One Multilocus Genomic Variation Is Responsible for a Severe Charcot–Marie–Tooth Axonal Form
Abstract
:1. Introduction
2. Case Presentation
3. Materials and Methods
3.1. DNA Extraction
3.2. Sequencing
3.3. Bioinformatics Analysis
3.4. Array-Comparative Genomic Hybridization (aCGH)
3.5. Quantitative Real-Time PCR (Q-PCR)
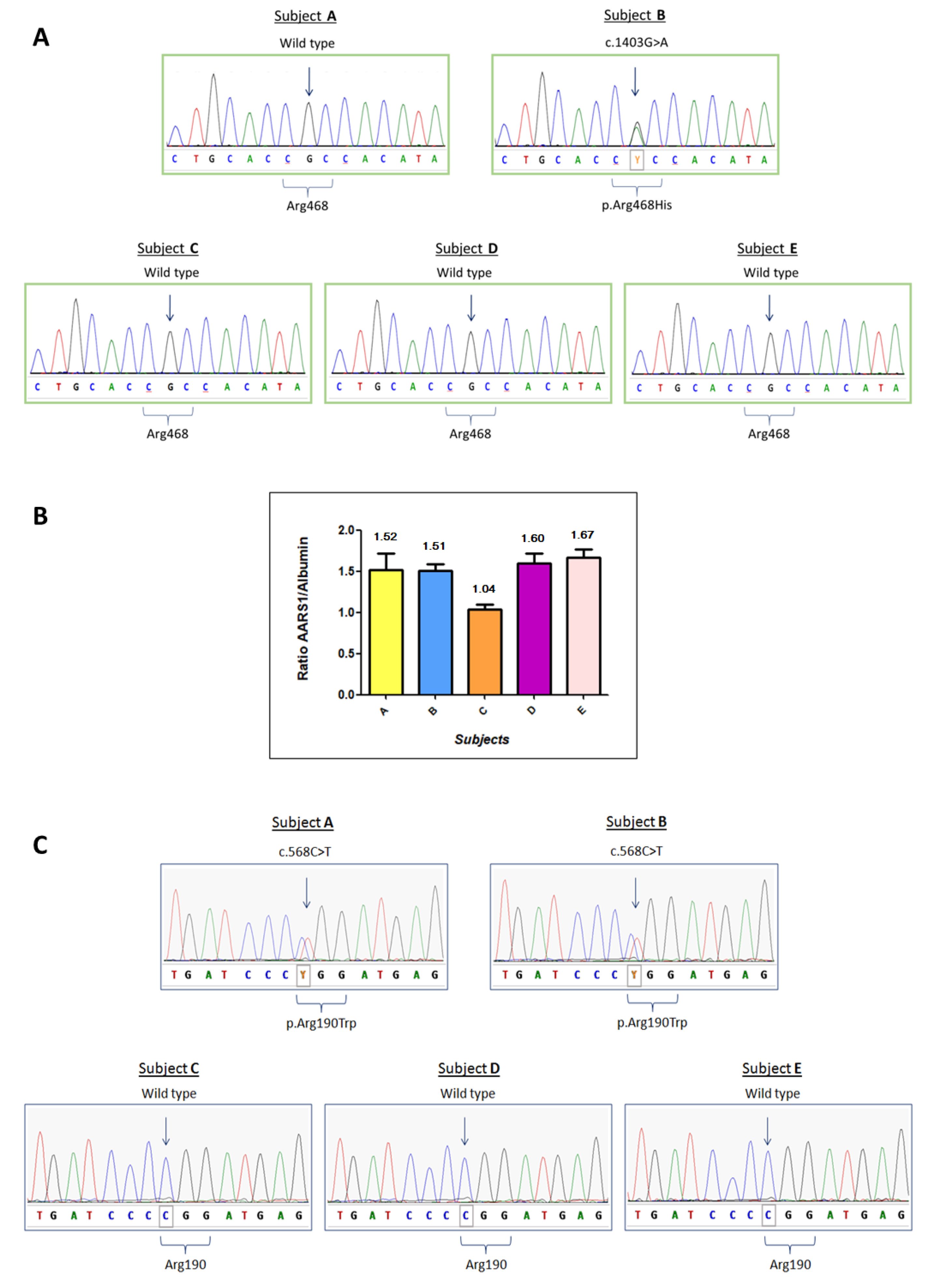
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Posey, J.E.; Harel, T.; Liu, P.; Rosenfeld, J.A.; James, R.A.; Akdemir, Z.H.C.; Walkiewicz, M.; Bi, W.; Xiao, R.; Ding, Y.; et al. Resolution of Disease Phenotypes Resulting from Multilocus Genomic Variation. N. Engl. J. Med. 2017, 376, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Derouault, P.; Parfait, B.; Moulinas, R.; Barrot, C.-C.; Sturtz, F.; Merillou, S.; Lia, A.-S. “COV’COP” allows to detect CNVs responsible for inherited diseases among amplicons sequencing data. Bioinformatis 2017, 33, 1586–1588. [Google Scholar] [CrossRef] [PubMed]
- Derouault, P.; Chauzeix, J.; Rizzo, D.; Miressi, F.; Magdelaine, C.; Bourthoumieu, S.; Durand, K.; Dzugan, H.; Feuillard, J.; Sturtz, F.; et al. CovCopCan: An efficient tool to detect Copy Number Variation from amplicon sequencing data in inherited diseases and cancer. PLoS Comput. Biol. 2020, 16, e1007503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevilla, T.; Lupo, V.; Martínez-Rubio, D.; Sancho, P.; Sivera, R.; Chumillas, M.J.; García-Romero, M.; Pascual, S.I.P.; Muelas, N.; Dopazo, J.; et al. Mutations in theMORC2gene cause axonal Charcot–Marie–Tooth disease. Brain 2015, 139, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Tchasovnikarova, I.A.; Timms, R.T.; Douse, C.H.; Roberts, R.C.; Dougan, G.; Kingston, R.E.; Modis, Y.; Lehner, P.J. Hyperactivation of HUSH complex function by Charcot–Marie–Tooth disease mutation in MORC2. Nat. Genet. 2017, 49, 1035–1044. [Google Scholar] [CrossRef]
- Douse, C.H.; Bloor, S.; Liu, Y.; Shamin, M.; Tchasovnikarova, I.A.; Timms, R.T.; Lehner, P.J.; Modis, Y. Neuropathic MORC2 mutations perturb GHKL ATPase dimerization dynamics and epigenetic silencing by multiple structural mechanisms. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, D.Q.; Nair, S.S.; Ohshiro, K.; Kumar, A.; Nair, V.S.; Pakala, S.B.; Reddy, S.D.N.; Gajula, R.P.; Eswaran, J.; Aravind, L.; et al. MORC2 Signaling Integrates Phosphorylation-Dependent, ATPase-Coupled Chromatin Remodeling during the DNA Damage Response. Cell Rep. 2012, 2, 1657–1669. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.H.; Zhang, Y.; Dong, W.J.; Shao, Z.M.; Li, D.Q. Chromatin remodeling protein MORC2 promotes breast cancer invasion and metastasis through a PRD domain-mediated interaction with CTNND1. Oncotarget 2017, 8, 97941–97954. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.L.; Cao, J.; Xie, H.Y.; Sun, R.; Yang, L.F.; Shao, Z.-M.; Li, D.Q. Cancer-Associated MORC2-Mutant M276I Regulates an hnRNPM-Mediated CD44 Splicing Switch to Promote Invasion and Metastasis in Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 5780–5792. [Google Scholar] [CrossRef] [Green Version]
- Laššuthová, P.; Brožková, D. Šafka; Krůtová, M.; Mazanec, R.; Züchner, S.; Gonzalez, M.A.; Seeman, P. Severe axonal Charcot-Marie-Tooth disease with proximal weakness caused byde novomutation in theMORC2gene. Brain 2016, 139, e26. [Google Scholar] [CrossRef] [Green Version]
- Sancho, P.; Bartesaghi, L.; Miossec, O.; García-García, F.; Ramírez-Jiménez, L.; Siddell, A.; Åkesson, E.; Hedlund, E.; Laššuthová, P.; Pascual-Pascual, S.I.; et al. Characterization of molecular mechanisms underlying the axonal Charcot–Marie–Tooth neuropathy caused by MORC2 mutations. Hum. Mol. Genet. 2019, 28, 1629–1644. [Google Scholar] [CrossRef] [PubMed]
- Engelfried, K.; Vorgerd, M.; Hagedorn, M.; Haas, G.; Gilles, J.; Epplen, J.T.; Meins, M. Charcot-Marie-Tooth neuropathy type 2A: Novel mutations in the mitofusin 2 gene (MFN2). BMC Med. Genet. 2006, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casasnovas, C.; Banchs, I.; Cassereau, J.; Gueguen, N.; Chevrollier, A.; Martinez-Matos, J.A.; Bonneau, D.; Volpini, V. Phenotypic spectrum of MFN2 mutations in the Spanish population. J. Med. Genet. 2009, 47, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassereau, J.; Casasnovas, C.; Gueguen, N.; Reynier, P.; Amati-Bonneau, P.; Banchs, I.; Volpini, V.; Procaccio, V.; Chevrollier, A.; Malinge, M.-C.; et al. Simultaneous MFN2 and GDAP1 mutations cause major mitochondrial defects in a patient with CMT. Neurology 2011, 76, 1524–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.W.; Sunwoo, I.N.; Kim, S.M.; Park, K.D.; Kim, W.K.; Kim, T.S.; Koo, H.; Cho, M.; Lee, J.; Choi, B.O. Two missense mutations of EGR2 R359W and GJB1 V136A in a Charcot–Marie–Tooth disease family. Neurogenetics 2005, 6, 159–163. [Google Scholar] [CrossRef]
- Vital, A.; Latour, P.; Solé, G.; Ferrer, X.; Rouanet, M.; Tison, F.; Vital, C.; Goizet, C. A French family with Charcot–Marie–Tooth disease related to simultaneous heterozygous MFN2 and GDAP1 mutations. Neuromuscul. Disord. 2012, 22, 735–741. [Google Scholar] [CrossRef]
- Yoshimura, A.; Yuan, J.H.; Hashiguchi, A.; Ando, M.; Higuchi, Y.; Nakamura, T.; Okamoto, Y.; Nakagawa, M.; Takashima, H. Genetic profile and onset features of 1005 patients with Charcot-Marie-Tooth disease in Japan. J. Neurol. Neurosurg. Psychiatry 2018, 90, 195–202. [Google Scholar] [CrossRef] [Green Version]
- McCorquodale, D.S.; Montenegro, G.; Peguero, A.; Carlson, N.; Speziani, F.; Price, J.; Taylor, S.W.; Melanson, M.; Vance, J.M.; Züchner, S. Mutation screening of mitofusin 2 in Charcot-Marie-Tooth disease type 2. J. Neurol. 2011, 258, 1234–1239. [Google Scholar] [CrossRef] [Green Version]
- Latour, P.; Thauvin-Robinet, C.; Baudelet-Méry, C.; Soichot, P.; Cusin, V.; Faivre, L.; Locatelli, M.-C.; Mayençon, M.; Sarcey, A.; Broussolle, E.; et al. A Major Determinant for Binding and Aminoacylation of tRNAAla in Cytoplasmic Alanyl-tRNA Synthetase Is Mutated in Dominant Axonal Charcot-Marie-Tooth Disease. Am. J. Hum. Genet. 2010, 86, 77–82. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, H.M.; Sakaguchi, R.; Giblin, W.; Program, N.C.S.; Wilson, T.E.; Biesecker, L.; Lupski, J.R.; Talbot, K.; Vance, J.M.; Züchner, S.; et al. A Recurrent loss-of-function alanyl-tRNA synthetase (AARS) mutation in patients with charcot-marie-tooth disease type 2N (CMT2N). Hum. Mutat. 2011, 33, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Bánsági, B.; Antoniadi, T.; Burton-Jones, S.; Murphy, S.M.; McHugh, J.; Alexander, M.; Wells, R.; Davies, J.; Hilton-Jones, D.; Lochmüller, H.; et al. Genotype/phenotype correlations in AARS-related neuropathy in a cohort of patients from the United Kingdom and Ireland. J. Neurol. 2015, 262, 1899–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boczonadi, V.; Jennings, M.J.; Horvath, R. The role of tRNA synthetases in neurological and neuromuscular disorders. FEBS Lett. 2018, 592, 703–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motley, W.W.; Seburn, K.L.; Nawaz, M.H.; Miers, K.E.; Cheng, J.; Antonellis, A.; Green, E.D.; Talbot, K.; Yang, X.L.; Fischbeck, K.H.; et al. Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels. PLoS Genet. 2011, 7, e1002399. [Google Scholar] [CrossRef] [PubMed]
- Kousi, M.; Katsanis, N. Genetic Modifiers and Oligogenic Inheritance. Cold Spring Harb. Perspect. Med. 2015, 5, a017145. [Google Scholar] [CrossRef] [Green Version]
- Bis-Brewer, D.M.; Fazal, S.; Züchner, S. Genetic modifiers and non-Mendelian aspects of CMT. Brain Res. 2020, 1726, 146459. [Google Scholar] [CrossRef]

| Subjects | Peroneal | Sural | Median | Ulnar | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | |||||||
| CMAP | CMAP | SNAP | SNAP | CMAP | CV | SNAP | CMAP | CV | SNAP | CMAP | SNAP | CMAP | SNAP | |
| Amp (mV) | Amp (mV) | Amp (µV) | Amp (µV) | Amp (mV) | (m/s) | Amp (µV) | Amp (mV) | (m/s) | Amp (µV) | Amp (mV) | Amp (µV) | Amp (mV) | Amp (µV) | |
| Patient A | 0.5 | 0.5 | NR | NR | 0.9 | 50 | 1.5 | 2.1 | 45 | 2.5 | 2.3 | 2.3 | 1.3 | NR |
| Patient B | 2.7 | NR | NR | NR | 4.7 | 50 | 3.2 | 3.2 | 45 | 2.8 | 4.1 | 1.2 | 5.7 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miressi, F.; Magdelaine, C.; Cintas, P.; Bourthoumieux, S.; Nizou, A.; Derouault, P.; Favreau, F.; Sturtz, F.; Faye, P.-A.; Lia, A.-S. One Multilocus Genomic Variation Is Responsible for a Severe Charcot–Marie–Tooth Axonal Form. Brain Sci. 2020, 10, 986. https://doi.org/10.3390/brainsci10120986
Miressi F, Magdelaine C, Cintas P, Bourthoumieux S, Nizou A, Derouault P, Favreau F, Sturtz F, Faye P-A, Lia A-S. One Multilocus Genomic Variation Is Responsible for a Severe Charcot–Marie–Tooth Axonal Form. Brain Sciences. 2020; 10(12):986. https://doi.org/10.3390/brainsci10120986
Chicago/Turabian StyleMiressi, Federica, Corinne Magdelaine, Pascal Cintas, Sylvie Bourthoumieux, Angélique Nizou, Paco Derouault, Frédéric Favreau, Franck Sturtz, Pierre-Antoine Faye, and Anne-Sophie Lia. 2020. "One Multilocus Genomic Variation Is Responsible for a Severe Charcot–Marie–Tooth Axonal Form" Brain Sciences 10, no. 12: 986. https://doi.org/10.3390/brainsci10120986
APA StyleMiressi, F., Magdelaine, C., Cintas, P., Bourthoumieux, S., Nizou, A., Derouault, P., Favreau, F., Sturtz, F., Faye, P.-A., & Lia, A.-S. (2020). One Multilocus Genomic Variation Is Responsible for a Severe Charcot–Marie–Tooth Axonal Form. Brain Sciences, 10(12), 986. https://doi.org/10.3390/brainsci10120986






