Effects of Transcranial Direct Current Stimulation on Hand Dexterity in Multiple Sclerosis: A Design for a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
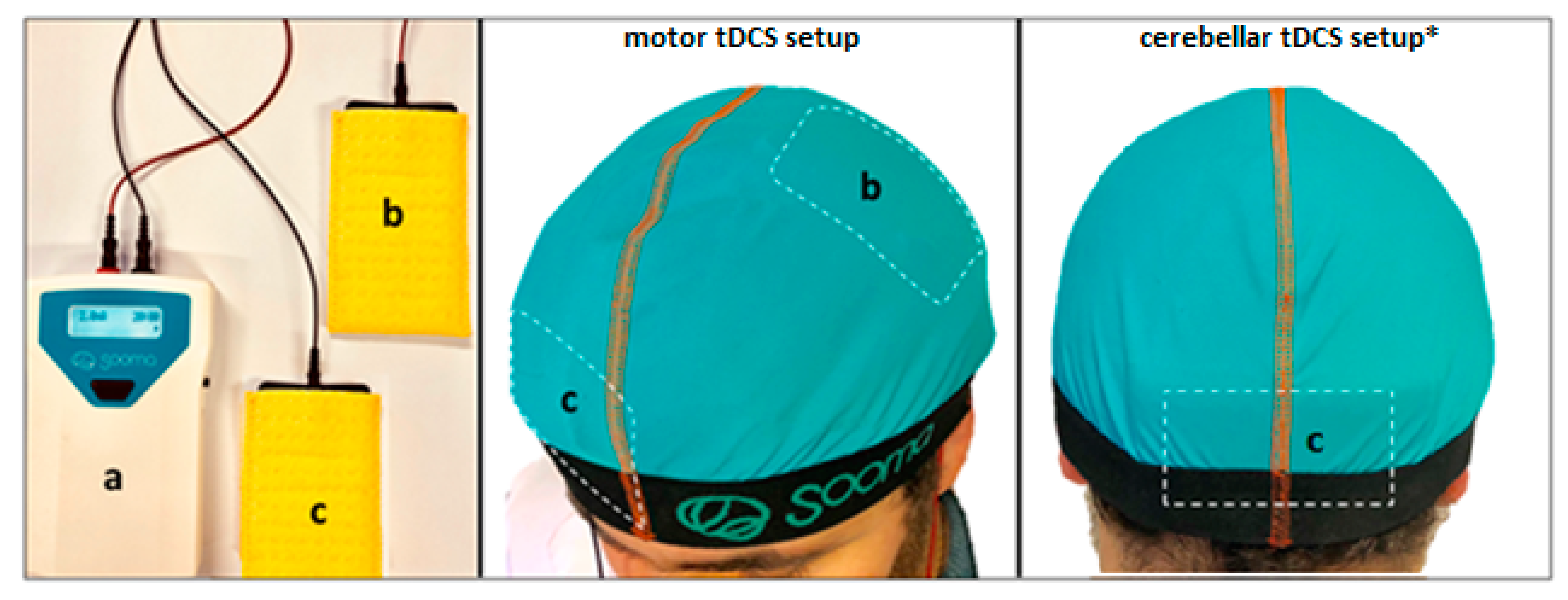
2.2. tDCS
2.3. Sample Size, Randomization and Blinding Effectiveness
2.4. Description of the Clinical Evaluation
2.4.1. Primary outcome: hand dexterity
2.4.2. Secondary outcomes
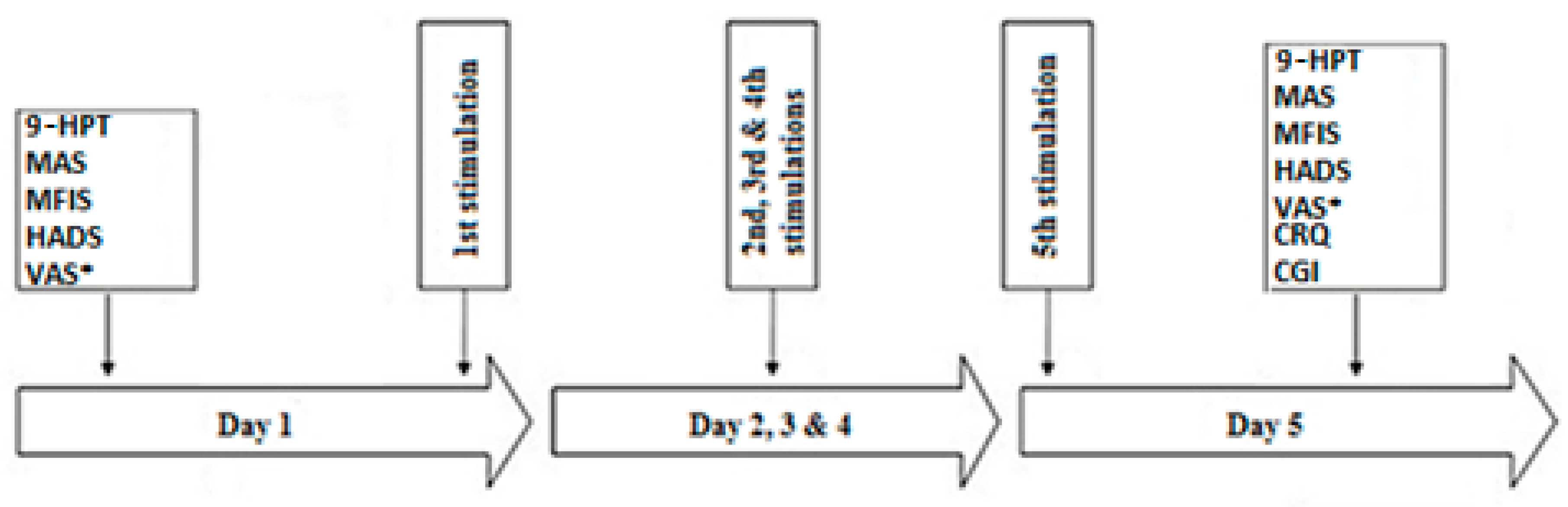
2.5. Experimental Protocol
2.6. Patient Withdrawal, Risks and Benefits
2.7. Data Management
2.8. Safety and Adverse Event Reporting
2.9. Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef]
- Feys, P.; Duportail, M.; Kos, D.; Van Asch, P.; Ketelaer, P. Validity of the TEMPA for the measurement of upper limb function in multiple sclerosis. Clin. Rehabil. 2002, 16, 166–173. [Google Scholar] [CrossRef]
- Marwaha, R.; Hall, S.J.; Knight, C.A.; Jaric, S. Load and grip force coordination in static bimanual manipulation tasks in multiple sclerosis. Motor Control 2006, 10, 160–177. [Google Scholar] [CrossRef]
- Krishnan, V.; de Freitas, P.B.; Jaric, S. Impaired object manipulation in mildly involved individuals with multiple sclerosis. Motor Control 2008, 12, 3–20. [Google Scholar] [CrossRef][Green Version]
- McPhee, S.D. Extension block splinting for the proximal interphalangeal joint. Am. J. Occup. Ther. 1987, 41, 389–390. [Google Scholar] [CrossRef][Green Version]
- Carroll, J.B. Human Cognitive Abilities: A Survey of Factor Analytic Studies; Cambridge University Press: NewYork, NY, USA, 1993. [Google Scholar]
- Birznieks, I.; Jenmalm, P.; Goodwin, A.W.; Johansson, R.S. Encoding of direction of fingertip forces by human tactile afferents. J. Neurosci. 2001, 21, 8222–8237. [Google Scholar] [CrossRef]
- Johansson, R.S.; Flanagan, J.R. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Rev. Neurosci. 2009, 10, 345–359. [Google Scholar] [CrossRef]
- Valero-Cuevas, F.J.; Smaby, N.; Venkadesan, M.; Peterson, M.; Wright, T. The strength-dexterity test as a measure of dynamic pinch performance. J. Biomech. 2003, 36, 265–270. [Google Scholar] [CrossRef]
- Binkofski, F.; Buccino, G.; Posse, S.; Seitz, R.J.; Rizzolatti, G.; Freund, H. A fronto-parietal circuit for object manipulation in man: Evidence from an fMRI-study. Eur. J. Neurosci. 1999, 11, 3276–3286. [Google Scholar] [CrossRef]
- Binkofski, F.; Buccino, G.; Stephan, K.M.; Rizzolatti, G.; Seitz, R.J.; Freund, H.J. A parieto-premotor network for object manipulation: Evidence from neuroimaging. Exp. Brain Res. 1999, 128, 210–213. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Fagergren, A.; Jonsson, T.; Westling, G.; Johansson, R.S.; Forssberg, H. Cortical activity in precision—Versus power-grip tasks: An fMRI study. J. Neurophysiol. 2000, 83, 528–536. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Fagergren, E.; Forssberg, H. Differential fronto-parietal activation depending on force used in a precision grip task: An fMRI study. J. Neurophysiol. 2001, 85, 2613–2623. [Google Scholar] [CrossRef]
- Holmstrom, L.; de Manzano, O.; Vollmer, B.; Forsman, L.; Valero-Cuevas, F.J.; Ulle’n, F.; Forssberg, H. Dissociation of brain areas associated with force production and stabilization during manipulation of unstable objects. Exp. Brain Res. 2011, 215, 359–367. [Google Scholar] [CrossRef]
- Kawashima, R.; Matsumura, M.; Sadato, N.; Naito, E.; Waki, A.; Nakamura, S.; Matsunami, K.; Fukuda, H.; Yonekura, Y. Regional cerebral blood flow changes in human brain related to ipsilateral and contralateral complex hand movements—A PET study. Eur. J. Neurosci. 1998, 10, 2254. [Google Scholar] [CrossRef]
- Kuhtz-Buschbeck, J.P.; Ehrsson, H.H.; Forssberg, H. Human brain activity in the control of fine static precision grip forces: An fMRI study. Eur. J. Neurosci. 2001, 14, 382–390. [Google Scholar] [CrossRef]
- Kuhtz-Buschbeck, J.P.; Gilster, R.; Wolff, S.; Ulmer, S.; Siebner, H.; Jansen, O. Brain activity is similar during precision and power gripping with light force: An fMRI study. NeuroImage 2008, 4, 1469–1481. [Google Scholar] [CrossRef]
- Mosier, K.; Lau, C.; Wang, Y.; Venkadesan, M.; Valero-Cuevas, F.J. Controlling instabilities in manipulation requires specific cortical-striatal-cerebellar networks. J. Neurophysiol. 2011, 105, 1295–1305. [Google Scholar] [CrossRef]
- Proville, R.D.; Spolidoro, M.; Guyon, N.; Dugué, G.P.; Selimi, F.; Isope, P.; Popa, D.; Lna, C. Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat. Neurosci. 2014, 17, 1233–1239. [Google Scholar] [CrossRef]
- Eccles, J.C.; Ito, M.; Szentagothai, J. The Cerebellum as a Neuronal Machine; Springer: Berlin/Heidelberg, Germany, 1967. [Google Scholar]
- Schwarz, C.; Their, P. Binding of signals relevant for action: Towards a hypothesis of the functional role of the pontine nuclei. Trends Neurosci. 1999, 22, 443–451. [Google Scholar] [CrossRef]
- Liepert, J.; Kucinski, T.; Tüscher, O.; Pawlas, F.; Bäumer, T.; Weiller, C. Motor cortex excitability after cerebellar infarction. Stroke 2004, 35, 2484–2488. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Restuccia, D.; Molinari, M.; Leggio, M.G.; Nardone, R.; Fogli, D.; Tonali, P. Excitability of the motor cortex to magnetic stimulation in patients with cerebellar lesions. J. Neurol. Neurosurg. Psychiatry 1994, 57, 108–110. [Google Scholar] [CrossRef]
- Johansson, S.; Ytterberg, C.; Claesson, I.M.; Lindberg, J.; Hillert, J.; Andersson, M.; Holmqvist, L.W.; von Koch, L. High concurrent presence of disability in multiple sclerosis. Associations with perceived health. J. Neurol. 2007, 254, 767–773. [Google Scholar] [CrossRef]
- Poole, J.L.; Nakamoto, T.; McNulty, T.; Montoya, J.R.; Weill, D.; Dieruf, K.; Skipper, B. Dexterity, visual perception, and activities of daily living in persons with multiple sclerosis. Occup. Ther. Health Care 2010, 24, 159–170. [Google Scholar] [CrossRef]
- Valè, N.; Gandolfi, M.; Mazzoleni, S.; Battini, E.; Dimitrova, E.K.; Gajofatto, A.; Ferraro, F.; Castelli, M.; Camin, M.; Filippetti, M. Characterization of Upper Limb Impairments at Body Function, Activity, and Participation in Persons With Multiple Sclerosis by Behavioral and EMG Assessment: A Cross-Sectional Study. Front. Neurol. 2020, 10, 1395. [Google Scholar] [CrossRef]
- Palm, U.; Feichtner, K.B.; Hasan, A.; Gauglitz, G.; Langguth, B.; Nitsche, M.A.; Keeser, D.; Padberg, F. The Role of Contact Media at the Skin-electrode Interface during Transcranial Direct Current Stimulation (tDCS). Brain Stimul. 2014, 7, 757–769. [Google Scholar] [CrossRef]
- Priori, A.; Berardelli, A.; Rona, S.; Accornero, N.; Manfredi, M. Polarization of the human motor cortex through the scalp. Neuroreport 1998, 9, 2257–2260. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Nitsche, M.S.; Klein, C.C.; Tergau, F.; Rothwell, J.C.; Paulus, W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin. Neurophysiol. 2003, 114, 600–604. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A. Clinical research with transcranial direct current stimulation(tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef]
- Creutzfeldt, O.D.; Fromm, G.H.; Kapp, H. Influence of transcortical D-C currents on cortical neuronal activity. Exp. Neurol. 1962, 5, 436–452. [Google Scholar] [CrossRef]
- Purpura, D.P.; McMurtry, J.G. Intracellular activities and evoked potential changes during polarization of motor cortex. J. Neurophysiol. 1965, 28, 166–185. [Google Scholar] [CrossRef]
- Iodice, R.; Manganelli, F.; Dubbioso, R. The therapeutic use of non-invasive brain stimulation in multiple sclerosis—A review. Restor. Neurol. Neurosci. 2017, 35, 497–509. [Google Scholar] [CrossRef]
- Hui, D.; Zhukovsky, D.S.; Bruera, E. Which treatment is better? Ascertaining patient preferences with crossover randomized controlled trials. J. Pain Symptom Manag. 2015, 49, 625–631. [Google Scholar] [CrossRef]
- Chalah, M.A.; Riachi, N.; Ahdab, R.; Mhalla, A.; Abdellaoui, M.; Créange, A.; Lefaucheur, J.-P.; Ayache, S.S. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J. Neurol. Sci. 2017, 372, 131–137. [Google Scholar] [CrossRef]
- Barreca, S.; Gowland, C.K.; Stratford, P.; Huijbregts, M.; Griffiths, J.; Torresin, W.; Dunkley, M.; Miller, P.; Masters, L. Development of the Chedoke Arm and Hand Activity Inventory: Theoretical constructs, item generation, and selection. Top Stroke Rehabil. 2004, 11, 31–42. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Paternostro-Sluga, T.; Grim-Stieger, M.; Posch, M.; Schuhfried, O.; Vacariu, G.; Mittermaier, C.; Bittner, C.; Fialka-Moser, V. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J. Rehabil. Med. 2008, 40, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.; Smith, M. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206. [Google Scholar] [CrossRef]
- Alusi, S.H.; Worthington, J.; Glickman, S.; Bain, P.G. A study of tremor in multiple sclerosis. Brain 2001, 124, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.J. Identifying patients with diabetes mellitus who are at risk for lower-extremity complications: Use of Semmes-Weinstein monofilaments. Phys. Ther. 1996, 76, 68–71. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Vanbellingen, T.; Kersten, B.; Van de Winckel, A.; Bellion, M.; Baronti, F.; Müri, R.; Bohlhalter, S. A new bedside test of gestures in stroke: The apraxia screen of TULIA (AST). J. Neurol. Neurosurg. Psychiatry 2011, 82, 389–392. [Google Scholar] [CrossRef]
- Beck, A.T.; Brown, G.; Steer, R.A. Beck Depression Inventory II Manual; The Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Ayache, S.S.; Chalah, M.A. The place of transcranial direct current stimulation in the management of multiple sclerosis-related symptoms. Neurodegener. Dis. Manag. 2018, 8, 411–422. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blindsham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Ambrus, G.G.; Al-Moyed, H.; Chaieb, L.; Sarp, L.; Antal, A.; Paulus, W. The fade-in—Short stimulation—Fade out approach to sham tDCS—Reliable at 1mA for naïve and experienced subjects, but not investigators. Brain Stimul. 2012, 5, 499–504. [Google Scholar] [CrossRef]
- Ferrucci, R.; Priori, A. Transcranial cerebellar direct current stimulation (tcDCS): Motor control, cognition, learning and emotions. NeuroImage 2013, 85, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Brunoni, A.R.; Parazzini, M.; Vergari, M.; Rossi, E.; Fumagalli, M.; Mameli, F.; Rosa, M.; Giannicola, G.; Zago, S. Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum 2013, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Giannicola, G.; Rosa, M.; Fumagalli, M.; Boggio, P.S.; Hallett, M.; Zago, S.; Priori, A. Cerebellum and processing of negative facial emotions: Cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn. Emot. 2012, 26, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Marceglia, S.; Vergari, M.; Cogiamanian, F.; Mrakic-Sposta, S.; Mameli, F.; Zago, S.; Barbieri, S.; Priori, A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J. Cogn. Neurosci. 2008, 20, 1687–1697. [Google Scholar] [CrossRef]
- Pope, P.A.; Miall, R.C. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul. 2012, 5, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, S.S.; Wang, J.; Chen, Y.; Chua, E. Transcranial Direct Current Stimulation to Primary Motor Area Improves Hand Dexterity and Selective Attention in Chronic Stroke. Am. J. Phys. Med. Rehabil. 2014, 93, 1057–1064. [Google Scholar] [CrossRef]
- Lefebvre, S.; Thonnard, J.L.; Laloux, P.; Peeters, A.; Jamart, J.; Vandermeeren, Y. Single session of dual-tDCS transiently improves precision grip and dexterity of the paretic hand after stroke. Neurorehabil. Neural Repair 2014, 28, 100–110. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A.G. Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Weber, K.; Kashman, N.; Volland, G. Adult norms for the nine-hole peg test of finger dexterity. Occup. Ther. J. Res. 1985, 5, 24–38. [Google Scholar] [CrossRef]
- Feys, P.; Lamers, I.; Francis, G.; Benedict, R.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Multiple Sclerosis Outcome Assessments Consortium. Multiple Sclerosis Outcome Assessments Consortium. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult. Scler. 2017, 23, 711–720. [Google Scholar] [CrossRef]
- Téllez, N.; Río, J.; Tintoré, M.; Nos, C.; Galán, I.; Montalban, X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult. Scler. 2005, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, B.J.; Tumani, H.; Otto, M.; Mursch, K.; Wiltfang, J.; Herrendorf, G.; Bittermann, H.-J.; Felgenhauer, K.; Paulus, W.; Markakis, E. Cisternal S100 protein and neuron-specific enolase are elevated and site-specific markers in intractable temporal lobe epilepsy. Epilepsy Res. 1999, 35, 75–82. [Google Scholar] [CrossRef]
- Mori, F.; Codecà, C.; Kusayanagi, H.; Monteleone, F.; Buttari, F.; Fiore, S.; Bernardi, G.; Koch, G.; Centonze, D. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J. Pain 2010, 11, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.S.; Palm, U.; Chalah, M.A.; Al-Ani, T.; Brignol, A.; Abdellaoui, M.; Dimitri, D.; Sorel, M.; Crange, A.; Lefaucheur, J.-P. Prefrontal tDCS Decreases Pain in Patients with Multiple Sclerosis. Front. Neurosci. 2016, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Nicoletti, C.G.; Kusayanagi, H.; Foti, C.; Restivo, D.A.; Marciani, M.G.; Centonze, D. Transcranial direct current stimulation ameliorates tactile sensory deficit in multiple sclerosis. Brain Stimul. 2013, 6, 654–659. [Google Scholar] [CrossRef]
- Tecchio, F.; Cancelli, A.; Cottone, C.; Zito, G.; Pasqualetti, P.; Ghazaryan, A.; Rossini, P.M.; Filippi, M.M. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J. Neurol. 2014, 261, 1552–1558. [Google Scholar] [CrossRef]
- Ferrucci, R.; Vergari, M.; Cogiamanian, F.; Bocci, T.; Ciocca, M.; Tomasini, E.; De Riz, M.; Scarpini, E.; Priori, A. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation 2014, 34, 121–127. [Google Scholar] [CrossRef]
- Saiote, C.; Goldschmidt, T.; Timäus, C.; Steenwijk, M.D.; Opitz, A.; Antal, A.; Paulus, W.; Nitsche, M.A. Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restor. Neurol. Neurosci. 2014, 32, 423–436. [Google Scholar] [CrossRef]
- Ayache, S.S.; Lefaucheur, J.P.; Chalah, M.A. Long term effects of prefrontal tDCS on multiple sclerosis fatigue: A case study. Brain Stimul. 2017, 10, 1001–1002. [Google Scholar] [CrossRef]
- Chalah, M.A.; Lefaucheur, J.P.; Ayache, S.S. Long-term effects of tDCS on fatigue, mood and cognition in multiple sclerosis. Clin. Neurophysiol. 2017, 128, 2179–2180. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Grigorescu, C.; Padberg, F.; Kümpfel, T.; Palm, U.; Ayache, S.S. Bifrontal transcranial direct current stimulation modulates fatigue in multiple sclerosis: A randomized sham-controlled study. J. Neural. Transm. (Vienna) 2020. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Rossi, S.; Prosperetti, C.; Codecà, C.; Monteleone, F.; Petrosini, L.; Bernardi, G.; Centonze, D. Improvement of hand dexterity following motor cortex rTMS in multiple sclerosis patients with cerebellar impairment. Mult. Scler. 2008, 14, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Elzamarany, E.; Afifi, L.; El-Fayoumy, N.M.; Salah, H.; Nada, M. Motor cortex rTMS improves dexterity 716 in relapsing-remitting and secondary progressive multiple sclerosis. Acta Neurol. Belg. 2016, 116, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Gironell, A.; Kulisevsky, J.; Lorenzo, J.; Barbanoj, M.; Pascual-Sedano, B.; Otermin, P. Transcranial magnetic stimulation of the cerebellum in essential tremor: A controlled study. Arch Neurol. 2002, 59, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Charvet, L.E.; Shaw, M.T.; Haider, L.; Melville, P.; Krupp, L.B. Remotely-delivered cognitive remediation in multiple sclerosis (MS): Protocol and results from a pilot study. Mult. Scler. J. Exp. Transl. Clin. 2015, 1, 2055217315609629. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Argyropoulos, G.P.; Bastian, A.; Cortes, M.; Davis, N.J.; Edwards, D.J.; Ferrucci, R.; Fregni, F.; Galea, J.M.; Hamada, M. Cerebellar Transcranial Direct Current Stimulation (ctDCS): A Novel Approach to Understanding Cerebellar Function in Health and Disease. Neuroscientist 2016, 22, 83–97. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayache, S.S.; Riachi, N.; Ahdab, R.; Chalah, M.A. Effects of Transcranial Direct Current Stimulation on Hand Dexterity in Multiple Sclerosis: A Design for a Randomized Controlled Trial. Brain Sci. 2020, 10, 185. https://doi.org/10.3390/brainsci10030185
Ayache SS, Riachi N, Ahdab R, Chalah MA. Effects of Transcranial Direct Current Stimulation on Hand Dexterity in Multiple Sclerosis: A Design for a Randomized Controlled Trial. Brain Sciences. 2020; 10(3):185. https://doi.org/10.3390/brainsci10030185
Chicago/Turabian StyleAyache, Samar S., Naji Riachi, Rechdi Ahdab, and Moussa A. Chalah. 2020. "Effects of Transcranial Direct Current Stimulation on Hand Dexterity in Multiple Sclerosis: A Design for a Randomized Controlled Trial" Brain Sciences 10, no. 3: 185. https://doi.org/10.3390/brainsci10030185
APA StyleAyache, S. S., Riachi, N., Ahdab, R., & Chalah, M. A. (2020). Effects of Transcranial Direct Current Stimulation on Hand Dexterity in Multiple Sclerosis: A Design for a Randomized Controlled Trial. Brain Sciences, 10(3), 185. https://doi.org/10.3390/brainsci10030185






