Non-Survivor Ischemic Stroke Patients Maintain High Serum Caspase-Cleaved Cytokeratin-18 Levels
Abstract
1. Introduction
2. Methods
2.1. Design and Patients
2.2. Recorded Variables
2.3. Blood Samples and Determination of Serum CCCK-18 Concentration
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Katzan, I.L.; Spertus, J.; Bettger, J.P.; Bravata, D.M.; Reeves, M.J.; Smith, E.E.; Bushnell, C.; Higashida, R.T.; Hinchey, J.A.; Holloway, R.G.; et al. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 918–944. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, O.Y.; Glushakov, A.V.; Miller, E.R.; Valadka, A.B.; Hayes, R.L. Biomarkers for acute diagnosis and management of stroke in neurointensive care units. Brain Circ. 2016, 2, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Liberale, L.; Vecchié, A.; Casula, M.; Carbone, F.; Dallegri, F.; Montecucco, F. Update on Inflammatory Biomarkers and Treatments in Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 1967. [Google Scholar] [CrossRef] [PubMed]
- Radak, D.; Katsiki, N.; Resanovic, I.; Jovanovic, A.; Sudar-Milovanovic, E.; Zafirovic, S.; Mousad, S.A.; Isenovic, E.R. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr. Vasc. Pharmacol. 2017, 15, 115–122. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.R.; Wang, J.X.; Wang, J.; Xu, R.; Zhao, J.K.; Wang, D.S. Ischemia induces endoplasmic reticulum stress and cell apoptosis in human brain. Neurosci. Lett. 2010, 475, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Lorberboym, M.; Blankenberg, F.G.; Sadeh, M.; Lampl, Y. In vivo imaging of apoptosis in patients with acute stroke: Correlation with blood-brain barrier permeability. Brain Res. 2006, 1103, 13–19. [Google Scholar] [CrossRef]
- Qi, J.P.; Wu, A.P.; Wang, D.S.; Wang, L.F.; Li, S.X.; Xu, F.L. Correlation between neuronal injury and Caspase-3 after focal ischemia in human hippocampus. Chin. Med. J. 2004, 117, 1507–1512. [Google Scholar]
- Love, S.; Barber, R.; Wilcock, G.K. Neuronal death in brain infarcts in man. Neuropathol. Appl. Neurobiol. 2000, 26, 55–66. [Google Scholar] [CrossRef]
- Rami, A.; Sims, J.; Botez, G.; Winckler, J. Spatial resolution of phospholipid scramblase 1 (PLSCR1), caspase-3 activation and DNA-fragmentation in the human hippocampus after cerebral ischemia. Neurochem. Int. 2003, 43, 79–87. [Google Scholar] [CrossRef]
- Mitsios, N.; Gaffney, J.; Krupinski, J.; Mathias, R.; Wang, Q.; Hayward, S.; Rubio, F.; Kumar, P.; Kumar, S.; Slevin, M. Expression of signaling molecules associated with apoptosis in human ischemic stroke tissue. Cell Biochem. Biophys. 2007, 47, 73–86. [Google Scholar] [CrossRef]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Caulín, C.; Salvesen, G.S.; Oshima, R.G. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J. Cell Biol. 1997, 138, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- Ari, F.; Aztopal, N.; Oran, S.; Bozdemir, S.; Celikler, S.; Ozturk, S.; Ulukaya, E. Parmelia sulcata Taylor and Usnea filipendula Stirt induce apoptosis-like cell death and DNA damage in cancer cells. Cell Prolif. 2014, 47, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Luciani, P.; Gelmini, S.; Ferrante, E.; Lania, A.; Benvenuti, S.; Baglioni, S.; Mantovani, G.; Cellai, I.; Ammannati, F.; Spada, A.; et al. Expression of the antiapoptotic gene seladin-1 and octreotide-induced apoptosis in growth hormone-secreting and nonfunctioning pituitary adenomas. J. Clin. Endocrinol. Metab. 2005, 90, 6156–6161. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Argueso, M.; Ramos, L.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; Borreguero-León, J.M. Serum levels of caspase-cleaved cytokeratin-18 in patients with severe traumatic brain injury are associated with mortality: A pilot study. PLoS ONE 2015, 10, e0121739. [Google Scholar] [CrossRef]
- Gu, S.J.; Lu, M.; Xuan, H.F.; Chen, X.Z.; Dong, W.F.; Yan, X.F.; Si, Y.; Gao, G.L.; Hu, D.X.; Miao, J.Q. Predictive value of serum caspase-cleaved cytokeratin-18 concentrations after acute intracerebral hemorrhage. Clin. Chim. Acta 2016, 452, 124–128. [Google Scholar] [CrossRef]
- Yuan, Z.G.; Wang, J.L.; Jin, G.L.; Yu, X.B.; Li, J.Q.; Qiu, T.L.; Dai, R.X. Serum caspase-cleaved cytokeratin-18 levels and outcomes after aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 2015, 359, 298–304. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. Association between serum levels of caspase-cleaved cytokeratin-18 and early mortality in patients with severe spontaneous intracerebral hemorrhage. BMC Neurosci. 2018, 19, 23. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. High serum levels of caspase-cleaved cytokeratin-18 are associated with malignant middle cerebral artery infarction patient mortality. BMC Neurol. 2018, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Molnar, T.; Borocz, K.; Berki, T.; Szapary, L.; Szolics, A.; Janszky, J.; Illes, Z.; Csecsei, P. Subacute Elevation of Plasma Level of Caspase-Cleaved Cytokeratin-18 is Associated with Hemorrhagic Transformation and Functional Outcome in Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessement of coma and impaired conciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Chen, B.; Wang, G.; Li, W.; Liu, W.; Lin, R.; Tao, J.; Jiang, M.; Chen, L.; Wang, Y. Memantine attenuates cell apoptosis by suppressing the calpain-caspase-3 pathway in an experimental model of ischemic stroke. Exp. Cell Res. 2017, 351, 163–172. [Google Scholar] [CrossRef]
- Cho, Y.S.; Shin, M.S.; Ko, I.G.; Kim, S.E.; Kim, C.J.; Sung, Y.H.; Yoon, H.S.; Lee, B.J. Ulinastatin inhibits cerebral ischemia-induced apoptosis in the hippocampus of gerbils. Mol. Med. Rep. 2015, 12, 1796–1802. [Google Scholar] [CrossRef]
- Liu, D.M.; Wang, Z.H.; Liu, L.; Zhang, X.M.; Lou, F.L. Acetylpuerarin increases cell viability and reduces apoptosis in rat hippocampal neurons following oxygen-glucose deprivation/reperfusion. Mol. Med. Rep. 2013, 8, 1453–1459. [Google Scholar] [CrossRef]
| Non-Survivors (n = 34) | Survivors (n = 34) | p-Value | |
|---|---|---|---|
| Age (years)—median (p 25–75) | 63 (53–70) | 59 (47–68) | 0.36 |
| Female—n (%) | 13 (38.2) | 14 (41.2) | 0.99 |
| Heart failure—n (%) | 1 (2.9) | 1 (2.9) | 0.99 |
| Diabetes mellitus—n (%) | 9 (26.5) | 4 (11.8) | 0.22 |
| COPD—n (%) | 1 (2.9) | 1 (2.9) | 0.99 |
| Chronic renal failure—n (%) | 2 (5.9) | 2 (5.9) | 0.99 |
| Arterial hypertension—n (%) | 16 (47.1) | 19 (55.9) | 0.63 |
| GCS score—median (p 25–75) | 6 (3–7) | 7 (6–8) | 0.01 |
| APACHE–II score—median (p 25–75) | 22 (19–27) | 20 (16–25) | 0.06 |
| Lactic acid (mmol/L)–median (p 25–75) | 1.55 (1.00–2.70) | 1.20 (0.90–1.70) | 0.05 |
| Temperature (°C)—median (p 25–75) | 36.9 (36.0–37.3) | 36.4 (36.0–37.0) | 0.15 |
| Bilirubin (mg/dL)—median (p 25–75) | 0.60 (0.33–1.10) | 0.60 (0.40–0.83) | 0.95 |
| Glycemia (g/dL)—median (p 25–75) | 136 (118–162) | 127 (100–170) | 0.40 |
| Creatinine (mg/dL)—median (p 25–75) | 1.00 (0.70–1.25) | 0.80 (0.60–1.13) | 0.19 |
| Sodium (mEq/L)– median (p 25–75) | 140 (139–145) | 139 (136–145) | 0.38 |
| PaO2 (mmHg)—median (p 25–75) | 115 (94–267) | 156 (105–293) | 0.26 |
| PaO2/FI02 ratio—median (p 25–75) | 254 (192–325) | 300 (198–369) | 0.24 |
| INR—median (p 25–75) | 1.20 (1.01–1.31) | 1.06 (1.00–1.20) | 0.07 |
| aPTT (seconds)—median (p 25–75) | 27 (26–32) | 28 (25–30) | 0.91 |
| Platelets—median × 103/mm3 (p 25–75) | 175 (136–216) | 202 (171–265) | 0.02 |
| Fibrinogen (mg/dl)—median (p 25–75) | 419 (337–631) | 443 (416–489) | 0.90 |
| Leukocytes–median × 103/mm3 (p 25–75) | 13.9 (9.7–20.1) | 12.4 (9.6–16.9) | 0.32 |
| Hemoglobin (g/dL)—median (p 25–75) | 12.5 (11.0–14.8) | 12.1 (11.4–14.0) | 0.81 |
| Thrombolysis—n (%) | 10 (29.4) | 11 (32.4) | 0.99 |
| Haemorrhagic transformation—n (%) | 6 (17.6) | 7 (20.6) | 0.99 |
| Volumen infarction (mL)—median (p 25–75) | 180 (60–277) | 173 (100–231) | 0.64 |
| Midline shift (mm)—median (p 25–75) | 9.0 (3.5–15.0) | 6.0 (2.5–11.5) | 0.43 |
| Decompressive craniectomy–n (%) | 7 (20.6) | 9 (26.5) | 0.78 |
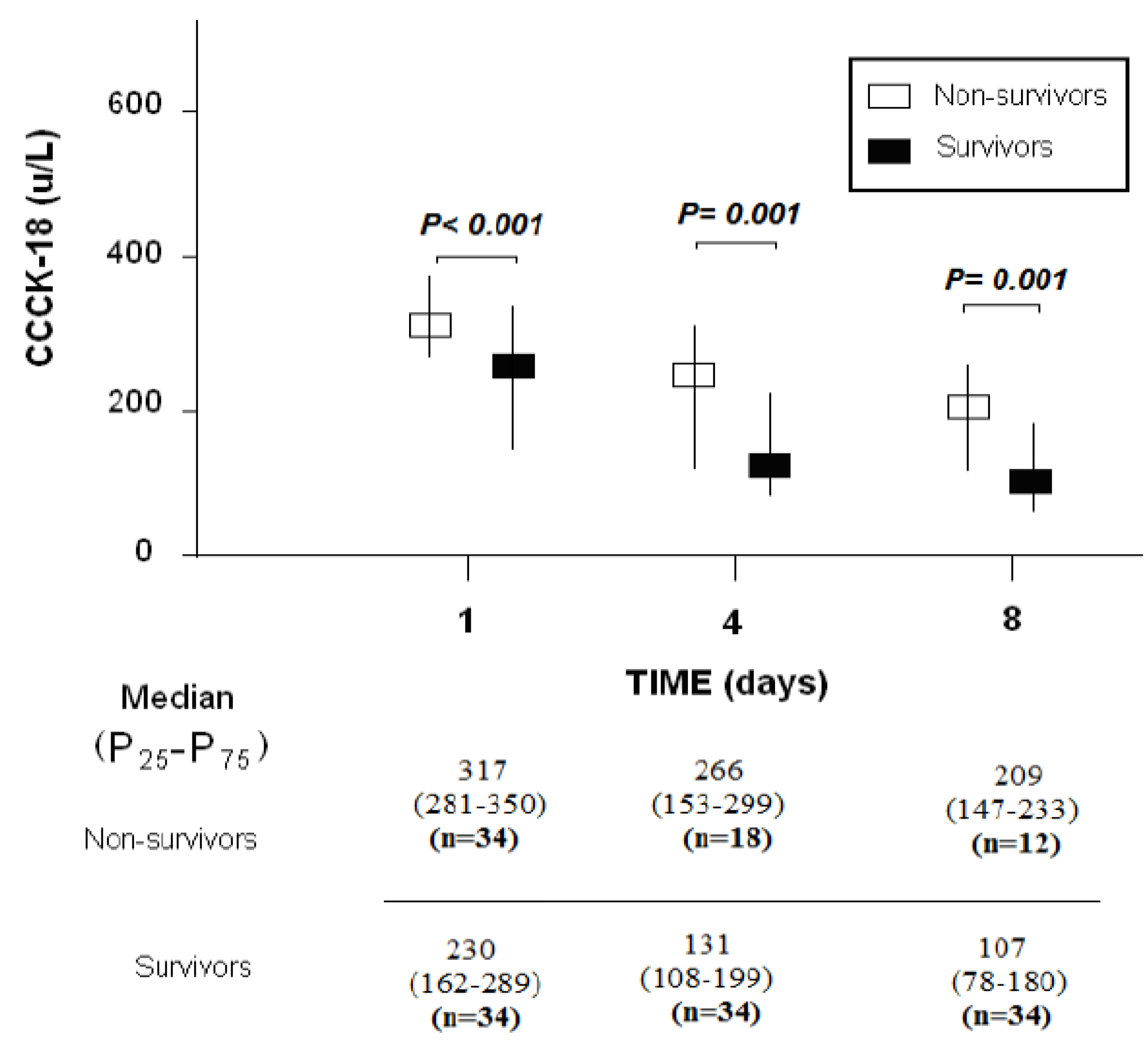
| CCCK–18 (U/L)—median (p 25–75) | 317 (281–350) | 230 (162–289) | <0.001 |
| Day 1 | Day 4 | Day 8 | |
|---|---|---|---|
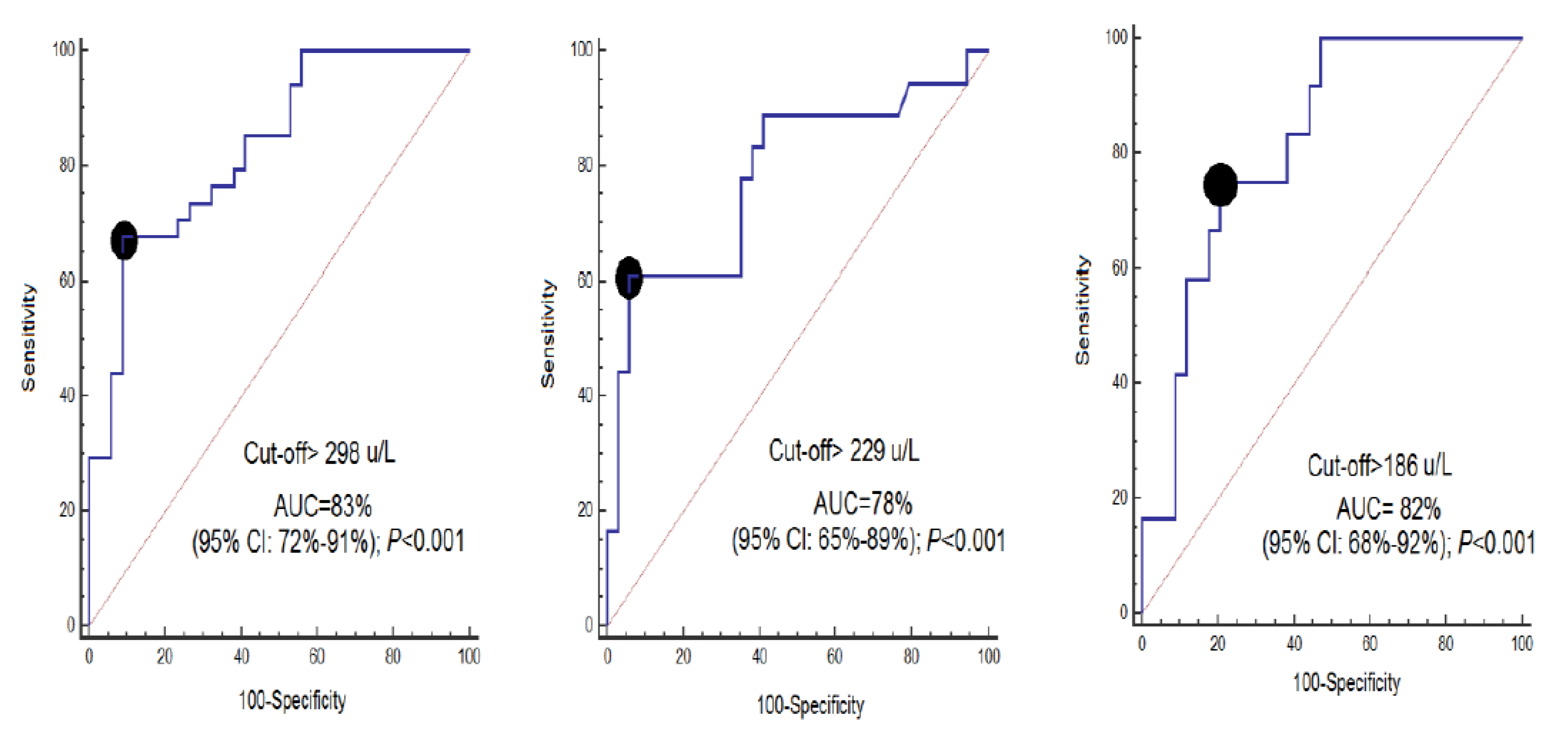
| Cut-off of CCCK-18 (U/L) | >298 | >229 | >186 |
| Specificity and 95% CI | 68% (50–83%) | 61% (36–83%) | 75% (43–95%) |
| Sensitivity and 95% CI | 91% (76–98%) | 94% (80–99%) | 79% (62–91%) |
| Positive likelihood ratio and 95% CI | 7.7 (2.5–23.2) | 10.4 (2.6–41.9) | 3.6 (1.7–7.6) |
| Negative likelihood ratio and 95% CI | 0.4 (0.2–0.6) | 0.4 (0.2–0.7) | 0.3 (0.1–0.9) |
| Positive predicted value and 95% CI | 89% (72–96%) | 85% (58–96%) | 56% (38–73%) |
| Negative predicted value and 95% CI | 74% (63–82%) | 82% (72–89%) | 90% (77–96%) |
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Glasgow Coma Scale (points) | 0.749 | 0.515–1.087 | 0.13 |
| Lactic acid (mmol/L) | 1.050 | 0.543–2.031 | 0.89 |
| Platelet count (each 1,000/mm3) | 0.987 | 0.976–0.998 | 0.02 |
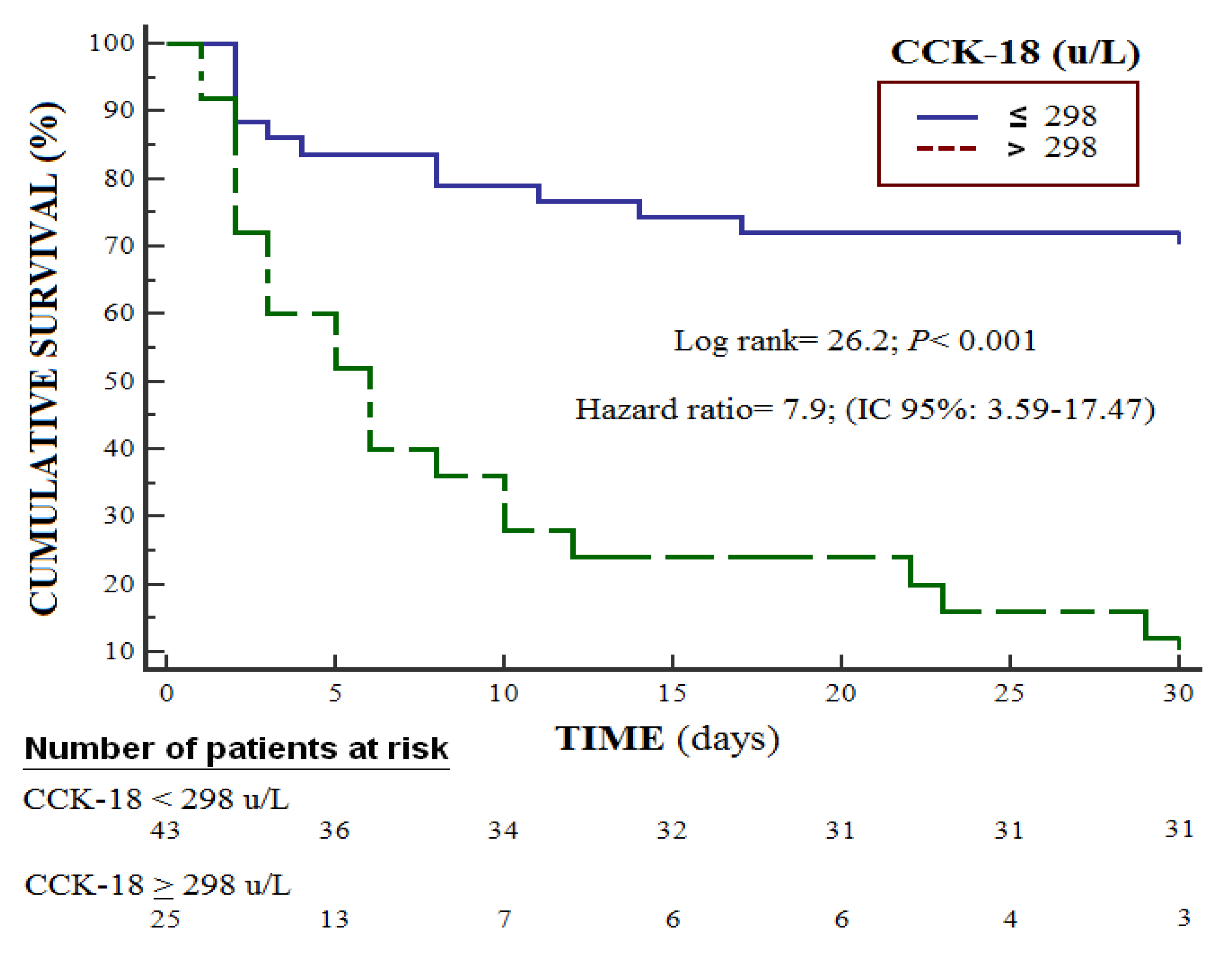
| Serum CCCK-18 levels (U/L) | 1.025 | 1.011–1.039 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; González-Rivero, A.F.; Sabatel, R.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; et al. Non-Survivor Ischemic Stroke Patients Maintain High Serum Caspase-Cleaved Cytokeratin-18 Levels. Brain Sci. 2020, 10, 132. https://doi.org/10.3390/brainsci10030132
Lorente L, Martín MM, Pérez-Cejas A, González-Rivero AF, Sabatel R, Ramos L, Argueso M, Solé-Violán J, Cáceres JJ, Jiménez A, et al. Non-Survivor Ischemic Stroke Patients Maintain High Serum Caspase-Cleaved Cytokeratin-18 Levels. Brain Sciences. 2020; 10(3):132. https://doi.org/10.3390/brainsci10030132
Chicago/Turabian StyleLorente, Leonardo, María M. Martín, Antonia Pérez-Cejas, Agustín F González-Rivero, Rafael Sabatel, Luis Ramos, Mónica Argueso, Jordi Solé-Violán, Juan J. Cáceres, Alejandro Jiménez, and et al. 2020. "Non-Survivor Ischemic Stroke Patients Maintain High Serum Caspase-Cleaved Cytokeratin-18 Levels" Brain Sciences 10, no. 3: 132. https://doi.org/10.3390/brainsci10030132
APA StyleLorente, L., Martín, M. M., Pérez-Cejas, A., González-Rivero, A. F., Sabatel, R., Ramos, L., Argueso, M., Solé-Violán, J., Cáceres, J. J., Jiménez, A., & García-Marín, V. (2020). Non-Survivor Ischemic Stroke Patients Maintain High Serum Caspase-Cleaved Cytokeratin-18 Levels. Brain Sciences, 10(3), 132. https://doi.org/10.3390/brainsci10030132





