The Significance of Tau Aggregates in the Human Brain
Abstract
1. Introduction
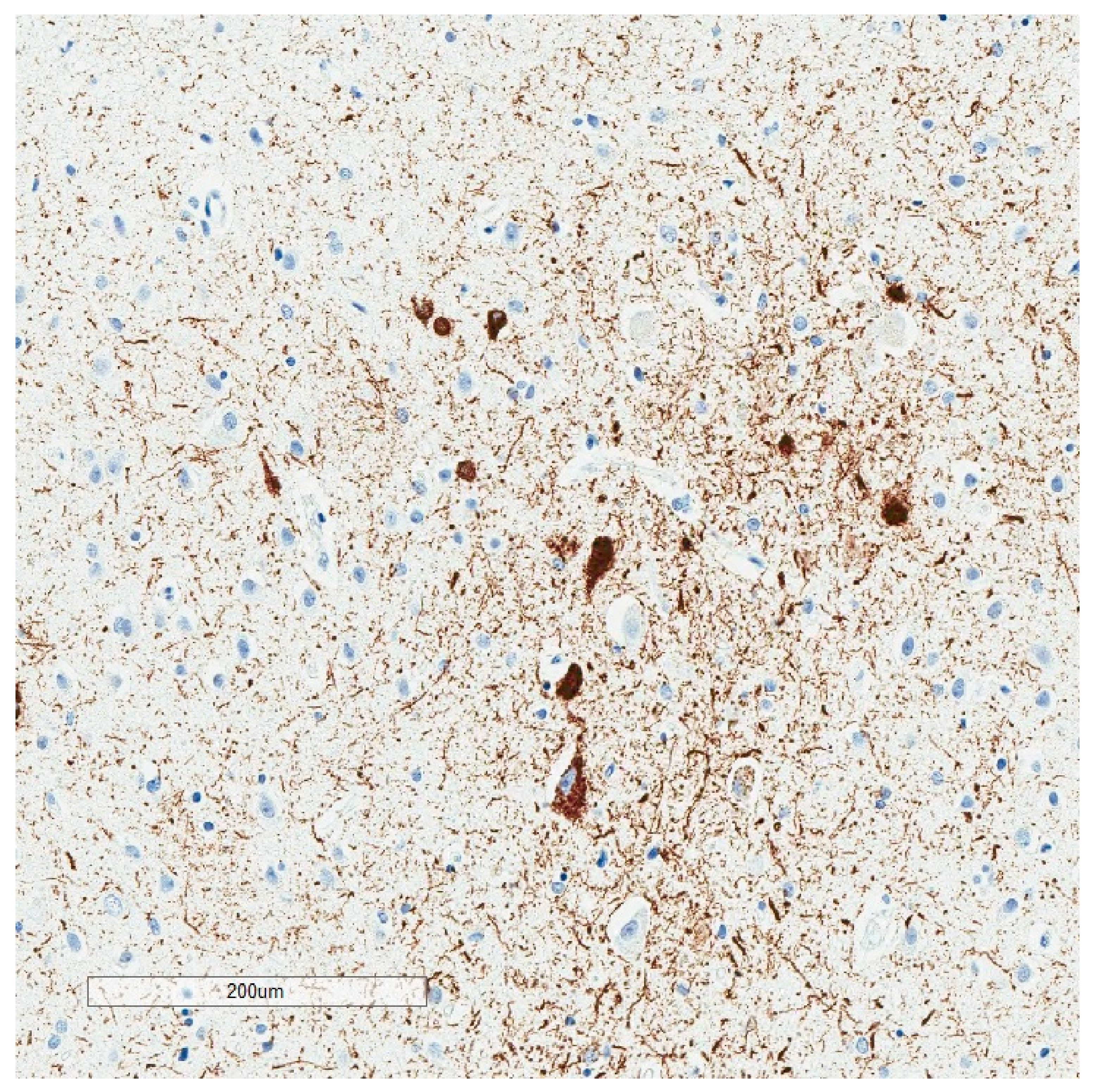
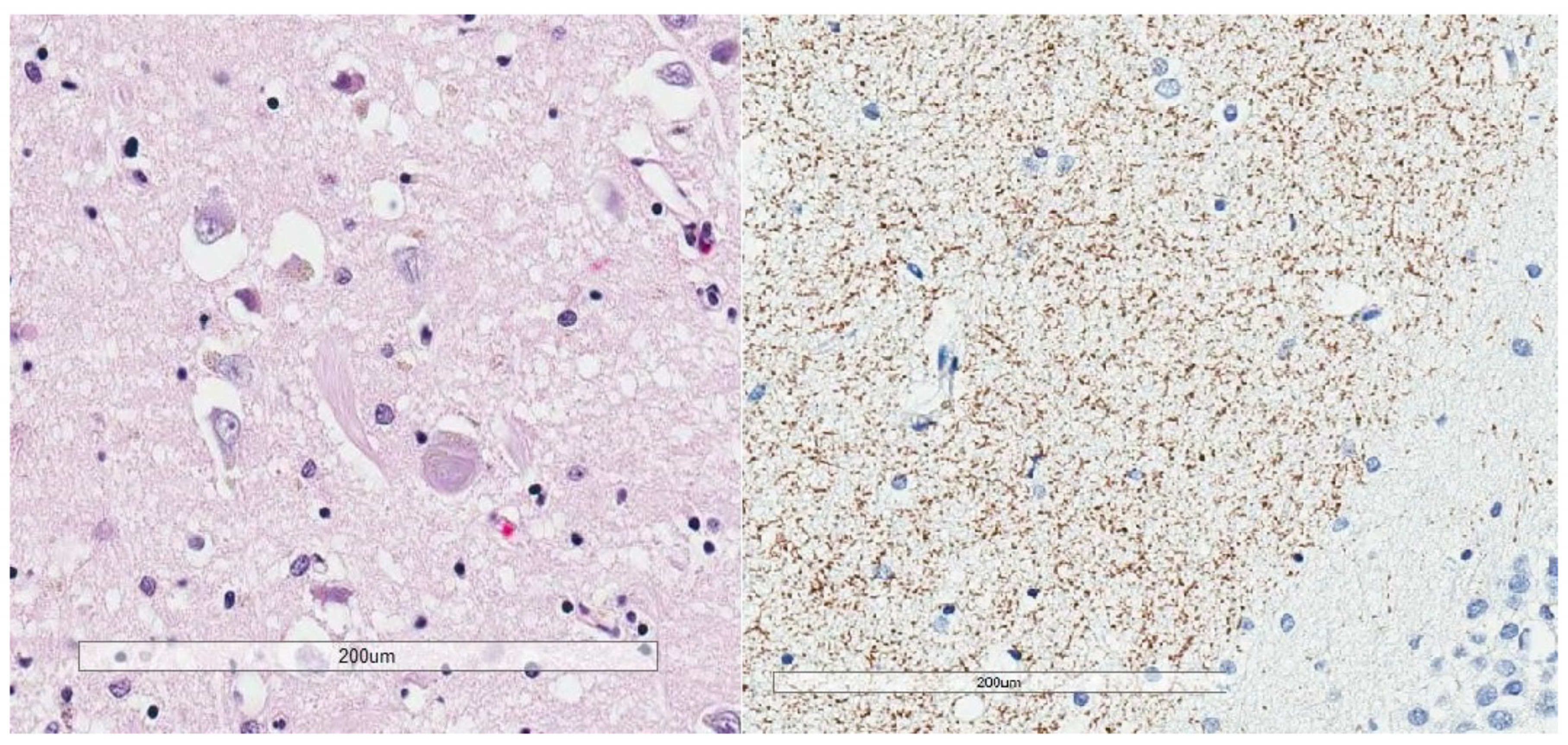
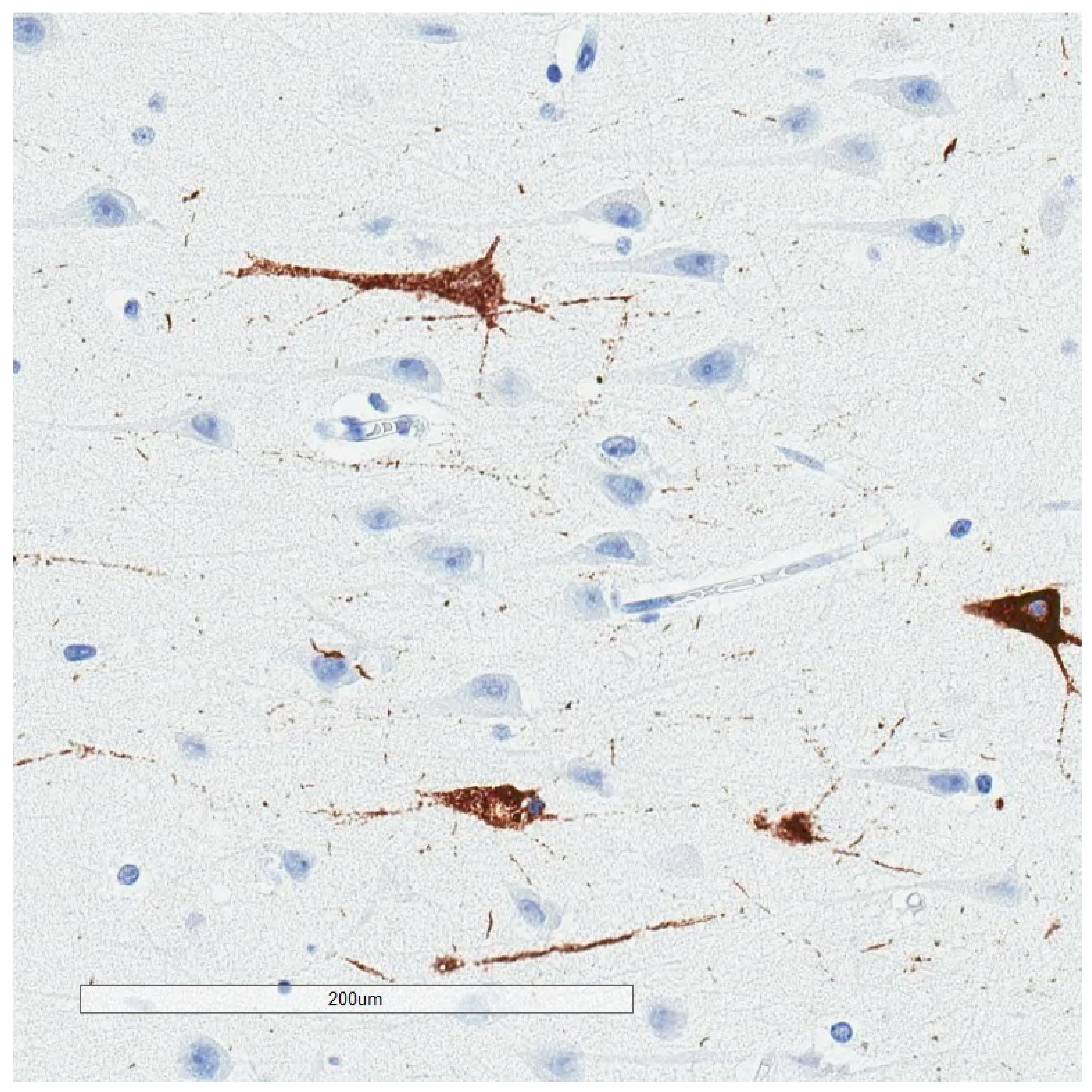
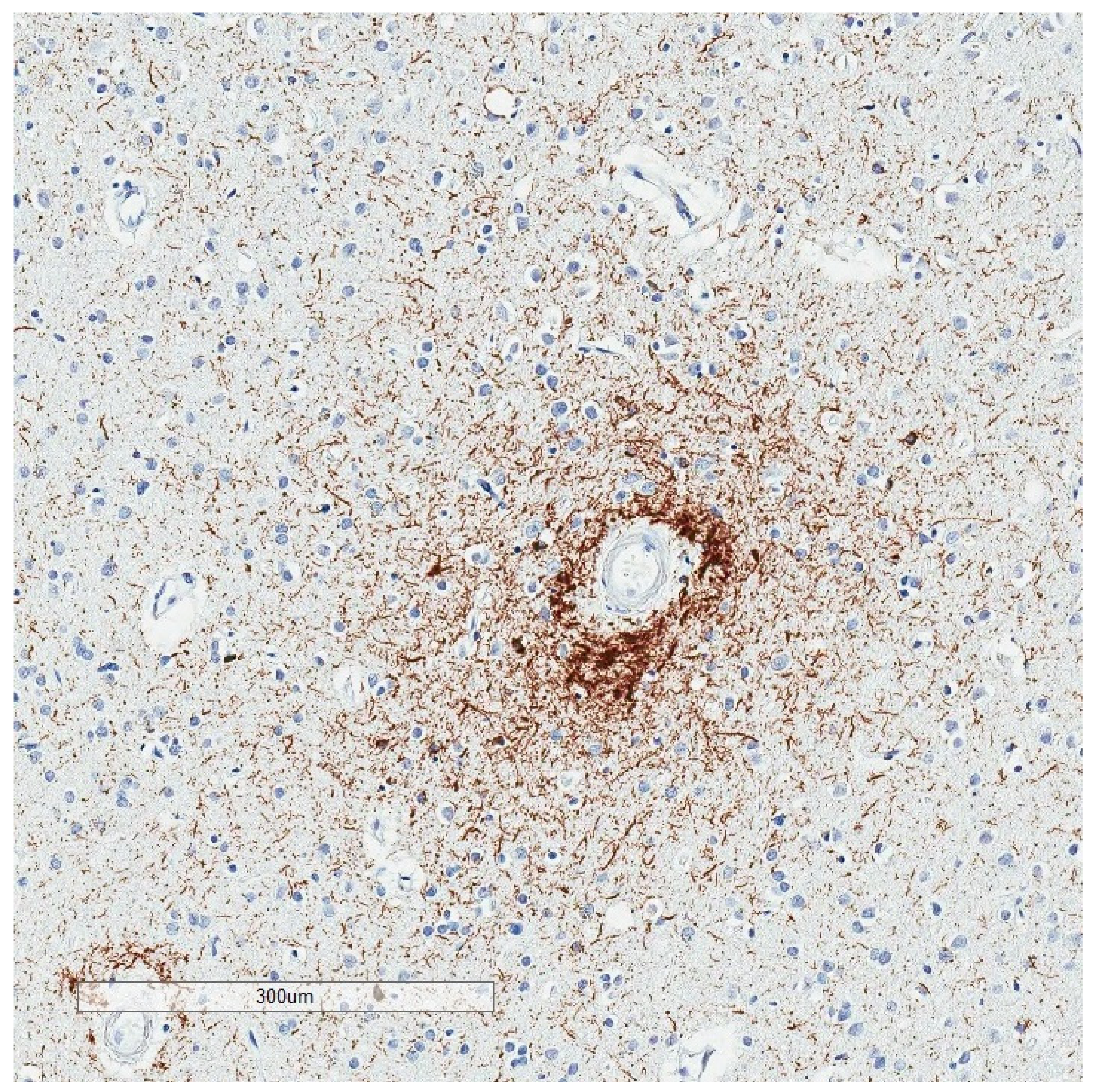
2. Phosphorylated Tau Assessment In Situ
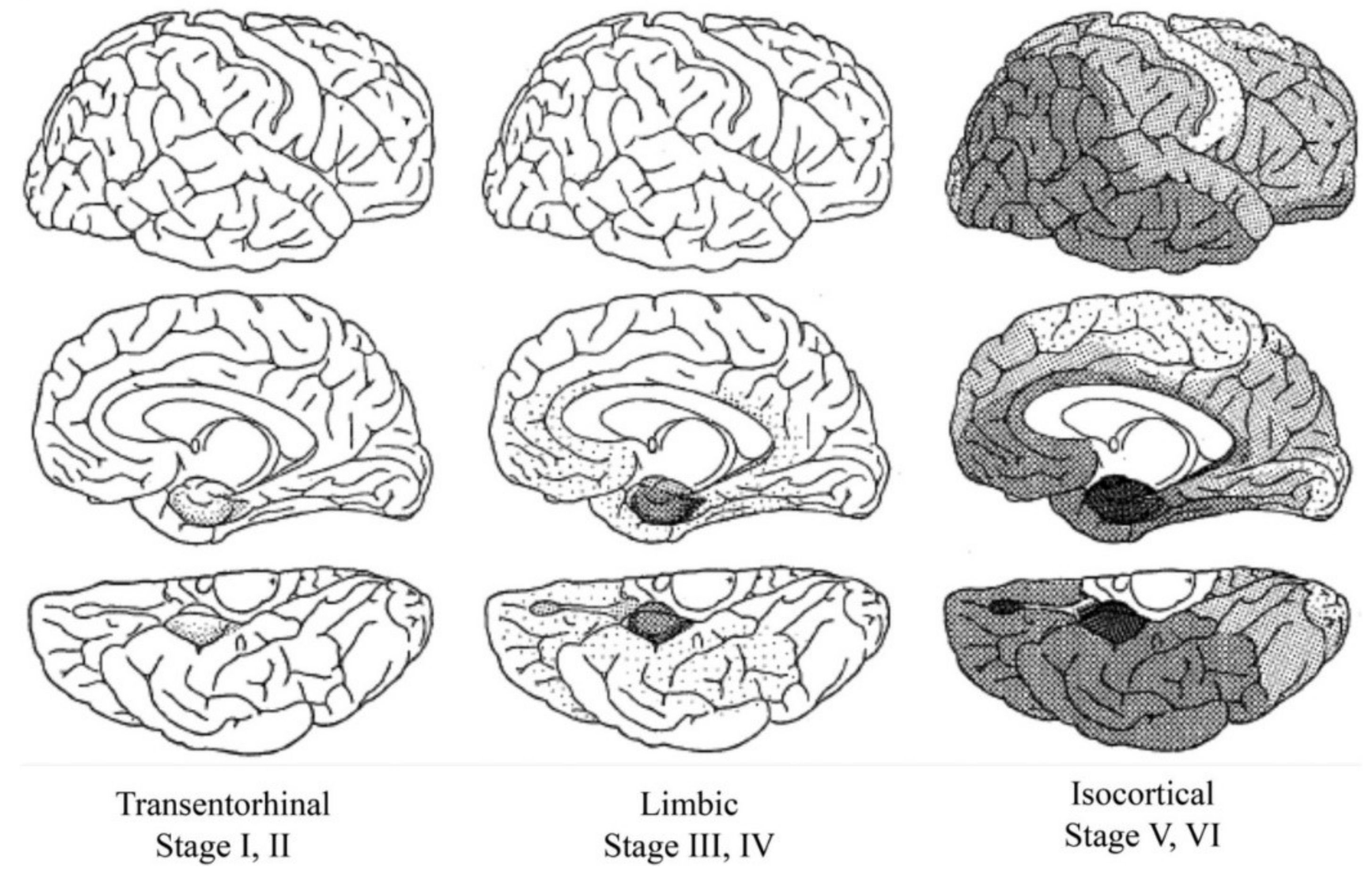
3. Tau in Aging and Alzheimer’s Disease
4. Selective Vulnerability
5. To What Extent Is p-Tau Neuropathology an Artifact of Decomposition?
6. Are p-Tau Aggregates in Part Evanescent?
7. P-Tau Lesions as Targets for Translational Research
8. Consensus Guidelines for p-Tau Research: Alzheimer’s Disease versus Chronic Traumatic Encephalopathy
9. Conclusions
Funding
Conflicts of Interest
References
- Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Zeitschr. Psychiatr. 1907, 64, 146–148. [Google Scholar]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bielschowsky, M. Histopathology of nerve cells. In Cytology and Cellular Pathology of the Nervous System; W. Penfield, McGill University. Paul B. Hoeber, Inc.: New York, NY, USA, 1932; p. 132. [Google Scholar]
- Malamud, W.L.K. Alzheimer’s Disease. Archiv. Neurol. Psychiatry 1929, 21, 805–827. [Google Scholar] [CrossRef]
- McMenemey, W. Alzheimer’s disease: A report of six cases. J. Neurol. Pscyhiatry 1940, 3, 211–240. [Google Scholar] [CrossRef][Green Version]
- King, L. Pathology of senile brains I. Silver-reducing structures in the hippocampus. Archiv. Neurol. Psychiatry 1942, 48, 241–256. [Google Scholar] [CrossRef]
- Kidd, M. Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 1963, 197, 192–193. [Google Scholar] [CrossRef]
- Terry, R.D. The Fine Structure of Neurofibrillary Tangles in Alzheimer’s Disease. J. Neuropathol. Exp. Neurol. 1963, 22, 629–642. [Google Scholar] [CrossRef]
- Terry, R.D.; Gonatas, N.K.; Weiss, M. Ultrastructural Studies in Alzheimer’s Presenile Dementia. Am. J. Pathol. 1964, 44, 269–297. [Google Scholar]
- Hirano, A.; Zimmerman, H.M. Alzheimer’s neurofibrillary changes. A topographic study. Arch. Neurol. 1962, 7, 227–242. [Google Scholar] [CrossRef]
- Brion, J.P.; Nunez, J.H.; Flament-Durand, J. Mise en ’evidence immunologique de la prot´eine tau au niveau des l´esions de d´eg´en´erescence neurofibrillaire de la maladie d’Alzheimer. Arch. Biol. 1985, 95, 229–352. [Google Scholar]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Iqbal, K.; Quinlan, M.; Tung, Y.C.; Zaidi, M.S.; Wisniewski, H.M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 1986, 261, 6084–6089. [Google Scholar] [PubMed]
- Hagestedt, T.; Lichtenberg, B.; Wille, H.; Mandelkow, E.M. Tau protein becomes long and stiff upon phosphorylation: Correlation between paracrystalline structure and degree of phosphorylation. J. Cell Biol. 1989, 109, 1643–1651. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M.; Crowther, R.A.; Murrell, J.R.; Farlow, M.R.; Ghetti, B. Familial multiple system tauopathy with presenile dementia: A disease with abundant neuronal and glial tau filaments. Proc. Natl. Acad. Sci. USA 1997, 94, 4113–4118. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Suemoto, C.K.; Molina, M.; Nascimento, C.F.; Leite, R.E.P.; de Lucena Ferretti-Rebustini, R.E.; Farfel, J.M.; Heinsen, H.; Nitrini, R.; Ueda, K.; et al. Argyrophilic Grain Disease: Demographics, Clinical, and Neuropathological Features from a Large Autopsy Study. J. Neuropathol. Exp. Neurol. 2016, 75, 628–635. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Ferrer, I.; Grinberg, L.T.; Alafuzoff, I.; Attems, J.; Budka, H.; Cairns, N.J.; Crary, J.F.; Duyckaerts, C.; Ghetti, B.; et al. Aging-related tau astrogliopathy (ARTAG): Harmonized evaluation strategy. Acta Neuropathol. 2016, 131, 87–102. [Google Scholar] [CrossRef]
- Crary, J.F.; Trojanowski, J.Q.; Schneider, J.A.; Abisambra, J.F.; Abner, E.L.; Alafuzoff, I.; Arnold, S.E.; Attems, J.; Beach, T.G.; Bigio, E.H.; et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol. 2014, 128, 755–766. [Google Scholar] [CrossRef]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Keene, C.D.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.G.; The TBI/CTE Group; et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016, 131, 75–86. [Google Scholar] [CrossRef]
- Iverson, P.G.L.; Gardner, A.J.; Shultz, S.R.; Solomon, G.S.; McCrory, P.; Zafonte, R.; Perry, G.; Hazrati, L.-N.; Keene, C.D.; Castellani, R.J. Chronic traumatic encephalopathy neuropathology might not be inexorably progressive or unique to repetitive neurotrauma. Brain 2019, 142, 3672–3693. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Kouri, N.; Murray, M.E.; Josephs, K.A. Neuropathology of Frontotemporal Lobar Degeneration-Tau (FTLD-Tau). J. Mol. Neurosci. 2011, 45, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K.A. Current Understanding of Neurodegenerative Diseases Associated With the Protein Tau. Mayo Clin. Proc. 2017, 92, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; del Tredici, K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011, 121, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef]
- Iverson, G.L.; Luoto, T.M.; Karhunen, P.J.; Castellani, R.J. Mild Chronic Traumatic Encephalopathy Neuropathology in People with No Known Participation in Contact Sports or History of Repetitive Neurotrauma. J. Neuropathol. Exp. Neurol. 2019, 78, 615–625. [Google Scholar] [CrossRef]
- Morishima-Kawashima, M.; Hasegawa, M.; Takio, K.; Suzuki, M.; Yoshida, H.; Watanabe, A.; Titani, K.; Ihara, Y. Hyperphosphorylation of tau in PHF. Neurobiol. Aging 1995, 16, 71–80. [Google Scholar] [CrossRef]
- Hanger, D.P.; Byers, H.L.; Wray, S.; Leung, K.-Y.; Saxton, M.J.; Seereeram, A.; Reynolds, C.H.; Ward, M.A.; Anderton, B.H. Novel Phosphorylation Sites in Tau from Alzheimer Brain Support a Role for Casein Kinase 1 in Disease Pathogenesis. J. Biol. Chem. 2007, 282, 23645–23654. [Google Scholar] [CrossRef]
- Augustinack, J.C.; Schneider, A.; Mandelkow, E.M.; Hyman, B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002, 103, 26–35. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G. Tau Biology, Tauopathy, Traumatic Brain Injury, and Diagnostic Challenges. J. Alzheimer’s Dis. 2019, 67, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hirano, A. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer’s neurofibrillary tangles. Neuropathol. Appl. Neurobiol. 1986, 12, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Vance, A.M. The Bodian stain: Its use and method. Am. J. Med. Technol. 1960, 26, 360–362. [Google Scholar] [PubMed]
- Hattori, M.; Hashizume, Y.; Yoshida, M.; Iwasaki, Y.; Hishikawa, N.; Ueda, R.; Ojika, K. Distribution of astrocytic plaques in the corticobasal degeneration brain and comparison with tuft-shaped astrocytes in the progressive supranuclear palsy brain. Acta Neuropathol. 2003, 106, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.L.; Kril, J.J.; Halliday, G.M. Cellular and regional vulnerability in frontotemporal tauopathies. Acta Neuropathol. 2019, 138, 705–727. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.G.; Giese, A.; Grossman, M.; Kurz, C.; et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Jones, B.E. Noradrenergic locus coeruleus neurons: Their distant connections and their relationship to neighboring (including cholinergic and GABAergic) neurons of the central gray and reticular formation. Prog. Brain Res. 1991, 88, 15–30. [Google Scholar]
- Haroutunian, V.; Purohit, D.P.; Perl, D.P.; Marin, D.; Khan, K.; Lantz, M.; Davis, K.L.; Mohs, R.C. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch. Neurol. 1999, 56, 713–718. [Google Scholar] [CrossRef]
- Ganz, A.B.; Beker, N.; Hulsman, M.; Sikkes, S.; Bank, N.B.; Scheltens, P.; Smit, A.B.; Rozemuller, A.J.M.; Hoozemans, J.J.M.; Holstege, H. Neuropathology and cognitive performance in self-reported cognitively healthy centenarians. Acta Neuropathol. Commun. 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Parisi, J.E.; Salviati, A.; Floriach-Robert, M.; Boeve, B.F.; Ivnik, R.J.; Smith, G.E.; Dickson, D.W.; Johnson, K.A.; Petersen, L.E.; et al. Neuropathology of Cognitively Normal Elderly. J. Neuropathol. Exp. Neurol. 2003, 62, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, K.S.; Sonnen, J.A.; Pezhouh, M.K.; Desrosiers, M.F.; Nelson, P.T.; Tyas, S.L. Alzheimer Disease Pathology in Subjects Without Dementia in 2 Studies of Aging: The Nun Study and the Adult Changes in Thought Study. J. Neuropathol. Exp. Neurol. 2011, 70, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; del Tredici, K.; et al. Correlation of Alzheimer Disease Neuropathologic Changes with Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Giannakopoulos, P.; Herrmann, F.R.; Bussiere, T. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003, 60, 495–500. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson-Carlgren, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Larner, A.J. The cerebellum in Alzheimer’s disease. Dement Geriatr. Cogn. 1997, 8, 203–209. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G. The complexities of the pathology–pathogenesis relationship in Alzheimer disease. Biochem. Pharmacol. 2014, 88, 671–676. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A.; Mayford, M.; Barzilai, A.; Keller, F.; Schacher, S.; Kandel, E. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Matsuo, E.S.; Shin, R.-W.; Billingsley, M.L.; van Devoorde, A.; O’Connor, M.; Trojanowski, J.Q.; Lee, V.M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau. Neuron 1994, 13, 989–1002. [Google Scholar] [CrossRef]
- Song, J.; Combs, C.K.; Pilcher, W.H.; Song, L.Y.; Utal, A.K.; Coleman, P.D. Low Initial Tau Phosphorylation in Human Brain Biopsy Samples. Neurobiol. Aging 1997, 18, 475–481. [Google Scholar] [CrossRef]
- Kopeikina, K.J.; Hyman, B.T.; Spires-Jones, T.L. Soluble forms of tau are toxic in Alzheimer’s disease. Transl. Neurosci. 2012, 3, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Marquié, M.; Normandin, M.D.; Ba, A.C.M.; Ba, M.S.T.C.; Bs, N.V.A.; Bs, A.A.; Klunk, W.E.; Mathis, C.A.; Ikonomovic, M.D.; Debnath, M.; et al. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann. Neurol. 2017, 81, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies—Still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Harada, R.; Okamura, N.; Furumoto, S.; Tago, T.; Yanai, K.; Arai, H.; Kudo, Y. Characteristics of Tau and Its Ligands in PET Imaging. Biomololecules 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Takashima, A. Tau oligomers. Adv. Exp. Med. Biol. 2014, 1184, 373–380. [Google Scholar] [CrossRef]
- Kaufman, S.K.; Sanders, D.W.; Thomas, T.L.; Ruchinskas, A.J.; Vaquer-Alicea, J.; Sharma, A.M.; Miller, T.M.; Diamond, M.I. Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 2016, 92, 796–812. [Google Scholar] [CrossRef]
- Morsch, R.; Simon, W.; Coleman, P.D. Neurons May Live for Decades with Neurofibrillary Tangles. J. Neuropathol. Exp. Neurol. 1999, 58, 188–197. [Google Scholar] [CrossRef]
- Braak, E.; Braak, H. Alzheimer’s disease: Transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon’s horn. Acta Neuropathol. 1997, 93, 323–325. [Google Scholar] [CrossRef]
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef]
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread Peroxynitrite-Mediated Damage in Alzheimer’s Disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Harris, P.L.R.; Sayre, L.M. Active glycation in neurofibrillary pathology of Alzheimer disease: N-epsilon-(carboxymethyl) lysine and hexitol-lysine. Free Radical. Bio. Med. 2001, 31, 175–180. [Google Scholar] [CrossRef]
- Sayre, L.M.; Zelasko, D.A.; Harris, P.L.R.; Perry, G.; Salomon, R.G.; Smith, M.A. 4-hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 1997, 68, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, O.; Zhu, X.; Castellani, R.J.; Harris, P.L.R.; Smith, M.A. Increased p27, an essential component of mitotic re-entry in Alzheimer disease. J. Neuropath. Exp. Neur 2002, 61, 455. [Google Scholar]
- Smith, M.A.; Kutty, R.K.; Richey, P.L.; Yan, S.D.; Stern, D.; Chader, G.J.; Wiggert, B.; Petersen, R.B.; Perry, G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer’s disease. Am. J. Pathol. 1994, 145, 42–47. [Google Scholar]
- Tang, Z.; Bereczki, E.; Zhang, H.Y. Mammalian Target of Rapamycin (mTor) Mediates Tau Protein Dyshomeostasis Imlication for Alzheimer Disease. J. Biol. Chem. 2013, 288, 15556–15570. [Google Scholar] [CrossRef]
- Rissman, R.A.; Poon, W.W.; Blurton-Jones, M.; Oddo, S.; Torp, R.; Vitek, M.P.; la Ferla, F.M.; Rohn, T.T.; Cotman, C.W. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Investig. 2004, 114, 121–130. [Google Scholar] [CrossRef]
- Arnold, C.S.; Johnson, G.V.W.; Cole, R.N.; Dong, D.L.-Y.; Lee, M.; Hart, G.W. The Microtubule-associated Protein Tau Is Extensively Modified withO-linkedN-acetylglucosamine. J. Biol. Chem. 1996, 271, 28741–28744. [Google Scholar] [CrossRef]
- Smith, M.A.; Taneda, S.; Richey, P.L.; Miyata, S.; Yan, S.D.; Stern, D.; Sayre, L.M.; Monnier, V.M.; Perry, G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc. Natl. Acad. Sci. USA 1994, 91, 5710–5714. [Google Scholar] [CrossRef]
- Mori, H.; Kondo, J.; Ihara, Y. Ubiquitin Is a Component of Paired Helical Filaments in Alzheimers-Disease. Science 1987, 235, 1641–1644. [Google Scholar] [CrossRef]
- Horiguchi, T.; Uryu, K.; Giasson, B.I.; Ischiropoulos, H.; Lightfoot, R.; Bellmann, C.; Richter-Landsberg, C.; Lee, V.M.-Y.; Trojanowski, J.Q. Nitration of Tau Protein Is Linked to Neurodegeneration in Tauopathies. Am. J. Pathol. 2003, 163, 1021–1031. [Google Scholar] [CrossRef]
- Cohen, T.J.; Guo, J.L.; Hurtado, D.E.; Kwong, L.K.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M.Y. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011, 2, 252. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Greenwood, A.; Binder, L. Proline Isomer-Specific Antibodies Reveal the Early Pathogenic Tau Conformation in Alzheimer’s Disease. Cell 2012, 149, 232–424. [Google Scholar] [CrossRef] [PubMed]
- Wischik, C.M.; Novak, M.; Thogersen, H.C.; Edwards, P.C.; Runswick, M.J.; Jakes, R.; Walker, J.E.; Milstein, C.; Roth, M.; Klug, A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1988, 85, 4506–4510. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative Damage Is the Earliest Event in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef]
- Raina, A.K.; Hochman, A.; Zhu, X.W. Abortive apoptosis in Alzheimer’s disease. Acta Neuropathol. 2001, 101, 305–310. [Google Scholar] [CrossRef]
- Castellani, R.J.; Gupta, Y.; Sheng, B. A novel origin for granulovacuolar degeneration in aging and Alzheimer’s disease: Parallels to stress granules. Lab. Investig. 2011, 91, 1777–1786. [Google Scholar] [CrossRef][Green Version]
- Santa-Cruz, K.; Lewis, J.; Spires, T. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef]
- Castellani, R.J.; Plascencia-Villa, G.; Perry, G. The amyloid cascade and Alzheimer’s disease therapeutics: Theory versus observation. Lab. Investig. 2019, 99, 958–970. [Google Scholar] [CrossRef]
- van deVrede, L.; Boxer, A.L.; Polydoro, M. Targeting tau: Clinical trials and novel therapeutic approaches. Neurosci. Lett. 2020, 731, 134919. [Google Scholar] [CrossRef]
- Mirra, S.S.; Heyman, A.; McKeel, D.; Sumi, S.M.; Crain, B.J.; Brownlee, L.M.; Vogel, F.S.; Hughes, J.P.; van Belle, G.; Berg, L.; et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 41, 479. [Google Scholar] [CrossRef] [PubMed]
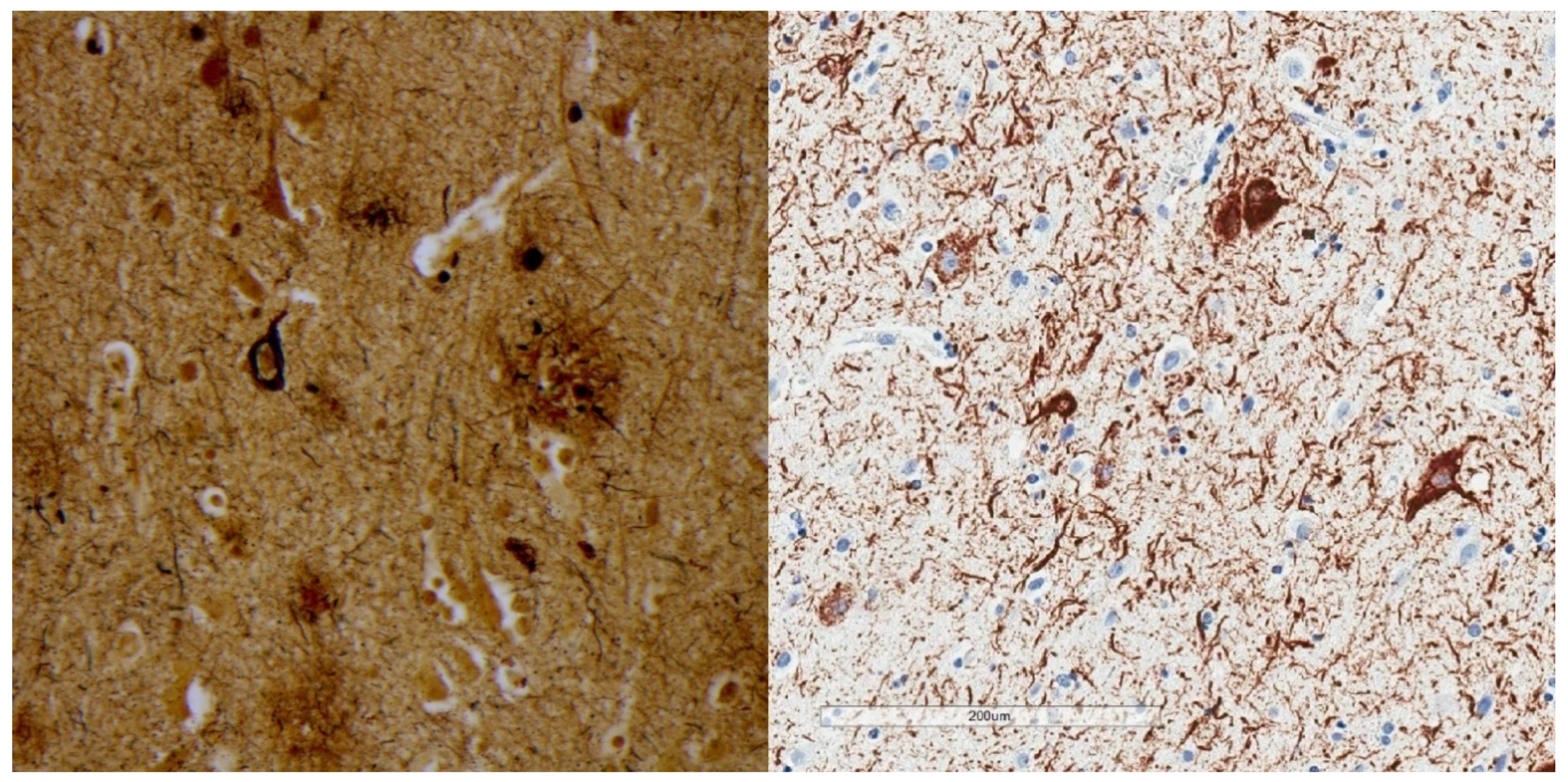
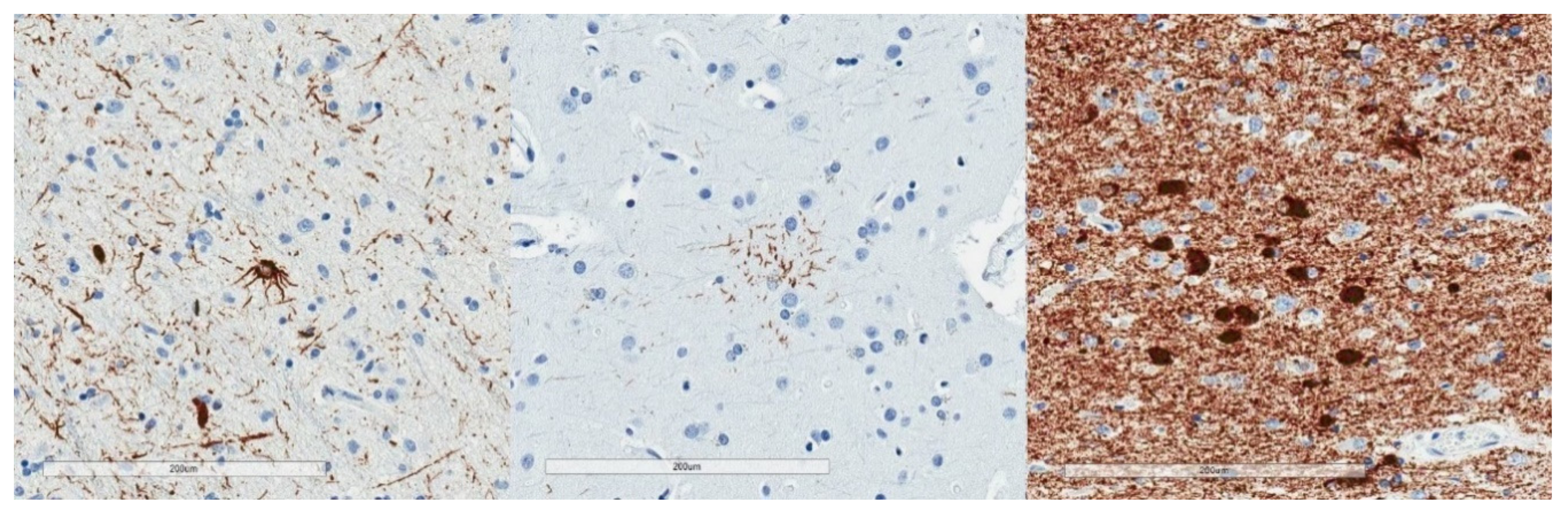
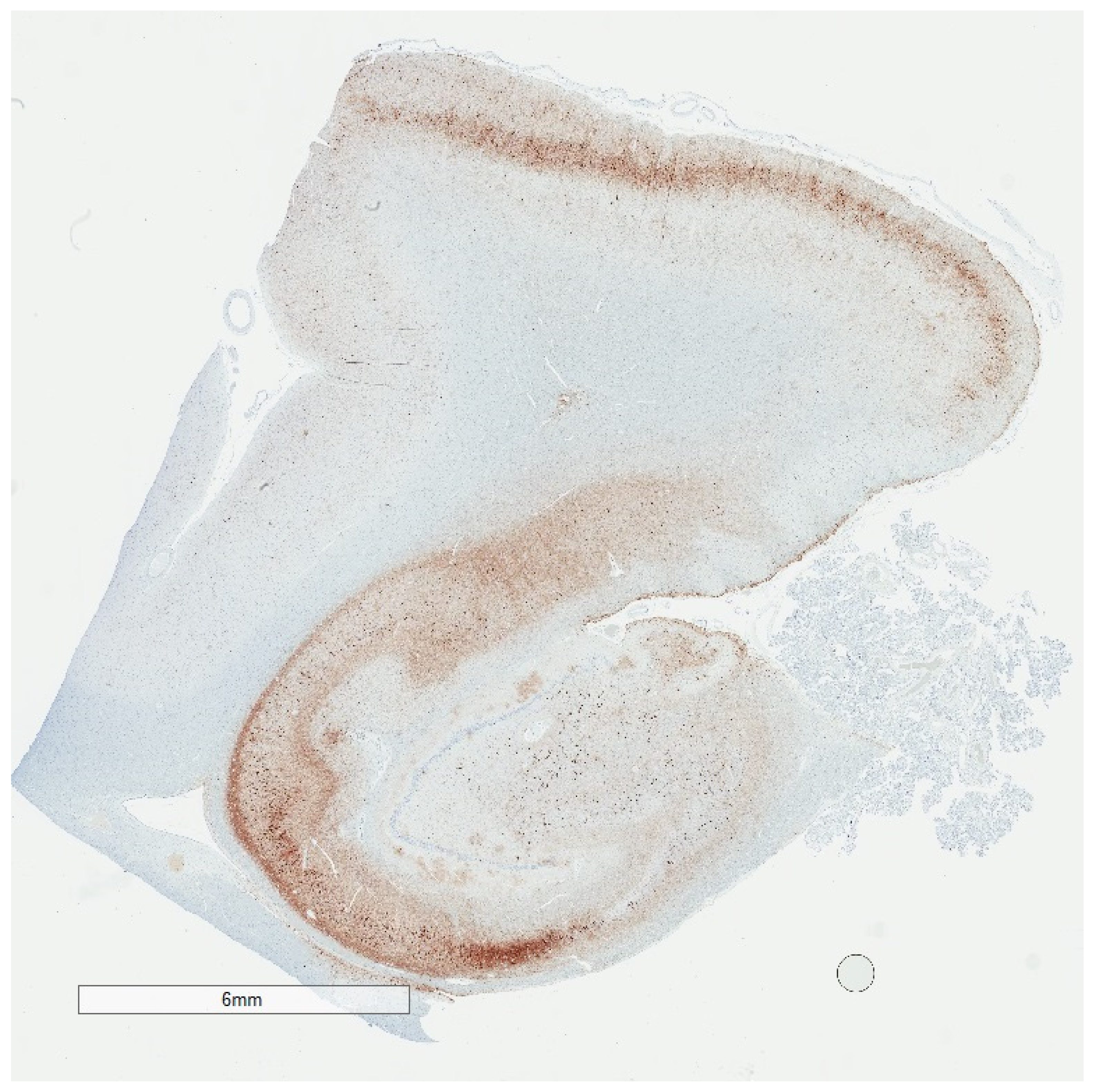
| Neurodegenerative Tauopathies | Subclinical Tauopathies |
|---|---|
| Progressive supranuclear palsy | Primary age-related tauopathy |
| Corticobasal degeneration | Aging-related tau astrogliopathy |
| Pick disease | Argyrophilic grain disease |
| Chromosome-17-linked dementia | Chronic traumatic encephalopathy |
| Guamanian dementia |
| Neurofibrillary Tangle |
|---|
| Ghost (extracellular) tangle |
| Pretangle |
| Dystrophic neurite |
| Neuropil thread |
| Grain |
| Pick body |
| Tufted astrocyte |
| Equivocal tufted astrocyte |
| Coiled body |
| Astrocytic plaque |
| Globular glial inclusion |
| Ramified astrocyte |
| Thorny astrocyte |
| Bushy astrocyte |
| Fuzzy astrocyte |
| Tauopathy | Isoform |
|---|---|
| Pick disease | 3R |
| Parkinson-Dementia complex of Guam | 3R+4R |
| Progressive supranuclear palsy | 4R |
| Corticobasal degeneration | 4R |
| Frontotemporal dementia & Parkinsonism linked to chromosome 17 | Mixed |
| Primary age-related tauopathy | 3R+4R |
| Chronic traumatic encephalopathy | 3R+4R |
| Aging-related tau astrogliopathy | 4R |
| Argyrophilic grain disease | 4R |
| Consensus Guidelines for p-Tau Assessment at Autopsy | NIA-AA 2012 AD Consensus Guidelines | NINDS/NIBIB 2016 Consensus Criteria for CTE |
|---|---|---|
| Lower threshold of p-tau for clinical correlation? | Yes | No |
| Upper limit for sampling? | Yes | No |
| Clinical context required? | Yes | No |
| Other disease processes exclusionary? | Yes | No |
| Diagnosis implies mechanism? | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellani, R.J. The Significance of Tau Aggregates in the Human Brain. Brain Sci. 2020, 10, 972. https://doi.org/10.3390/brainsci10120972
Castellani RJ. The Significance of Tau Aggregates in the Human Brain. Brain Sciences. 2020; 10(12):972. https://doi.org/10.3390/brainsci10120972
Chicago/Turabian StyleCastellani, Rudy J. 2020. "The Significance of Tau Aggregates in the Human Brain" Brain Sciences 10, no. 12: 972. https://doi.org/10.3390/brainsci10120972
APA StyleCastellani, R. J. (2020). The Significance of Tau Aggregates in the Human Brain. Brain Sciences, 10(12), 972. https://doi.org/10.3390/brainsci10120972




