Abnormal Emotional Processing and Emotional Experience in Patients with Peripheral Facial Nerve Paralysis: An MEG Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Stimuli and Design
2.3. Data Acquisition and Preprocessing
2.4. Feature Extraction
2.4.1. Features Based on ERFs
2.4.2. Features Based on Power Spectrums
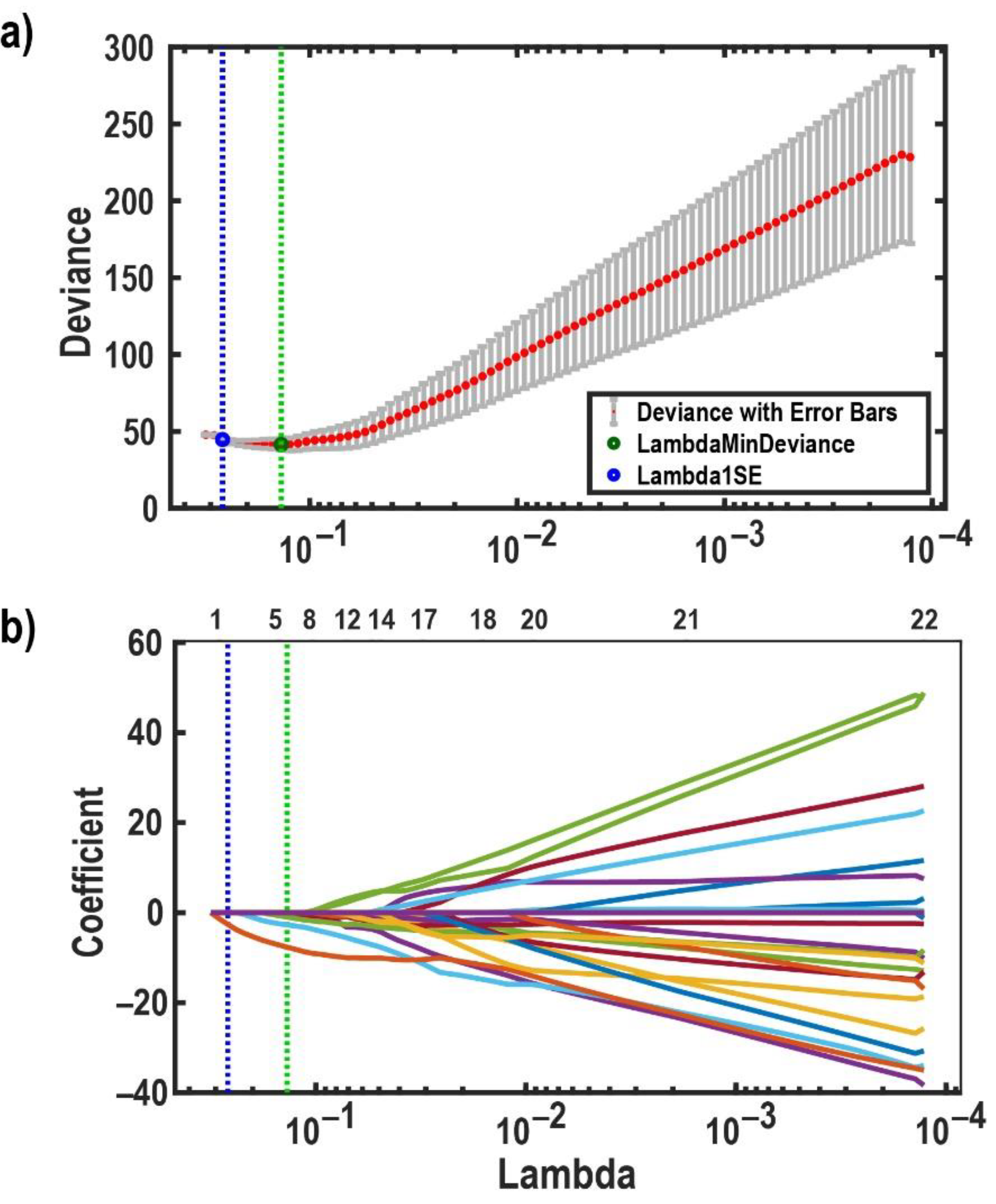
2.5. Feature Subset Selection and Classification
3. Results
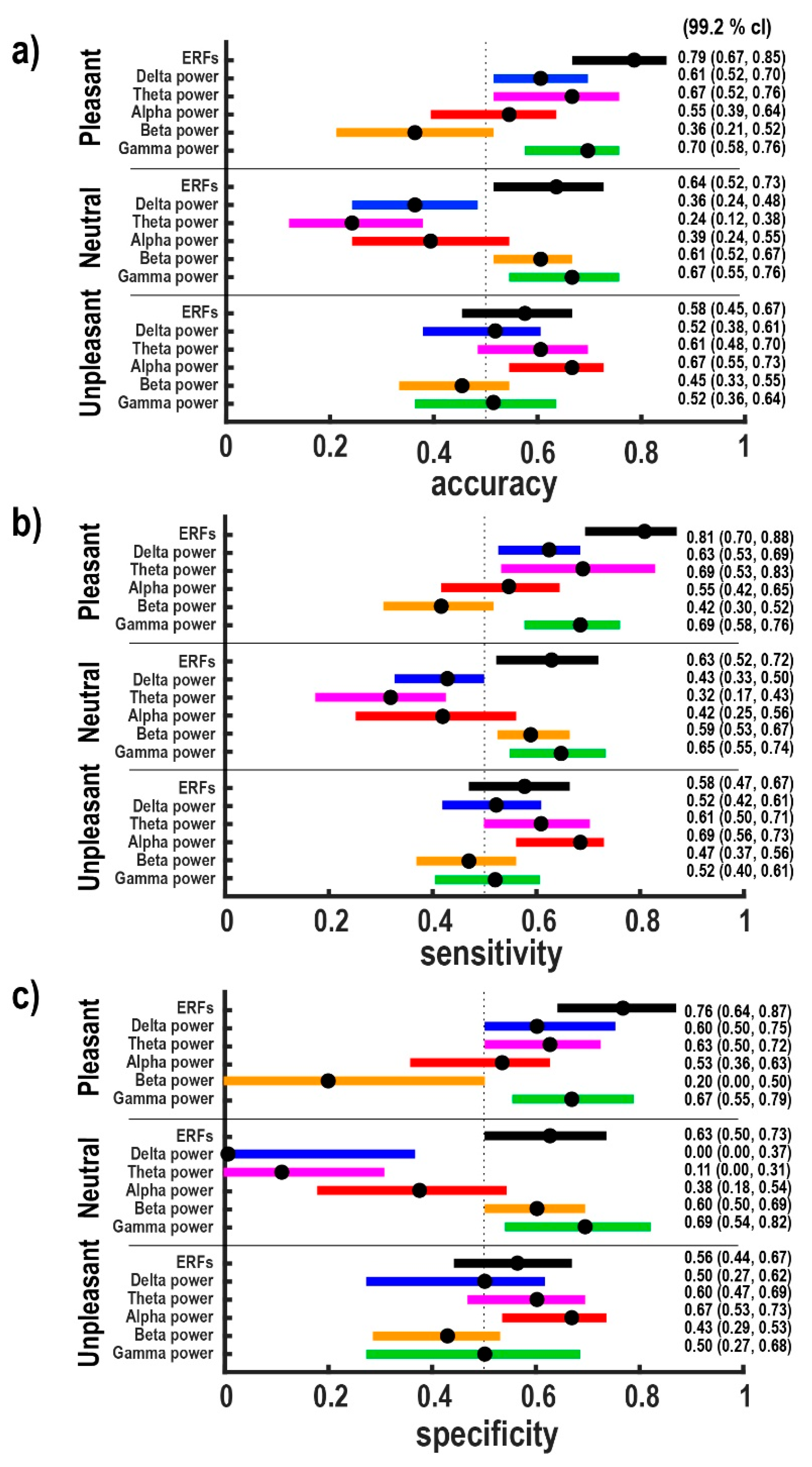
3.1. Classification Results
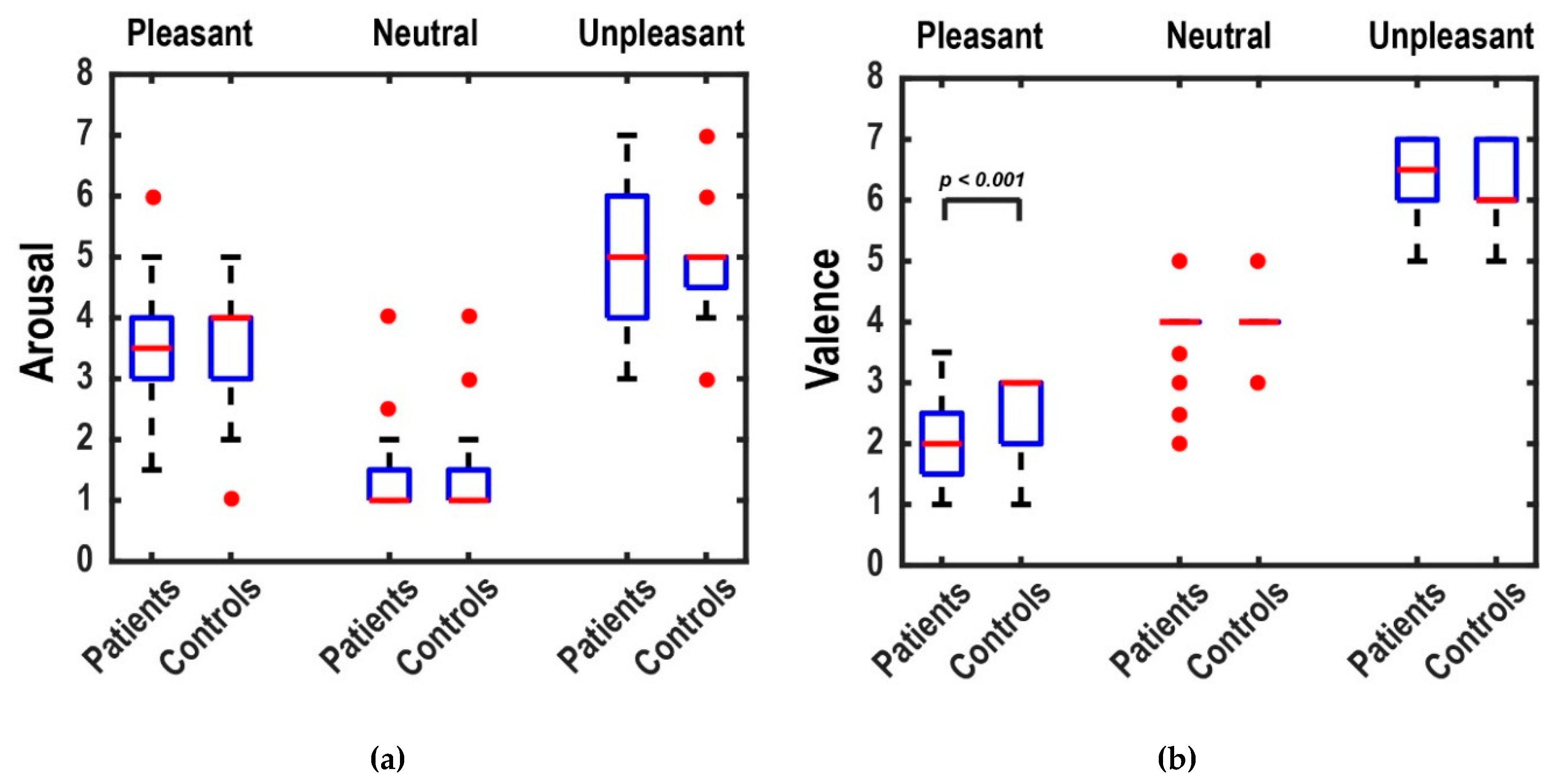
3.2. Ratings Results
4. Discussion
5. Limitations in the Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrew, R.J. The origins of facial expressions. Sci. Am. 1965, 213, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ekman, P.; Friesen, W.V.; O’Sullivan, M. Smiles when lying. In What the Face Reveals; Oxford University Press: New York, NY, USA, 1997; pp. 201–216. [Google Scholar]
- Hennenlotter, A.; Dresel, C.; Castrop, F.; Ceballos-Baumann, A.O.; Wohlschläger, A.M.; Haslinger, B. The link between facial feedback and neural activity within central circuitries of emotion—New insights from Botulinum toxin–induced denervation of frown muscles. Cereb. Cortex 2008, 19, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C.R. The Expression of the Emotions in Man and Animals; Appleton: New York, NY, USA, 1896. [Google Scholar]
- Tomkins, S.S. Affect as amplification: Some Modifications in Theory. In Theories of Emotion; Academic Press: Cambridge, MA, USA, 1980; pp. 141–164. [Google Scholar]
- Izard, C.E. The Face of Emotion; Appleton-Century-Crofts: New York, NY, USA, 1971. [Google Scholar]
- Izard, C.E. Differential emotions theory and the facial feedback hypothesis of emotion activation: Comments on Tourangeau and Ellsworth’s “The role of facial response in the experience of emotion”. J. Personal. Soc. Psychol. 1981, 58, 487–498. [Google Scholar] [CrossRef]
- Carr, L.; Iacoboni, M.; Dubeau, M.C.; Mazziotta, J.C.; Lenzi, G.L. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. USA 2003, 100, 5497–5502. [Google Scholar] [CrossRef]
- Wild, B.; Erb, M.; Eyb, M.; Bartels, M.; Grodd, W. Why are smiles contagious? An fMRI study of the interaction between perception of facial affect and facial movements. Psychiatry Res. Neuroimaging 2003, 123, 17–36. [Google Scholar] [CrossRef]
- Dapretto, M.; Davies, M.S.; Pfeifer, J.H.; Scott, A.A.; Sigman, M.; Bookheimer, S.Y.; Iacoboni, M. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006, 9, 28. [Google Scholar] [CrossRef]
- Lee, T.W.; Josephs, O.; Dolan, R.J.; Critchley, H.D. Imitating expressions: Emotion-specific neural substrates in facial mimicry. Soc. Cogn. Affect. Neurosci. 2006, 1, 122–135. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Coulson, S.E.; O’dwyer, N.J.; Adams, R.D.; Croxson, G.R. Expression of emotion and quality of life after facial nerve paralysis. Otol. Neurotol. 2004, 25, 1014–1019. [Google Scholar] [CrossRef]
- Spencer, R.C.; Irving, M.R. Causes and management of facial nerve palsy. Br. J. Hosp. Med. 2016, 77, 686–691. [Google Scholar] [CrossRef]
- Hussain, A.; Nduka, C.; Moth, P.; Malhotra, R. Bell’s facial nerve palsy in pregnancy: A clinical review. J. Obstet. Gynaecol. 2017, 37, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Sessle, B.J.; Yao, D.; Nishiura, H.; Yoshino, K.; Lee, J.C.; Martin, R.E.; Murray, G.M. Properties and plasticity of the primate somatosensory and motor cortex related to orofacial sensorimotor function. Clin. Exp. Pharmacol. Physiol. 2005, 32, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Svensson, P.; Romaniello, A.; Wang, K.; Arendt-Nielsen, L.; Sessle, B.J. One hour of tongue-task training is associated with plasticity in corticomotor control of the human tongue musculature. Exp. Brain Res. 2006, 173, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Klingner, C.M.; Volk, G.F.; Maertin, A.; Brodoehl, S.; Burmeister, H.P.; Guntinas-Lichius, O.; Witte, O.W. Cortical reorganization in Bell’s palsy. Restor. Neurol. Neurosci. 2011, 29, 203–214. [Google Scholar] [CrossRef]
- Klingner, C.M.; Volk, G.F.; Brodoehl, S.; Burmeister, H.P.; Witte, O.W.; Guntinas-Lichius, O. Time course of cortical plasticity after facial nerve palsy: A single-case study. Neurorehabilit. Neural Rep. 2012, 26, 197–203. [Google Scholar] [CrossRef]
- Klingner, C.M.; Volk, G.F.; Brodoehl, S.; Witte, O.W.; Guntinas-Lichius, O. The effects of deefferentation without deafferentation on functional connectivity in patients with facial palsy. Neuroimage Clin. 2014, 6, 26–31. [Google Scholar] [CrossRef]
- Klingner, C.M.; Brodoehl, S.; Witte, O.W.; Guntinas-Lichius, O.; Volk, G.F. The impact of motor impairment on the processing of sensory information. Behav. Brain Res. 2019, 359, 701–708. [Google Scholar] [CrossRef]
- Fridlund, A.J. Human Facial Expression: An Evolutionary View; Academic Press: New York, NY, USA, 1994. [Google Scholar]
- Keillor, J.M.; Barrett, A.M.; Crucian, G.P.; Kortenkamp, S.; Heilman, K.M. Emotional experience and perception in the absence of facial feedback. J. Int. Neuropsychol. Soc. 2002, 8, 130–135. [Google Scholar] [CrossRef]
- Giannini, A.J.; Tamulonis, D.; Giannini, M.C.; Loiselle, R.H.; Spirtos, G. Defective response to social cues in Möbius’ syndrome. J. Nerv. Ment. Dis. 1984, 172, 174–175. [Google Scholar] [CrossRef]
- Calder, A.J.; Keane, J.; Cole, J.; Campbell, R.; Young, A.W. Facial expression recognition by people with Möbius syndrome. Cogn. Neuropsychol. 2000, 17, 73–87. [Google Scholar] [CrossRef]
- Rives Bogart, K.; Matsumoto, D. Facial mimicry is not necessary to recognize emotion: Facial expression recognition by people with Moebius syndrome. Soc. Neurosci. 2010, 5, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Li, F.; Lebanon, G.; Essa, I. Beyond sentiment: The manifold of human emotions. In Proceedings of the 16th International Conference on Artificial Intelligence and Statistics (AISTATS), Scottsdale, AZ, USA, 29 April–1 May 2013; Volume 31, pp. 360–369. [Google Scholar]
- Fan, M.; Chou, C.A. Recognizing affective state patterns using regularized learning with nonlinear dynamical features of EEG. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018; pp. 137–140. [Google Scholar]
- Caicedo-Acosta, J.; Cárdenas-Peña, D.; Collazos-Huertas, D.; Padilla-Buritica, J.I.; Castaño-Duque, G.; Castellanos-Dominguez, G. Multiple-instance lasso regularization via embedded instance selection for emotion recognition. In Proceedings of the International Work-Conference on the Interplay Between Natural and Artificial Computation, Almeria, Spain, 3–7 June 2019; pp. 244–251. [Google Scholar]
- Meuleman, B.; Scherer, K.R. Nonlinear appraisal modeling: An application of machine learning to the study of emotion production. IEEE Trans. Affect. Comput. 2013, 4, 398–411. [Google Scholar] [CrossRef]
- Kauppi, J.P.; Kandemir, M.; Saarinen, V.M.; Hirvenkari, L.; Parkkonen, L.; Klami, A.; Hari, R.; Kaski, S. Towards brain-activity-controlled information retrieval: Decoding image relevance from MEG signals. NeuroImage 2015, 112, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Addington, J.; Farris, M.; Stowkowy, J.; Santesteban-Echarri, O.; Metzak, P.; Kalathil, M.S. Predictors of transition to psychosis in individuals at clinical high risk. Curr. Psychiatry Rep. 2019, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J. Behavioral treatment and bio-behavioral assessment: Computer applications. Technol. Ment. Health Care Deliv. Syst. 1980, 119–137. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International affective picture system (IAPS): Technical manual and affective ratings. Nimh Cent. Study Emot. Atten. 1997, 1, 39–58. [Google Scholar]
- Taulu, S.; Simola, J.; Kajola, M. Applications of the signal space separation method. IEEE Trans. Signal Process. 2005, 53, 3359–3372. [Google Scholar] [CrossRef]
- Garcés, P.; López-Sanz, D.; Maestú, F.; Pereda, E. Choice of magnetometers and gradiometers after signal space separation. Sensors 2017, 17, 2926. [Google Scholar] [CrossRef]
- García-Pacios, J.; Garces, P.; del Río, D.; Maestu, F. Tracking the effect of emotional distraction in working memory brain networks: Evidence from an MEG study. Psychophysiology 2017, 54, 1726–1740. [Google Scholar] [CrossRef]
- Taulu, S.; Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006, 51, 1759. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Chandra, B.; Gupta, M. An efficient statistical feature selection approach for classification of gene expression data. J. Biomed. Inform. 2011, 44, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Algamal, Z.Y.; Lee, M.H. Penalized logistic regression with the adaptive LASSO for gene selection in high-dimensional cancer classification. Expert Syst. Appl. 2015, 42, 9326–9332. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Gothard, K.M. The amygdalo-motor pathways and the control of facial expressions. Front. Neurosci. 2014, 8, 43. [Google Scholar] [CrossRef]
- Han, X.; Li, H.; Wang, X.; Zhu, Y.; Song, T.; Du, L.; Sun, S.; Guo, R.; Liu, J.; Shi, S.; et al. Altered brain fraction amplitude of low frequency fluctuation at resting state in patients with early left and right Bell’s palsy: Do they have differences? Front. Neurosci. 2018, 12, 797. [Google Scholar] [CrossRef]
- Petrantonakis, P.C.; Hadjileontiadis, L.J. A novel emotion elicitation index using frontal brain asymmetry for enhanced EEG-based emotion recognition. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 737–746. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wang, C.H.; Jung, T.P.; Wu, T.L.; Jeng, S.K.; Duann, J.R.; Chen, J.H. EEG-based emotion recognition in music listening. IEEE Trans. Biomed. Eng. 2010, 57, 1798–1806. [Google Scholar]
- Schaaff, K.; Schultz, T. Towards emotion recognition from electroencephalographic signals. In Proceedings of the 2009 IEEE 3rd International Conference on Affective Computing and Intelligent Interaction and Workshops, Amsterdam, The Netherlands, 10–12 September 2009. [Google Scholar]
- Takahashi, K. Remarks on emotion recognition from bio-potential signals. In Proceedings of the 2nd International Conference on Autonomous Robots and Agents, Palmerston North, New Zealand, 13–15 December 2004; pp. 1148–1153. [Google Scholar]
- Chanel, G.; Kronegg, J.; Grandjean, D.; Pun, T. Emotion assessment: Arousal evaluation using EEG’s and peripheral physiological signals. In Proceedings of the International Workshop on Multimedia Content Representation, Classification and Security, Istambul, Turkey, 11–13 September 2006; pp. 530–537. [Google Scholar]
- Fan, X.A.; Bi, L.Z.; Chen, Z.L. Using EEG to detect drivers’ emotion with Bayesian Networks. In Proceedings of the IEEE 2010 International Conference on Machine Learning and Cybernetics, Qingdao, China, 11–14 July 2010; pp. 1177–1181. [Google Scholar]
- Abadi, M.K.; Subramanian, R.; Kia, S.M.; Avesani, P.P.I.; Sebe, N. DECAF: MEG-Based Multimodal Database for Decoding Affective Physiological Responses. IEEE Trans. Affect. Comput. 2015, 6, 209–222. [Google Scholar] [CrossRef]
- Wallbott, H.G.; Scherer, K.R. Assessing emotion by questionnaire. In The Measurement of Emotions; Academic Press: Cambridge, MA, USA, 1989; pp. 55–82. [Google Scholar]
- Kensinger, E.A. Remembering emotional experiences: The contribution of valence and arousal. Rev. Neurosc. 2004, 15, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.I.; Senghas, A.; Ochsner, K.N. How does facial feedback modulate emotional experience? J. Res. Personal. 2009, 43, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C.; Kalcher, J. 40-Hz oscillations during motor behavior in man. Neurosci. Lett. 1993, 164, 179–182. [Google Scholar] [CrossRef]
- Crone, N.E.; Miglioretti, D.L.; Gordon, B.; Lesser, R.P. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain A J. Neurol. 1998, 121, 2301–2315. [Google Scholar] [CrossRef]
- Schack, B.; Vath, N.; Petsche, H.; Geissler, H.G.; Möller, E. Phase-coupling of theta–gamma EEG rhythms during short-term memory processing. Int. J. Psychophysiol. 2002, 44, 143–163. [Google Scholar] [CrossRef]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef]
- Voytek, B.; Canolty, R.T.; Shestyuk, A.; Crone, N.; Parvizi, J.; Knight, R.T. Shifts in gamma phase–amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front. Hum. Neurosci. 2010, 4, 191. [Google Scholar] [CrossRef]
- Maris, E.; van Vugt, M.; Kahana, M. Spatially distributed patterns of oscillatory coupling between high-frequency amplitudes and low-frequency phases in human iEEG. Neuroimage 2011, 54, 836–850. [Google Scholar] [CrossRef]
- Lisman, J.E.; Jensen, O. The theta-gamma neural code. Neuron 2013, 77, 1002–1016. [Google Scholar] [CrossRef]
- Woolley, D.E.; Timiras, P.S. Prepyriform electrical activity in the rat during high altitude exposure. Electroencephalogr. Clin. Neurophysiol. 1965, 18, 680–690. [Google Scholar] [CrossRef]
- Fujisawa, S.; Buzsáki, G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron 2011, 72, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Bradley, M.M.; Hauk, O.; Rockstroh, B.; Elbert, T.; Lang, P.J. Large-scale neural correlates of affective picture processing. Psychophysiology 2002, 39, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.K.; Niles, N.; Gore, S.; Ebrahimi, A.; McGuinness, J.; Clark, J.R. Social perception of morbidity in facial nerve paralysis. Head Neck 2016, 38, 1158–1163. [Google Scholar] [CrossRef]
- Nellis, J.C.; Ishii, L.E.; Boahene, K.D.; Byrne, P.J. Psychosocial Impact of Facial Paralysis. Curr. Otorhinolaryngol. Rep. 2018, 6, 151–160. [Google Scholar] [CrossRef]
| Patient Number. | Gender 1 | Side | Duration of Having Facial Paralysis in Month | Degree of Paralysis | Type of Paralysis | Reason for Facial Paralysis 2 | Becks Depression Inventory (BDI) | Depression’s Severity According to BDI |
|---|---|---|---|---|---|---|---|---|
| 1 | W | Left | 72 | Complete | Chronic | 3 | 10 | Mild |
| 2 | W | Right | 58 | Complete | Chronic | 1 | 12 | Mild |
| 3 | W | Right | 101 | Complete | Chronic | 1 | 1 | Minimal |
| 4 | W | Left | 40 | Complete | Chronic | 1 | 44 | Severe |
| 5 | W | Left | 29 | Complete | Chronic | 1 | 25 | Moderate |
| 6 | W | Right | 79 | Complete | Chronic | 1 | 3 | Minimal |
| 7 | M | Left | 35 | Complete | Acute | 2 | 6 | Minimal |
| 8 | W | Right | 23 | Complete | Acute | 1 | 7 | Minimal |
| 9 | W | Right | 71 | Complete | Chronic | 1 | 8 | Minimal |
| 10 | W | Left | 25 | Complete | Chronic | 1 | 13 | Mild |
| 11 | W | Right | 22 | Complete | Chronic | 2 | 3 | Minimal |
| 12 | W | Right | 21 | Complete | Acute | 1 | 17 | Mild |
| 13 | M | Right | 17 | Complete | Chronic | 1 | 2 | Minimal |
| 14 | W | Left | 99 | Complete | Chronic | 3 | 6 | Minimal |
| 15 | W | Left | 19 | Complete | Acute | 2 | 4 | Minimal |
| 16 | W | Left | 16 | Complete | Chronic | 3 | 15 | Mild |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheirkhah, M.; Brodoehl, S.; Leistritz, L.; Götz, T.; Baumbach, P.; Huonker, R.; Witte, O.W.; Volk, G.F.; Guntinas-Lichius, O.; Klingner, C.M. Abnormal Emotional Processing and Emotional Experience in Patients with Peripheral Facial Nerve Paralysis: An MEG Study. Brain Sci. 2020, 10, 147. https://doi.org/10.3390/brainsci10030147
Kheirkhah M, Brodoehl S, Leistritz L, Götz T, Baumbach P, Huonker R, Witte OW, Volk GF, Guntinas-Lichius O, Klingner CM. Abnormal Emotional Processing and Emotional Experience in Patients with Peripheral Facial Nerve Paralysis: An MEG Study. Brain Sciences. 2020; 10(3):147. https://doi.org/10.3390/brainsci10030147
Chicago/Turabian StyleKheirkhah, Mina, Stefan Brodoehl, Lutz Leistritz, Theresa Götz, Philipp Baumbach, Ralph Huonker, Otto W. Witte, Gerd Fabian Volk, Orlando Guntinas-Lichius, and Carsten M. Klingner. 2020. "Abnormal Emotional Processing and Emotional Experience in Patients with Peripheral Facial Nerve Paralysis: An MEG Study" Brain Sciences 10, no. 3: 147. https://doi.org/10.3390/brainsci10030147
APA StyleKheirkhah, M., Brodoehl, S., Leistritz, L., Götz, T., Baumbach, P., Huonker, R., Witte, O. W., Volk, G. F., Guntinas-Lichius, O., & Klingner, C. M. (2020). Abnormal Emotional Processing and Emotional Experience in Patients with Peripheral Facial Nerve Paralysis: An MEG Study. Brain Sciences, 10(3), 147. https://doi.org/10.3390/brainsci10030147







