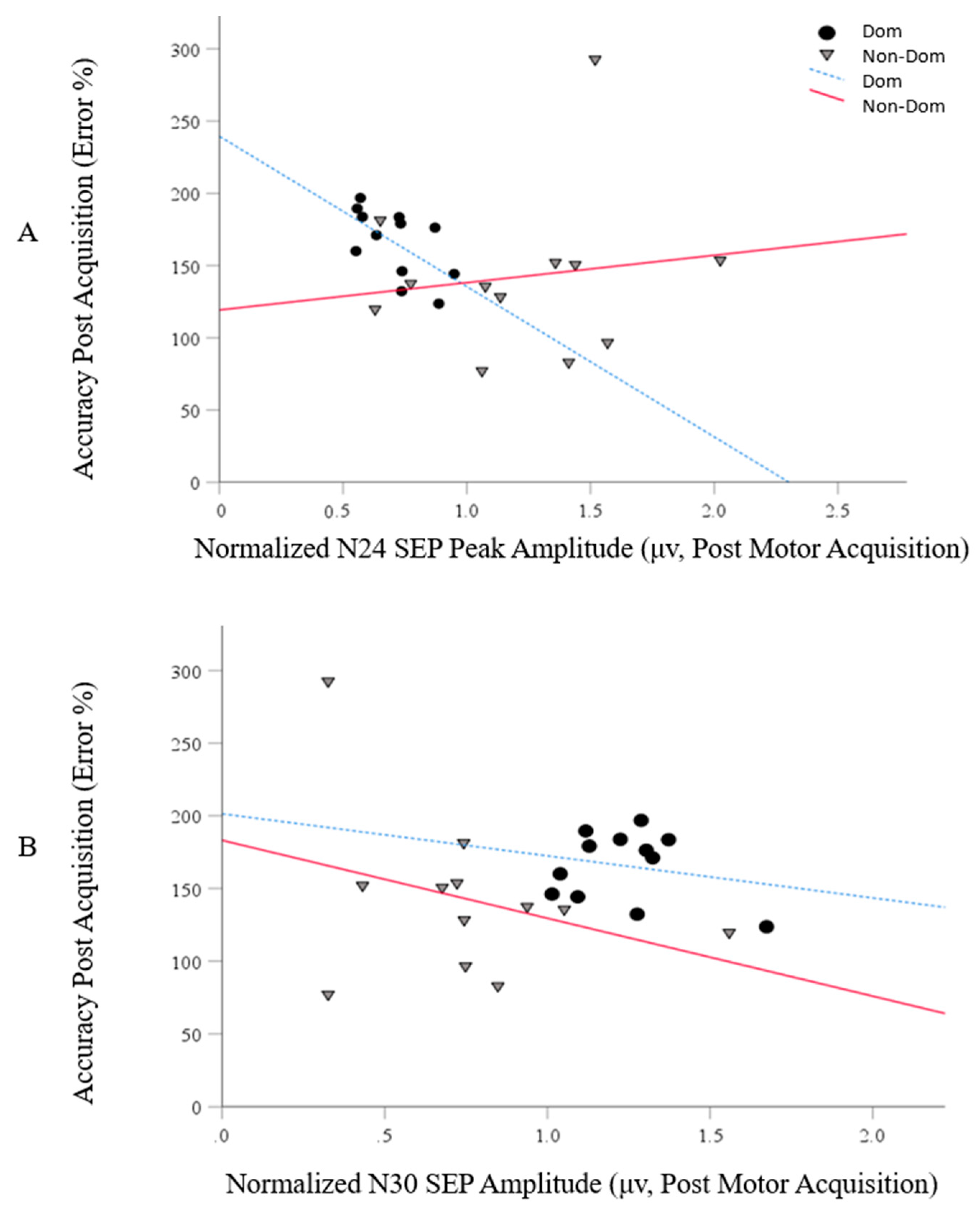
5.3. Correlations between the Motor Skill Acquisition and SEP Peak (N24 and N30) Data
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mutha, P.K.; Haaland, K.Y.; Sainburg, R.L. Rethinking Motor Lateralization: Specialized but Complementary Mechanisms for Motor Control of Each Arm. PLoS ONE 2013, 8, e58582. [Google Scholar] [CrossRef] [PubMed]
- Schabowsky, C.N.; Hidler, J.M.; Lum, P.S. Greater reliance on impedance control in the nondominant arm compared with the dominant arm when adapting to a novel dynamic environment. Exp. Brain Res. 2007, 182, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Sainburg, R. Motor lateralization is characterized by a serial hybrid control scheme. Neurosci. 2011, 196, 153–167. [Google Scholar] [CrossRef]
- Goble, D.J.; Lewis, C.A.; Brown, S.H. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp. Brain Res. 2005, 168, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Mutha, P.K.; Haaland, K.Y.; Sainburg, R.L. The effects of brain lateralization on motor control and adaptation. J. Mot. Behav. 2012, 44, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Sainburg, R.L. Limb Dominance Results from Asymmetries in Predictive and Impedance Control Mechanisms. PLoS ONE 2014, 9, e93892. [Google Scholar] [CrossRef]
- Mehta, B.; Schaal, S. Forward models in visuomotor control. J. Neurophysiol. 2002, 88, 942–953. [Google Scholar] [CrossRef]
- Shadmehr, R.; Smith, M.A.; Krakauer, J.W. Error Correction, Sensory Prediction, and Adaptation in Motor Control. Annu. Rev. Neurosci. 2010, 33, 89–108. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z.; Jordan, M. An internal model for sensorimotor integration. Science 1995, 269, 1880–1882. [Google Scholar] [CrossRef]
- Milner, T.; Franklin, D.W. Impedance control and internal model use during the initial stage of adaptation to novel dynamics in humans. J. Physiol. 2005, 567, 651–664. [Google Scholar] [CrossRef]
- Sainburg, R.L.; Kalakanis, D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J. Neurophysiol. 2000, 83, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Daligadu, J.; Haavik, H.; Yielder, P.C.; Baarbé, J.; Murphy, B.A. Alterations in Cortical and Cerebellar Motor Processing in Subclinical Neck Pain Patients Following Spinal Manipulation. J. Manip. Physiol. Ther. 2013, 36, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.; Murphy, B.A.; Passmore, S.; Yielder, P. Differences in Corticomotor Excitability Between Hemispheres Following Performance of a Novel Motor Training Task. Neurosci. Biomed. Eng. 2018, 5, 116–125. [Google Scholar] [CrossRef]
- Tousi, M.M.; Emami, T.; Hoseini, S.M. The Effect of Initial Practice with Dominant and Non-Dominant Hand on Acquisition, Retention and Transfer of a Complex Motor Task. Biosci. Biotechnol. Res. Asia 2017, 14, 1067. [Google Scholar] [CrossRef]
- Pereira, E.A.H.; Raja, K.; Gangavelli, R. Effect of Training on Interlimb Transfer of Dexterity Skills in Healthy Adults. Am. J. Phys. Med. Rehabil. 2011, 90, 25–34. [Google Scholar] [CrossRef]
- Miall, R.C.; Reckess, G.Z.; Imamizu, H. The cerebellum coordinates eye and hand tracking movements. Nat. Neurosci. 2001, 4, 638–644. [Google Scholar] [CrossRef]
- Panouillères, M.; Miall, R.C.; Jenkinson, N. The role of the posterior cerebellum in saccadic adaptation: A transcranial direct current stimulation study. J. Neurosci. 2015, 35, 5471–5479. [Google Scholar] [CrossRef]
- Nixon, P.; Passingham, R. Predicting sensory events. Exp. Brain Res. 2001, 138, 251–257. [Google Scholar] [CrossRef]
- Dancey, E.; Murphy, B.A.; Andrew, D.; Yielder, P. Interactive effect of acute pain and motor learning acquisition on sensorimotor integration and motor learning outcomes. J. Neurophysiol. 2016, 116, 2210–2220. [Google Scholar] [CrossRef]
- Taylor, H.H.; Murphy, B.A. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef]
- Andrew, D.; Yielder, P.; Murphy, B.A. Do pursuit movement tasks lead to differential changes in early somatosensory evoked potentials related to motor learning compared with typing tasks? J. Neurophysiol. 2015, 113, 1156–1164. [Google Scholar] [CrossRef]
- Dancey, E.; Murphy, B.A.; Srbely, J.; Yielder, P.C. The effect of experimental pain on motor training performance and sensorimotor integration. Exp. Brain Res. 2014, 232, 2879–2889. [Google Scholar] [CrossRef] [PubMed]
- Andrew, D.; Haavik, H.; Dancey, E.; Yielder, P.; Murphy, B.A. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin. Neurophysiol. 2015, 126, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Hoshiyama, M.; Kakigi, R. Changes of somatosensory evoked potentials during writing with the dominant and non-dominant hands. Brain Res. 1999, 833, 10–19. [Google Scholar] [CrossRef]
- Passmore, S.R.; Murphy, B.; Lee, T.D. The origin, and application of somatosensory evoked potentials as a neurophysiological technique to investigate neuroplasticity. J. Can. Chiropr. Assoc. 2014, 58, 170–183. [Google Scholar]
- Mehrkanoon, S.; Boonstra, T.W.; Breakspear, M.; Hinder, M.; Summers, J.J. Upregulation of cortico-cerebellar functional connectivity after motor learning. NeuroImage 2016, 128, 252–263. [Google Scholar] [CrossRef]
- Taylor, H.H.; Murphy, B.A. Altered cortical integration of dual somatosensory input following the cessation of a 20 min period of repetitive muscle activity. Exp. Brain Res. 2006, 178, 488–498. [Google Scholar] [CrossRef]
- A Murphy, B.; Taylor, H.H.; Wilson, S.; Oliphant, G.; Mathers, K. Rapid reversible changes to multiple levels of the human somatosensory system following the cessation of repetitive contractions: A somatosensory evoked potential study. Clin. Neurophysiol. 2003, 114, 1531–1537. [Google Scholar] [CrossRef]
- Bosse, J. Motor Training and Cervical Spine Manipulation: Effects On Sensorimotor Integration; UOIT: Oshawa, ON, Canada, 2012. [Google Scholar]
- Haavik, H.; Murphy, B.A. Selective changes in cerebellar-cortical processing following motor training. Exp. Brain Res. 2013, 231, 397–403. [Google Scholar] [CrossRef]
- Restuccia, D.; Della Marca, G.; Valeriani, M.; Leggio, M.; Molinari, M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch-negativity study. Brain 2006, 130, 276–287. [Google Scholar] [CrossRef]
- Rossi, S.; Della Volpe, R.; Ginanneschi, F.; Ulivelli, M.; Bartalini, S.; Spidalieri, R.; Rossi, A. Early somatosensory processing during tonic muscle pain in humans: Relation to loss of proprioception and motor ‘defensive’ strategies. Clin. Neurophysiol. 2003, 114, 1351–1358. [Google Scholar] [CrossRef]
- Waberski, T.D.; Buchner, H.; Perkuhn, M.; Gobbelé, R.; Wagner, M.; Kücker, W.; Silny, J. N30 and the effect of explorative finger movements: A model of the contribution of the motor cortex to early somatosensory potentials. Clin. Neurophysiol. 1999, 110, 1589–1600. [Google Scholar] [CrossRef]
- Miall, R.C.; Weir, D.J.; Wolpert, D.M.; Stein, J.F. Is the Cerebellum a Smith Predictor? J. Mot. Behav. 1993, 25, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, D. The cerebellum and sensorimotor coupling: Looking at the problem from the perspective of vestibular reflexes. Cerebellum 2007, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E. GPOWER: A Priori-, Post Hoc-, and Compromise Power Analyses for MS-DOS; Bonn University: Bonn, Germany, 1992. [Google Scholar]
- Cohen, L.A. ROLE OF EYE AND NECK PROPRIOCEPTIVE MECHANISMS IN BODY ORIENTATION AND MOTOR COORDINATION. J. Neurophysiol. 1961, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karni, A.; Meyer, G.; Rey-Hipolito, C.; Jezzard, P.; Adams, M.M.; Turner, R.; Ungerleider, L.G. The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proc. Natl. Acad. Sci. USA 1998, 95, 861–868. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Aminoff, M.; Desmedt, J.; Eisen, A.A.; Goodin, U.; Matsuoka, S.; Mauguière, F.; Shibasaki, H.; Sutherling, W.; Vibert, J.-F. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 6–11. [Google Scholar] [CrossRef]
- Holland, L.; Murphy, B.A.; Passmore, S.; Yielder, P.C. Time course of corticospinal excitability changes following a novel motor training task. Neurosci. Lett. 2015, 591, 81–85. [Google Scholar] [CrossRef]
- Desmurget, M.; Grafton, S.T. Forward modeling allows feedback control for fast reaching movements. Trends Cogn. Sci. 2000, 4, 423–431. [Google Scholar] [CrossRef]
- Cebolla, A.; Palmero-Soler, E.; Dan, B.; Cheron, G. Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential. NeuroImage 2011, 54, 1297–1306. [Google Scholar] [CrossRef]
- Brown, M.J.; Staines, W. Somatosensory input to non-primary motor areas is enhanced during preparation of cued contraterlateral finger sequence movements. Behav. Brain Res. 2015, 286, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Baarbé, J.; Yielder, P.; Daligadu, J.; Behbahani, H.; Haavik, H.; Murphy, B. A novel protocol to investigate motor training-induced plasticity and sensorimotor integration in the cerebellum and motor cortex. J. Neurophysiol. 2014, 111, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Doyon, J.; Benali, H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005, 15, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Doyon, J.; Penhune, V.; Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003, 41, 252–262. [Google Scholar] [CrossRef]
- Sainburg, R. Evidence for a dynamic-dominance hypothesis of handedness. Exp. Brain Res. 2002, 142, 241–258. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Maeda, F.; Zaidel, E.; Mazziotta, J.; Iacoboni, M. Lateralization in motor facilitation during action observation: A TMS study. Exp. Brain Res. 2002, 144, 127–131. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Iacoboni, M.; Zaidel, E.; Wilson, S.; Mazziotta, J. Left hemisphere motor facilitation in response to manual action sounds. Eur. J. Neurosci. 2004, 19, 2609–2612. [Google Scholar] [CrossRef]
- Guadagnoli, M.A.; Lee, T.D. Challenge Point: A Framework for Conceptualizing the Effects of Various Practice Conditions in Motor Learning. J. Mot. Behav. 2004, 36, 212–224. [Google Scholar] [CrossRef]
- Wulf, G. Attentional focus and motor learning: A review of 10 years of research. E-J. Beweg. Train. 2007, 4, 1–4. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabihhosseinian, M.; Gilley, R.; Andrew, D.; Murphy, B.; Yielder, P. Differential Changes in Early Somatosensory Evoked Potentials between the Dominant and Non-Dominant Hand, Following a Novel Motor Tracing Task. Brain Sci. 2020, 10, 290. https://doi.org/10.3390/brainsci10050290
Zabihhosseinian M, Gilley R, Andrew D, Murphy B, Yielder P. Differential Changes in Early Somatosensory Evoked Potentials between the Dominant and Non-Dominant Hand, Following a Novel Motor Tracing Task. Brain Sciences. 2020; 10(5):290. https://doi.org/10.3390/brainsci10050290
Chicago/Turabian StyleZabihhosseinian, Mahboobeh, Ryan Gilley, Danielle Andrew, Bernadette Murphy, and Paul Yielder. 2020. "Differential Changes in Early Somatosensory Evoked Potentials between the Dominant and Non-Dominant Hand, Following a Novel Motor Tracing Task" Brain Sciences 10, no. 5: 290. https://doi.org/10.3390/brainsci10050290
APA StyleZabihhosseinian, M., Gilley, R., Andrew, D., Murphy, B., & Yielder, P. (2020). Differential Changes in Early Somatosensory Evoked Potentials between the Dominant and Non-Dominant Hand, Following a Novel Motor Tracing Task. Brain Sciences, 10(5), 290. https://doi.org/10.3390/brainsci10050290