Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment
Abstract
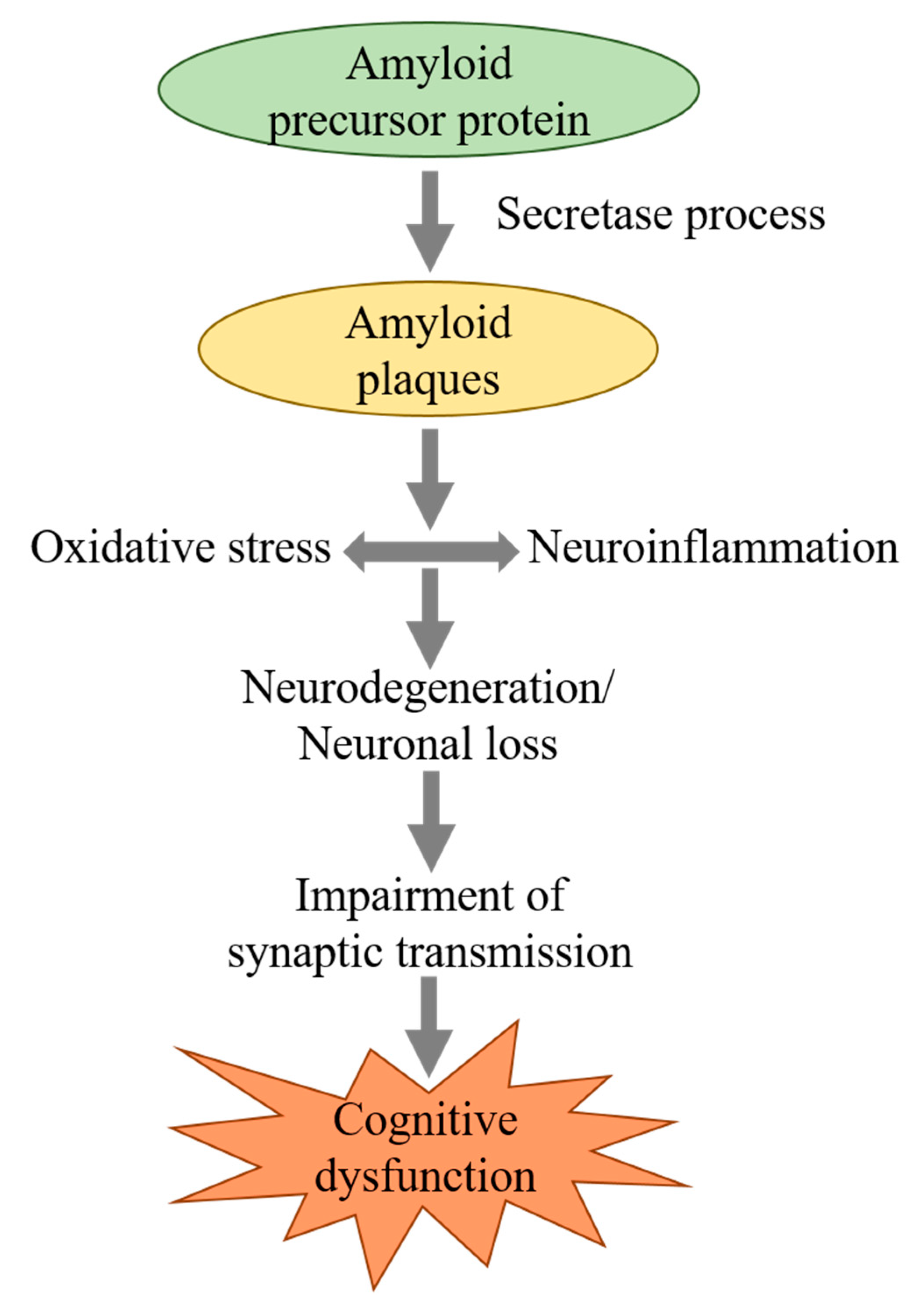
1. Introduction
2. Limitations of Currently Approved Cognition Enhancers
3. Herbal Medicines for Alzheimer’s Disease, Experimental and Clinical Evidence
3.1. Centella asiatica
3.2. Bacopa monnieri
3.3. Curcuma longa
3.4. Clitoria ternatea
3.5. Withania somnifera
3.6. Celastrus paniculatus
3.7. Evolvulus alsinoides
3.8. Desmodium gangeticum
3.9. Eclipta Species
3.10. Moringa oleifera
3.11. Convolvulus pluricaulis
4. Other Plants with Potential Memory Enhancing Activity
5. Methodology
6. Indian Herbal Formulations Studied in Alzheimer’s Disease
6.1. Mentat
6.2. Trasina
6.3. Memorin
6.4. Bramhi Ghrita
6.5. Abana
7. Herbal Drugs: Regulatory Status
8. Issues and Challenges with Herbal Drugs
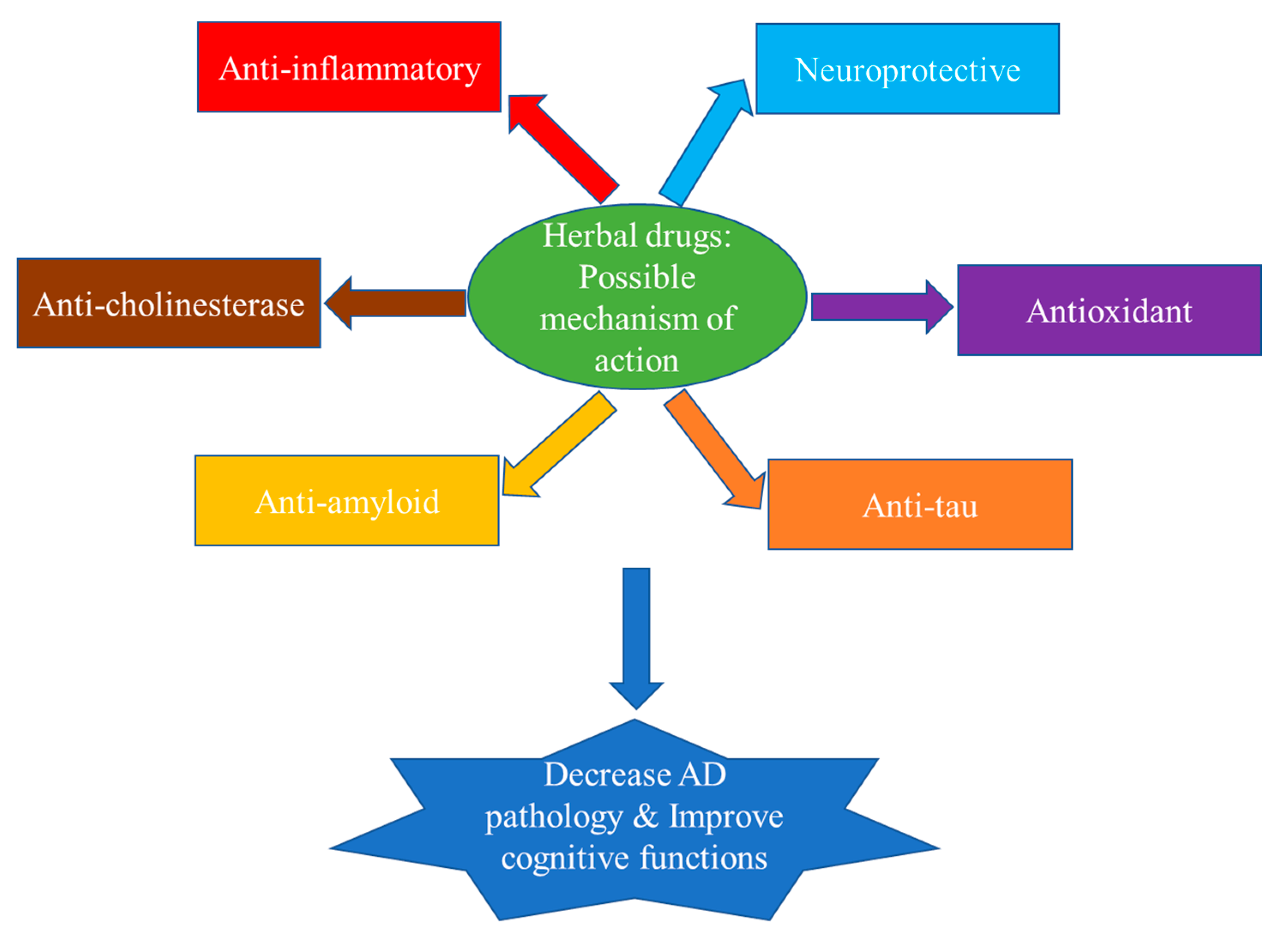
9. Conclusions and Future Prospectus
Funding
Conflicts of Interest
References
- Dua, J.S.; Prasad, D.N.; Tripathi, A.C.; Gupta, R. Role of traditional medicine in neuropsychopharmacology. Asian J. Pharm. Clin. Res. 2009, 2, 72–76. [Google Scholar]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. 2019, 4, 29. [Google Scholar] [CrossRef]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef]
- Shaji, K.S.; Jotheeswaran, A.T.; Girish, N.; Bharath, S.; Dias, A.; Pattabiraman, M.; Varghese, M. (Eds.) Alzheimer’s and Related Disorders Society of India; The Dementia India Report 2010, Prevalence, Impact, Costs and Services for Dementia; ARDSI: New Delhi, India, 2010; pp. 10–55. [Google Scholar]
- Kumar, A.; Sidhu, J.; Goyal, A.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed on 25 November 2020).
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef]
- Davies, P. Selective Loss of Central Cholinergic Neurons in Alzheimer’s Disease. Lancet 1976, 308, 1403. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Beyreuther, K.; Masters, C.L. Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease, precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1991, 1, 241–251. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G. Alzheimer’s disease, the amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Calabrese, C.; Gregory, W.L.; Leo, M.; Kraemer, D.; Bone, K.; Oken, B. Effects of a Standardized Bacopa monnieri Extract on Cognitive Performance, Anxiety, and Depression in the Elderly: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Altern. Complement. Med. 2008, 14, 707–713. [Google Scholar] [CrossRef]
- Mehla, J.; Pahuja, M.; Dethe, S.M.; Agarwal, A.; Gupta, Y. Amelioration of intracerebroventricular streptozotocin induced cognitive impairment by Evolvulus alsinoides in rats: In vitro and in vivo evidence. Neurochem. Int. 2012, 61, 1052–1064. [Google Scholar] [CrossRef]
- Bruce, A.J.; Malfroy, B.; Baudry, M. beta-Amyloid toxicity in organotypic hippocampal cultures: Protection by EUK-8, a synthetic catalytic free radical scavenger. Proc. Natl. Acad. Sci. USA 1996, 93, 2312–2316. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Sos, M.; Omar, R.A.; Bick, R.J.; Hickson-Bick, D.L.M.; Reiter, R.J.; Efthimiopoulos, S.; Robakis, N.K. Melatonin Prevents Death of Neuroblastoma Cells Exposed to the Alzheimer Amyloid Peptide. J. Neurosci. 1997, 17, 1683–1690. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, R.; Tang, Y.; Wen, S.; Wang, D.; Qi, J. Fuzhisan, a Chinese Herbal Medicine, Inhibits Beta-Amyloid-Induced Neurotoxicity and Tau Phosphorylation Through Calpain/Cdk5 Pathway in Cultured Cortical Neurons. Neurochem. Res. 2011, 36, 801–811. [Google Scholar] [CrossRef]
- Peterson, D.W.; George, R.C.; Scaramozzino, F.; LaPointe, N.E.; Anderson, R.A.; Donald, J.G.; John, L. Cinnamon extract inhibits tau aggregation associated with Alzheimer’s disease in vitro. J. Alzheimers Dis. 2009, 17, 585–597. [Google Scholar] [CrossRef]
- Dou, K.-X.; Tan, M.-S.; Tan, C.-C.; Cao, X.-P.; Hou, X.-H.; Guo, Q.-H.; Tan, L.; Mok, V.; Yu, J.-T. Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer’s disease: A network meta-analysis of 41 randomized controlled trials. Alzheimer’s Res. 2018, 10, 126. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ohnishi, T.; Nakagawa, R.; Yoshizawa, K. The comparative efficacy and safety of cholinesterase inhibitors in patients with mild-to-moderate Alzheimer’s disease: A Bayesian network meta-analysis. Int. J. Geriatr. Psychiatry 2016, 31, 892–904. [Google Scholar] [CrossRef]
- Inglis, F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int. J. Clin. Pract. Suppl. 2002, 127, 45–63. [Google Scholar]
- Quinn, J.; Kaye, J.; Montine, T.; Stackman, R. Phytochemicals in Alzheimer disease, the development of clinical trials. Pharm. Biol. 2004, 42, 64–73. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Li, Y.; Shi, X.; Ma, C. An overview on therapeutics attenuating amyloid β level in Alzheimer’s disease: Targeting neurotransmission, inflammation, oxidative stress and enhanced cholesterol levels. Am. J. Transl. Res. 2016, 8, 246–269. [Google Scholar]
- Bagi, Z.; Csekő, C.; Toth, E.; Koller, A. Oxidative stress-induced dysregulation of arteriolar wall shear stress and blood pressure in hyperhomocysteinemia is prevented by chronic vitamin C treatment. Am. J. Physiol. Circ. Physiol. 2003, 285, H2277–H2283. [Google Scholar] [CrossRef][Green Version]
- Cole, G.M.; Morihara, T.; Lim, G.P.; Yang, F.; Begum, A.; Frautschy, S.A. NSAID and antioxidant prevention of Alzheimer’s disease, lessons from in vitro and animal models. Ann. N. Y. Acad. Sci. 2004, 1035, 68–84. [Google Scholar] [CrossRef]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.M.; Breteler, M.M.B. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002, 287, 3223–3229. [Google Scholar] [CrossRef]
- Esposito, E.; Rotilio, D.; Di Matteo, V.; Di Giulio, C.; Cacchio, M.; Algeri, S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging 2002, 23, 719–735. [Google Scholar] [CrossRef]
- Moore, A.H.; O’Banion, M.K. Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2002, 54, 1627–1656. [Google Scholar] [CrossRef]
- Pavlik, V.N.; Doody, R.S.; Rountree, S.D.; Darby, E.J. Vitamin E use is associated with improved survival in an Alzheimer’s disease cohort. Dement. Geriatr. Cogn. Disord. 2009, 28, 536–540. [Google Scholar] [CrossRef]
- Yoon, B.-K.; Kim, D.K.; Kang, Y.; Kim, J.-W.; Shin, M.-H.; Na, D.L. Hormone replacement therapy in postmenopausal women with Alzheimer’s disease: A randomized, prospective study. Fertil. Steril. 2003, 79, 274–280. [Google Scholar] [CrossRef]
- Cummings, J.L. Alzheimer’s disease. N. Engl. J. Med. 2004, 351, 56–67. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C.; et al. Memantine for the Treatment of Dementia: A Review on its Current and Future Applications. J. Alzheimers Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef]
- Bullock, R. Efficacy and Safety of Memantine in Moderate-to-Severe Alzheimer Disease: The Evidence to Date. Alzheimer Dis. Assoc. Disord. 2006, 20, 23–29. [Google Scholar] [CrossRef]
- Grossberg, G.T.; Thomas, S.J. Memantine: A review of studies into its safety and efficacy in treating Alzheimer’s disease and other dementias. Clin. Interv. Aging 2009, 4, 367–377. [Google Scholar] [CrossRef]
- Youdim, K.A.; Josepha, J. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: A multiplicity of effects. Free Radic. Biol. Med. 2001, 30, 583–594. [Google Scholar] [CrossRef]
- Perry, N.; Court, G.; Bidet, N.; Court, J.; Perry, E. European Herbs with Cholinergic Activities: Potential in Dementia Therapy. Int. J. Geriatr. Psychiatry 1996, 11, 1063–1069. [Google Scholar] [CrossRef]
- Perry, N.S.; Bollen, C.; Perry, E.K.; Ballard, C. Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial. Pharm. Biochem. Behav. 2003, 75, 651–659. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease, a double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Manyam, B.V. Dementia in Ayurveda. J. Altern. Complement. Med. 1999, 5, 81–88. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br. J. Pharm. 2005, 144, 961–971. [Google Scholar] [CrossRef]
- Abourjaily, P. American Herbal Pharmacopoeia and Therapeutic Compendium (A botanical supplement monograph series). Nutr. Clin. Care 2001, 4, 221–222. [Google Scholar] [CrossRef]
- Holcomb, L.A.; Dhanasekaran, M.; Hitt, A.R.; Young, K.A.; Riggs, M.; Manyam, B.V. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J. Alzheimer’s Dis. 2006, 9, 243–251. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Withanoside IV and its active metabolite, sominone, attenuate Ab (25–35)-induced neurodegeneration. Eur. J. Neurosci. 2006, 23, 1417–1426. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, Y. Antioxidant property of Celastrus paniculatus Willd: A possible mechanism in enhancing cognition. Phytomedicine 2002, 9, 302–311. [Google Scholar] [CrossRef]
- Shinomol, G.K.; Bharath, M.M. Exploring the Role of “Brahmi” (Bacopa monnieri and Centella asiatica) in Brain Function and Therapy. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011, 5, 33–49. [Google Scholar] [CrossRef]
- Uabundit, N.; Wattanathorn, J.; Mucimapura, S.; Ingkaninan, K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J. Ethnopharmacol. 2010, 127, 26–31. [Google Scholar] [CrossRef]
- Kumar, M.H.V.; Gupta, Y. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin. Exp. Pharm. Physiol. 2003, 30, 336–342. [Google Scholar] [CrossRef]
- Yancheva, S.; Ihl, R.; Nikolova, G.; Panayotov, P.; Schlaefke, S.; Hoerr, R. GINDON Study Group. Ginkgo biloba extract EGb 761®, donepezil or both combined in the treatment of Alzheimer’s disease with neuropsychiatric features: A randomised, double-blind, exploratory trial. Aging Ment. Health 2009, 13, 183–190. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef]
- Rao, K.G.M.; Rao, S.M.; Rao, S.G. Centella asiatica (L.) Leaf Extract Treatment during the Growth Spurt Period Enhances Hippocampal CA3 Neuronal Dendritic Arborization in Rats. Evid. Based Complement. Altern. Med. 2006, 3, 349–357. [Google Scholar] [CrossRef]
- Kumar, M.V.; Gupta, Y. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J. Ethnopharmacol. 2002, 79, 253–260. [Google Scholar] [CrossRef]
- Rao, S.B.; Chetana, M.; Devi, P.U. Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol. Behav. 2005, 86, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Singh, S.; Patwardhan, K.; Gehlot, S.; Gambhir, I.S. Effect of Centella asiatica on mild cognitive impairment (MCI) and other common age-related clinical problems. Dig. J. Nanomat. Biostruct. 2008, 3, 215–220. [Google Scholar]
- Dhanasekaran, M.; Holcomb, L.A.; Hitt, A.R.; Tharakan, B.; Porter, J.W.; Young, K.A.; Manyam, B.V. Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer’s disease animal model. Phytother. Res. 2009, 23, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Nalini, K.; Karanth, K.S.; Rao, A.; Aroor, A.R. Effects of Celastrus paniculatus on passive avoidance performance and biogenic amine turnover in albino rats. J. Ethnopharmacol. 1995, 47, 101–108. [Google Scholar] [CrossRef]
- Sakina, M.R.; Dandiya, P.C. A psycho-neuropharmacological profile of Centella asiatica extract. Fitoterapia 1990, 61, 291–296. [Google Scholar]
- Soumyanath, A.; Zhong, Y.P.; Gold, S.A.; Yu, X.; Koop, D.R.; Bourdette, D.; Gold, B.G. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in vitro. J. Pharm. Pharmacol. 2005, 57, 1221–1229. [Google Scholar] [CrossRef]
- Kandel, E.R. The Molecular Biology of Memory Storage: A Dialogue between Genes and Synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef]
- Yamamoto-Sasaki, M.; Ozawa, H.; Saito, T.; Rösler, M.; Riederer, P. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res. 1999, 824, 300–303. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, Z.; Khan, I.; Luo, Y. Gotu Kola (Centella Asiatica) Extract Enhances Phosphorylation of Cyclic AMP Response Element Binding Protein in Neuroblastoma Cells Expressing Amyloid Beta Peptide. J. Alzheimer’s Dis. 2008, 13, 341–349. [Google Scholar] [CrossRef]
- Hausen, B.M. Centella asiatica (Indian pennywort), an effective therapeutic but a weak sensitizer. Contact Dermat. 1993, 29, 175–179. [Google Scholar] [CrossRef]
- James, J.T.; Dubery, I.A. Pentacyclic Triterpenoids from the Medicinal Herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.S.; Aslam, H.; Ali, S.T.; Khan, S.; Begum, S. Chemical constituents of Centella asiatica. J. Asian Nat. Prod. Res. 2007, 9, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, S.R.; Sung, S.H.; Lim, D.; Kim, H.; Choi, H.; Park, H.K.; Je, S.; Ki, Y.C. Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity. Res. Commun. Mol. Pathol. Pharmacol. 2000, 108, 75–86. [Google Scholar] [PubMed]
- Kim, S.R.; Koo, K.A.; Lee, M.K.; Park, H.-G.; Jew, S.-S.; Cha, K.-H.; Kim, Y.C. Asiatic acid derivatives enhance cognitive performance partly by improving acetylcholine synthesis. J. Pharm. Pharm. 2004, 56, 1275–1282. [Google Scholar] [CrossRef]
- Orhan, I.E. Centella asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evid. Based Complement. Altern. Med. 2012, 2012, 946259. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Mator, L.; Muchimapura, S.; Tongun, T.; Pasuriwong, O.; Piyawatkul, N.; Yimtae, K.; Sripanidkulchai, B.; Singkhoraard, J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J. Ethnopharmacol. 2008, 116, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Karting, T. Herbs, Spices and Medicinal Plants; Cracker, L.E., Simon, J.E., Eds.; Oryx Press: Phoenix, AZ, USA, 1998; Volume 3, pp. 145–173. [Google Scholar]
- Chivapat, S.; Chavalittumrong, P.; Attawish, A.; Boonruad, T.; Bansiddhi, J.; Phadungpat, S.; Punyamong, S.; Mingmuang, J. Toxicity study of Centella asiatica (L) urban. J. Thai Trad Alt Med. 2004, 2, 3–17. [Google Scholar]
- Oruganti, M.; Kumar Roy, B.; Kumar Singh, K.; Prasad, R.; Kumar, S. Safety assessment of Centella asiatica in albino rats. Phcog. J. 2010, 2, 5–11. [Google Scholar] [CrossRef]
- Aguiar, S.; Borowski, T. Neuropharmacological Review of the Nootropic Herb Bacopa monnieri. Rejuvenation Res. 2013, 16, 313–326. [Google Scholar] [CrossRef]
- Chaudhari, K.S.; Tiwari, N.R.; Tiwari, R.R.; Sharma, R.S. Neurocognitive effect of nootropic drug Brahmi (Bacopa monnieri) in Alzheimer’s disease. Ann. Neurosci. 2017, 24, 111–122. [Google Scholar] [CrossRef]
- Maheshwari, K.K.; Singh, M. Effect of bacosides, alcoholic extract of Bacopa monniera Linn. (brahmi), on experimental amnesia in mice. Indian J. Exp. Boil. 2005, 43, 640–645. [Google Scholar]
- Singh, M.; Murthy, V.; Ramassamy, C. Modulation of Hydrogen Peroxide and Acrolein-Induced Oxidative Stress, Mitochondrial Dysfunctions and Redox Regulated Pathways by the Bacopa Monniera Extract: Potential Implication in Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 21, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Parle, M. Brahmi rasayana Improves Learning and Memory in Mice. Evid. Based Complement. Altern. Med. 2006, 3, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.K.; Dhawan, B.N. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi). Indian J. Pharmacol. 1997, 29, S359–S365. [Google Scholar]
- Srinath, S. Memory enhancing medicinal herbs. J. Pharm. Sci. Res. 2014, 6, 331. [Google Scholar]
- Rastogi, M.; Ojha, R.P.; Prabu, P.C.; Devi, B.P.; Agrawal, A.; Dubey, G.P. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology 2012, 13, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Preethi, J.; Singh, H.K.; Charles, P.D.; Rajan, K.E. Participation of microRNA 124-CREB pathway: A parallel memory enhancing mechanism of standardised extract of Bacopa monniera (BESEB CDRI-08). Neurochem. Res. 2012, 37, 2167–2177. [Google Scholar] [CrossRef]
- Rajan, K.E.; Singh, H.K.; Parkavi, A.; Charles, P.D. Attenuation of 1-(m-Chlorophenyl)-Biguanide Induced Hippocampus-Dependent Memory Impairment by a Standardised Extract of Bacopa monniera (BESEB CDRI-08). Neurochem. Res. 2011, 36, 2136–2144. [Google Scholar] [CrossRef]
- Pandey, S.P.; Singh, H.K.; Prasad, S.B. Alterations in Hippocampal Oxidative Stress, Expression of AMPA Receptor GluR2 Subunit and Associated Spatial Memory Loss by Bacopa monnieri Extract (CDRI-08) in Streptozotocin-Induced Diabetes Mellitus Type 2 Mice. PLoS ONE 2015, 10, e0131862. [Google Scholar] [CrossRef]
- Jyoti, A.; Sethi, P.; Sharma, D. Bacopa monniera prevents from aluminium neuro- toxicity in the cerebral cortex of rat brain. J. Ethnopharmacol. 2007, 111, 56–62. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Kumar, A.; Ghosal, S. Effect of Bacopa monniera on animal models of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. In Molecular Aspects of Asian Medicines; Siva Sankar, D.V., Ed.; PJD Publications: New York, NY, USA, 2000. [Google Scholar]
- Saini, N.; Singh, D.; Sandhir, R. Neuroprotective Effects of Bacopa monnieri in Experimental Model of Dementia. Neurochem. Res. 2012, 37, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Vollala, V.R.; Upadhya, S.; Nayak, S. Enhanced dendritic arborization of hippocampal CA3 neurons by Bacopa monniera extract treatment in adult rats. Romanian J. Morphol. Embryol. 2011, 52, 879–886. [Google Scholar]
- Nathan, P.J.; Clarke, J.; Lloyd, J.; Hutchison, C.W.; Downey, L.; Stough, C. The acute effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy normal subjects. Hum. Psychopharmacol. Clin. Exp. 2001, 16, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Roodenrys, S.; Booth, D.; Bulzomi, S.; Phipps, A.; Micallef, C.; Smoker, J. Chronic Effects of Brahmi (Bacopa monnieri) on Human Memory. Neuropsychopharmacol. 2002, 27, 279–281. [Google Scholar] [CrossRef]
- Stough, C.; Lloyd, J.; Clarke, J.; Downey, L.A.; Hutchison, C.W.; Rodgers, T.; Nathan, P.J. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacol 2001, 156, 481–484. [Google Scholar] [CrossRef]
- Raghav, S.; Singh, H.; Dalal, P.K.; Srivastawa, J.S.; Asthana, O.P. Randomized controlled trial of standardized Bacopa monniera extract in age associated memory impairment. Indian J. Psychiatry. 2006, 48, 238–242. [Google Scholar]
- Morgan, A.; Stevens, J. Does Bacopa monnieri Improve Memory Performance in Older Persons? Results of a Randomized, Placebo-Controlled, Double-Blind Trial. J. Altern. Complement. Med. 2010, 16, 753–759. [Google Scholar] [CrossRef]
- Sharma, R.; Chaturvedi, C.; Tewari, P.V. Efficacy of Bacopa monnieri in revitalizing intellectual functions in children. J. Res. Educ. Indian Med. 1987, 1, 12. [Google Scholar]
- Negi, K.S.; Singh, Y.D.; Kushwaha, K.P.; Rastogi, C.K.; Rathi, A.K.; Srivastava, J.S. Clinical evaluation of memory enhancing properties of Memory Plus in children with attention deficit hyperactivity disorder. Indian J. Psychiatry. 2000, 42, 42–50. [Google Scholar]
- Martis, G.; Rao, A.; Karanth, K.S. Neuropharmacological activity of Herpestis monniera. Fitoterapia 1992, 63, 399–404. [Google Scholar]
- Majeed, M.; Badmaev, V.; Murrray, F. Turmeric and the Healing Curcuminoids; Keats Publishing, Inc.: New Canaan, CT, USA, 1996. [Google Scholar]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Russo, L.L.; De Lillo, A.; Laino, L.; Muzio, L.L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharm. 2020, 11. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Correction to Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2013, 8, 76–103. [Google Scholar] [CrossRef]
- Mourtas, S.; Canovi, M.; Zona, C.; Aurilia, D.; Niarakis, A.; La Ferla, B.; Salmona, M.; Nicotra, F.; Gobbi, M.; Antimisiaris, S.G. Curcumin-decorated nanoliposomes with very high affinity for amyloid-β1-42 peptide. Biomaterials 2011, 32, 1635–1645. [Google Scholar] [CrossRef]
- Taylor, M.; Moore, S.; Mourtas, S.; Niarakis, A.; Re, F.; Zona, C.; La Ferla, B.; Nicotra, F.; Masserini, M.; Antimisiaris, S.G.; et al. Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer’s Aβ peptide. Nanomedicine 2011, 7, 541–550. [Google Scholar] [CrossRef]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A. ApoE3 Mediated Poly(butyl) Cyanoacrylate Nanoparticles Containing Curcumin: Study of Enhanced Activity of Curcumin against Beta Amyloid Induced Cytotoxicity Using In Vitro Cell Culture Model. Mol. Pharm. 2010, 7, 815–825. [Google Scholar] [CrossRef]
- Caesar, I.; Jonson, M.; Nilsson, K.P.R.; Thor, S.; Hammarström, P. Curcumin Promotes A-beta Fibrillation and Reduces Neurotoxicity in Transgenic Drosophila. PLoS ONE 2012, 7, e31424. [Google Scholar] [CrossRef]
- Xiong, Z.; Hongmei, Z.; Lu, S.; Yu, L. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s disease. Pharm. Rep. 2011, 63, 1101–1108. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, H.; Tota, S.; Hanif, K.; Nath, C.; Shukla, R. Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life Sci. 2010, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Frautschy, S.A.; Hu, W.; Kim, P.; Miller, S.A.; Chu, T.; Harris-White, M.E.; Cole, G.M. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging 2002, 22, 993–1005. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Teng, Z.; Zhang, T.; Li, Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur. J. Pharm. 2014, 740, 312–320. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, S.Y.; Kim, J.Y. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1-42) insult. Neurosci. Lett. 2001, 303, 57–61. [Google Scholar] [CrossRef]
- Ramassamy, C. Faculty Opinions recommendation of Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. Fac. Opin. Post-Publ. Peer Rev. Biomed. Lit. 2008, 102, 1095–1104. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system, relevance to Alzheimer’s disease. J. Alzheimers Dis. 2005, 7, 45–61. [Google Scholar] [CrossRef]
- Schubert, M.; Brazil, D.P.; Burks, D.J.; Kushner, J.A.; Ye, J.; Flint, C.L.; Farhang-Fallah, J.; Dikkes, P.; Warot, X.M.; Rio, C.; et al. Insulin Receptor Substrate-2 Deficiency Impairs Brain Growth and Promotes Tau Phosphorylation. J. Neurosci. 2003, 23, 7084–7092. [Google Scholar] [CrossRef]
- Schubert, M.; Gautam, D.; Surjo, D.; Ueki, K.; Baudler, S.; Schubert, D.; Kondo, T.; Alber, J.; Galldiks, N.; Küstermann, E.; et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 2004, 101, 3100–3105. [Google Scholar] [CrossRef]
- Isik, A.T.; Celik, T.; Ulusoy, G.K.; Ongoru, O.; Elibol, B.; Doruk, H.; Bozoglu, E.; Kayir, H.; Mas, M.R.; Akman, S. Curcumin ameliorates impaired insulin/IGF signalling and memory deficit in a streptozotocin-treated rat model. AGE 2008, 31, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Mishra, B.; Tyagi, E.; Nath, C.; Shukla, R. Effect of curcumin on brain insulin receptors and memory functions in STZ (ICV) induced dementia model of rat. Pharm. Res. 2010, 61, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ishrat, T.; Hoda, M.N.; Khan, M.B.; Yousuf, S.; Ahmad, M.; Khan, M.M.; Ahmad, A.; Islam, F. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT). Eur. Neuropsychopharmacol. 2009, 19, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, M.V.; Aksenov, M.Y.; Butterfield, D.A.; Carney, J.M. alpha-1-antichymotrypsin interaction with a beta (1-40) inhibits fibril formation but does not affect the peptide toxicity. Neurosci. Lett. 1996, 211, 45–48. [Google Scholar] [CrossRef]
- Shoji, M.; Hirai, S.; Yamaguchi, H.; Harigaya, Y.; Ishiguro, K.; Matsubara, E. Alpha 1-antichymotrypsin is present in diffuse senile plaques. A comparative study of beta-protein and alpha 1-antichymotrypsin immunostaining in the Alzheimer brain. Am. J. Pathol. 1991, 138, 247–257. [Google Scholar]
- Beffert, U.; Cohn, J.S.; Petit-Turcotte, C.; Tremblay, M.; Aumont, N.; Ramassamy, C.; Davignon, J.; Poirier, J. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer’s disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999, 843, 87–94. [Google Scholar] [CrossRef]
- Weisgraber, K.H.; Mahley, R.W. Human apolipoprotein E, the Alzheimer’s disease connection. FASEB J. 1996, 10, 1485–1494. [Google Scholar] [CrossRef]
- Wisniewski, T.; Castano, E.M.; Golabek, A.; Vogel, T.; Frangione, B. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am. J. Pathol. 1994, 145, 1030–1035. [Google Scholar]
- Wisniewski, T.; Frangione, B. Apolipoprotein E: A pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci. Lett. 1992, 135, 235–238. [Google Scholar] [CrossRef]
- Friedlich, A.L.; Butcher, L.L. Involvement of free oxygen radicals in beta-amyloidosis, an hypothesis. Neurobiol. Aging 1994, 15, 443–455. [Google Scholar] [CrossRef]
- Hensley, K.; Carney, J.M.; Mattson, M.P.; Aksenova, M.; Harris, M.; Wu, J.F.; Floyd, R.A.; Butterfield, D.A. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: Relevance to Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 3270–3274. [Google Scholar] [CrossRef] [PubMed]
- Soudamini, K.K.; Unnikrishnan, M.C.; Soni, K.B.; Kuttan, R. Inhibition of lipid peroxidation and cholesterol levels in mice by curcumin. Indian J. Physiol. Pharmacol. 1992, 36, 239–243. [Google Scholar] [PubMed]
- Ahmed, T.; Gilani, A.H. A comparative study of curcuminoids to measure their effect on inflammatory and apoptotic gene expression in an Aβ plus ibotenic acid-infused rat model of Alzheimer’s disease. Brain Res. 2011, 1400, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Enam, S.A.; Gilani, A.H. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience 2010, 169, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Naidu, P.; Seghal, N.; Padi, S. Effect of Curcumin on Intracerebroventricular Colchicine-Induced Cognitive Impairment and Oxidative Stress in Rats. J. Med. Food 2007, 10, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, D.; Li, S.; Li, G.; Shyamala, S.G.; Barish, P.A.; Vernon, M.M.; Pan, J.; Ogle, W.O. Curcumin reverses impaired cognition and neuronal plasticity induced by chronic stress. Neuropharmacol 2009, 57, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, H.; Li, J.; Zhang, Y.; Han, B.; Zeng, Z.; Qiao, N.; Cui, X.; Lou, J.; Li, J. Amelioration of β-amyloid-induced cognitive dysfunction and hippocampal axon degeneration by curcumin is associated with suppression of CRMP-2 hyperphosphorylation. Neurosci. Lett. 2013, 557, 112–117. [Google Scholar] [CrossRef]
- Yin, H.L.; Wang, Y.L.; Lin, J.F.; Han, B.; Zhang, X.X.; Wang, Y.T.; Geng, S. Effects of curcumin on hippocampal expression of NgR and axonal regeneration in Abeta-induced cognitive disorder rats. Genet. Mol. Res. 2014, 13, 2039–2047. [Google Scholar] [CrossRef]
- Baum, L.; Alex, N.G. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimers Dis. 2004, 6, 367–377. [Google Scholar] [CrossRef]
- McClure, R.; Ong, H.; Janve, V.; Barton, S.; Zhu, M.; Li, B.; Dawes, M.; Jerome, W.G.; Anderson, A.; Massion, P.; et al. Aerosol Delivery of Curcumin Reduced Amyloid-β Deposition and Improved Cognitive Performance in a Transgenic Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 55, 797–811. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Qadri, N.M.; Ahmad, S.; Qureshi, S.; Badar, Y. Acute toxicological evaluation of the aqueous extract of Eclipta alba Hassk. Pak. J. Sci. Ind. Res. 2001, 44, 38–41. [Google Scholar]
- Mukherjee, P.K.; Kumar, V.; Kumar, N.S.; Heinrich, M. The Ayurvedic medicine Clitoria ternatea—From traditional use to scientific assessment. J. Ethnopharmacol. 2008, 120, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Murthy, K.; Karanth, K.; Nalini, K.; Rao, M.; Srinivasan, K. Clitoria ternatea root extract enhances acetylcholine content in rat hippocampus. Fitoterapia 2002, 73, 685–689. [Google Scholar] [CrossRef]
- Taranalli, A.D.; Cheeramkhuzhy, T.C. Influence of Clitoria ternatea extracts on memory and central cholinergic activity in rats. Pharm Biol. 2000, 38, 51–56. [Google Scholar] [CrossRef]
- Rai, K.S.; Murthy, K.D.; Karanth, K.S.; Rao, M.S. Clitoria ternatea (Linn) root extract treatment during growth spurt period enhances learning and memory in rats. Indian J. Physiol. Pharmacol. 2001, 45, 305–313. [Google Scholar] [PubMed]
- Rai, K.S.; Murthy, K.D.; Rao, M.S.; Karanth, K.S. Altered dendritic arborization of amygdale neurons in young adult rats orally intubated with Clitoria ternatea aqueous root extract. Phytother. Res. 2005, 19, 592–598. [Google Scholar] [CrossRef]
- Rai, K.S. Neurogenic potential of Clitoria ternatea aqueous root extract-a basis for enhancing learning and memory. World Acad. Sci. Eng. Technol. 2010, 46, 237–242. [Google Scholar]
- Damodaran, T.; Cheah, P.S.; Murugaiyah, V.; Hassan, Z. The nootropic and anticholinesterase activities of Clitoria ternatea Linn. root extract: Potential treatment for cognitive decline. Neurochem. Int. 2020, 139, 104785. [Google Scholar] [CrossRef]
- Mehla, J.; Pahuja, M.; Gupta, Y.K. Streptozotocin-induced sporadic Alzheimer’s disease: Selection of appropriate dose. J Alzheimers Dis. 2012, 33, 17–21. [Google Scholar] [CrossRef]
- Taur, D.J.; Patil, R.Y. Evaluation of antiasthmatic activity of Clitoria ternatea L. roots. J. Ethnopharmacol. 2011, 136, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Bone, K. Clinical Applications of Ayurvedic and Chinese Herbs. Monographs for the Western Herbal Practitioner; Phytotherapy Press: Queensland, Australia, 1996; pp. 137–141. [Google Scholar]
- Chatterjee, A.; Pakrashi, S.C. The Treatise on Indian Medicinal Plants. Council for Scientific and Industrial Research; Publications & Information Directorate: New Delhi, India, 1995; Volume 4, pp. 208–212. [Google Scholar]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal Lactones from Withania somnifera, an Ancient Plant for Novel Medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.C.; Singh, B.B.; Dagenais, S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): A review. Altern. Med. Rev. 2000, 5, 334–346. [Google Scholar]
- Kumar, V.; Dey, A.; Hadimani, M.B.; Marcović, T.; Emerald, M. Chemistry and pharmacology of Withania somnifera: An update. Tang (Humanit. Med.) 2015, 5, e1. [Google Scholar] [CrossRef]
- Malhotra, C.L.; Mehta, V.L.; Das, P.K.; Dhalla, N.S. Studies on Withania-ashwagandha, Kaul. V. The effect of total alkaloids (ashwagandholine) on the central nervous system. Indian J. Physiol. Pharmacol. 1965, 9, 127–136. [Google Scholar]
- Parihar, M.; Chaudhary, M.; Shetty, R.; Hemnani, T. Susceptibility of hippocampus and cerebral cortex to oxidative damage in streptozotocin treated mice: Prevention by extracts of Withania somnifera and Aloe vera. J. Clin. Neurosci. 2004, 11, 397–402. [Google Scholar] [CrossRef]
- Jain, S.; Shukla, S.D.; Sharma, K.; Bhatnagar, M. Neuroprotective effects of Withania somnifera Dunn. In hippocampal sub-regions of female albino rat. Phytother. Res. 2001, 15, 544–548. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Kumar, A.; Ghosal, S. Effects of glycowithanolides from Withania somnifera on an animal model of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. Phytother. Res. 1995, 9, 110–113. [Google Scholar] [CrossRef]
- Schliebs, R.; Liebmann, A.; Bhattacharya, S.K.; Kumar, A.; Ghosal, S.; Bigl, V. Systemic administration of defined extracts from Withania somnifera (Indian ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem. Int. 1997, 30, 181–190. [Google Scholar] [CrossRef]
- Sun, G.Y.; Li, R.; Cui, J.; Hannink, M.; Gu, Z.; Fritsche, K.L.; Lubahn, D.B.; Simonyi, A. Withania somnifera and Its Withanolides Attenuate Oxidative and Inflammatory Responses and Up-Regulate Antioxidant Responses in BV-2 Microglial Cells. Neuromolecular. Med. 2016, 18, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Joyashiki, E. Sominone enhances neurite outgrowth and spatial memory mediated by the neurotrophic factor receptor, RET. Br. J. Pharm. 2009, 157, 1427–1440. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Padmanabhan, K.; Nair, M.G. Withanamides in Withania somnifera fruit protect PC-12 cells from beta-amyloid responsible for Alzheimer’s disease. Phytother Res. 2010, 24, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.; Thakur, S.S.; Ravindranath, V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Konar, A.; Shah, N.; Singh, R.; Saxena, N.; Kaul, S.C.; Wadhwa, R.; Thakur, M.K. Protective Role of Ashwagandha Leaf Extract and Its Component Withanone on Scopolamine-Induced Changes in the Brain and Brain-Derived Cells. PLoS ONE 2011, 6, e27265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Harris, R.J.; Seal, C.J.; Okello, E.J. An Aqueous Extract of Withania somnifera Root Inhibits Amyloid β Fibril Formation In Vitro. Phytother. Res. 2011, 26, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Dhuley, J.N. Effect of ashwagandha on lipid peroxidation in stress-induced animals. J. Ethnopharmacol. 1998, 60, 173–178. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Evidence for free radical scavenging activity of Ashwagandha root powder in mice. Indian J. Physiol. Pharmacol. 1997, 41, 424–426. [Google Scholar]
- Pandey, A.; Bani, S.; Dutt, P.; Satti, N.K.; Suri, K.A.; Qazi, G.N. Multifunctional neuroprotective effect of Withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine 2018, 102, 211–221. [Google Scholar] [CrossRef]
- Halim, M.A.; Rosli, I.M.; Jaafar, S.S.M.; Ooi, H.; Leong, P.; Shamsuddin, S.; Najimudin, N.; Azzam, G. Withania somnifera showed neuroprotective effect and increase longevity in Drosophila Alzheimer’s disease model. bioRxiv 2020. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and Safety of Ashwagandha (Withania somnifera (L.) Dunal) Root Extract in Improving Memory and Cognitive Functions. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Loke, W.; Foo, N.X.; Tan, W.J.; Chan, H.W.; Lim, D.Y.; Yeo, W.S. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother. Res. 2019, 34, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Arseculeratne, S.N.; Gunatilaka, A.; Panabokke, R.G. Studies on medicinal plants of sri lanka. part 14: Toxicity of some traditional medicinal herbs. J. Ethnopharmacol. 1985, 13, 323–335. [Google Scholar] [CrossRef]
- Malhotra, C.L.; Mehta, V.L.; Prasad, K.; Das, P.K. Studies on Withania ashwagandha, Kaul. IV. The effect of total alkaloids on the smooth muscles. Indian J. Physiol. Pharmacol. 1965, 9, 9–15. [Google Scholar] [PubMed]
- Grandhi, A.; Mujumdar, A.; Patwardhan, B. A comparative pharmacological investigation of Ashwagandha and Ginseng. J. Ethnopharmacol. 1994, 44, 131–135. [Google Scholar] [CrossRef]
- Warrier, P.K.; Ramankutty, C.; Nambiar, V.P.K. Indian Medicinal Plants, A Compendium of 500 Species; Orient Longman Ltd.: Madras, India, 1997; Volume 2, p. 47. [Google Scholar]
- Debnath, M.; Biswas, M.; Shukla, V.K.; Nishteswar, K. Phytochemical and analytical evaluation of Jyotishmati (Celastrus paniculatus Willd.) leaf extracts. Ayu 2014, 35, 54–57. [Google Scholar] [CrossRef]
- Malik, J.; Karan, M.; Dogra, R. Ameliorating effect of Celastrus paniculatus standardized extract and its fractions on 3-nitropropionic acid induced neuronal damage in rats: Possible antioxidant mechanism. Pharm. Biol. 2017, 55, 980–990. [Google Scholar] [CrossRef]
- Ramaiah, C.V.; Kumar, G.S.; Rajendra, W. Traditional, Ethnomedical, and Pharmacological uses of Celastrus paniculatus. Asian J. Pharm. 2018, 12, S1119–S1126. [Google Scholar]
- Jakka, A.L. A study on nootropic activity of Celastrus paniculata willd whole plant methanolic extract in rats. Asian J. Pharmaceut. Clin. Res. 2016, 9, 336–341. [Google Scholar]
- Lekha, G.; Bhagya, P.; Kumar, S.; Rao, N.; Irudaya, A.; Karthik, M. Cognitive enhancement and Neuroprotective effect of Celastrus paniculatus Willd. seed oil (Jyothismati oil) on male Wistar rats. J. Pharma. Sci. Tech. 2010, 2, 130–138. [Google Scholar]
- Karanth, K.S.; Haridas, K.K.; Gunasundari, S.; Guruswami, M.N. Effect of Celastrus paniculatus on learning process. Arogya 1980, 6, 137–139. [Google Scholar]
- Gattu, M.; Boss, K.L.; Terry, A.V.; Buccafusco, J.J. Reversal of Scopolamine-Induced Deficits in Navigational Memory Performance by the Seed Oil of Celastrus paniculatus. Pharm. Biochem. Behav. 1997, 57, 793–799. [Google Scholar] [CrossRef]
- Bhanumathy, M.; Harish, M.; Shivaprasad, H.; Sushma, G. Nootropic activity of Celastrus paniculatus seed. Pharm. Biol. 2010, 48, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.S.; Marathe, P.A.; Rege, N.N.; Raut, S.B.; Parekar, R.R. Effect of Jyotiṣmatī seed oil on spatial and fear memory using scopolamine induced amnesia in mice. Anc. Sci. Life 2015, 34, 130–133. [Google Scholar] [CrossRef]
- Cervenka, F.; Koleckar, V.; Rehakova, Z.; Jahodar, L.; Kunes, J.; Opletal, L.; Hyspler, R.; Jun, D.; Kuca, K. Evaluation of natural substances from Evolvulus alsinoides L. with the purpose of determining their antioxidant potency. J. Enzym. Inhib. Med. Chem. 2008, 23, 574–578. [Google Scholar] [CrossRef]
- Chatterjee, A. Treatise of Indian Medicinal Plants; Council for Scientific and Industrial Research; Publications & Information Directorate: New Delhi, India, 1990; p. 327. [Google Scholar]
- Gomathi, D.; Kalaiselvi, M.; Ravikumar, G.; Devaki, K.; Uma, C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. J. Food Sci. Technol. 2015, 52, 1212–1217. [Google Scholar] [CrossRef]
- Auddy, B.; Ferreira, M.; Blasina, F.; Lafon, L.; Arredondo, F.; Dajas, F.; Tripathi, P.; Seal, T.; Mukherjee, B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 2003, 84, 131–138. [Google Scholar] [CrossRef]
- Ganju, L.; Karan, D.; Chanda, S.; Srivastava, K.; Sawhney, R.; Selvamurthy, W. Immunomodulatory effects of agents of plant origin. Biomed. Pharm. 2003, 57, 296–300. [Google Scholar] [CrossRef]
- Siripurapu, K.B.; Gupta, P.; Bhatia, G.; Maurya, R.; Nath, C.; Palit, G. Adaptogenic and anti-amnesic properties of Evolvulus alsinoides in rodents. Pharmacol. Biochem. Behav. 2005, 81, 424–432. [Google Scholar] [CrossRef]
- Asolkar, L.V.; Kakkar, K.K.; Chakre, O.J. Second Supplement to Glossary of India Medicinal Plants with Active Constituents; Council for Scientific and Industrial Research; Publications & Information Directorate: New Delhi, India, 1992; p. 1965. [Google Scholar]
- Nahata, A.; Patil, U.K.; Dixit, V.K. Anxiolytic activity of Evolvulus alsinoides and Convulvulus pluricaulis in rodents. Pharm Biol. 2009, 5, 444–451. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Nahata, A.; Singh, P.K.; Mishra, S. Neuropharmacological evaluation on four traditional herbs used as nervine tonic and commonly available as Shankhpushpi in India. J. Ayurveda Integr. Med. 2019, 10, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Singh, S.K.; Singh, M.; Mishra, S.S.; Singh, A.K.; Tripathi, J.S.; Tripathi, Y.B. Neuroprotective Activity of Evolvulus alsinoides & Centella asiatica Ethanolic Extracts in Scopolamine-Induced Amnesia in Swiss Albino Mice. Open Access Maced. J. Med Sci. 2019, 7, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Raghuwanshi, R.; Masood, M.; Acharya, A.; Jain, S.K. Medicinal plants with acetylcholinesterase inhibitory activity. Rev. Neurosci. 2018, 29, 491–529. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. An ethnomedicinal, phytochemical and pharmacological profile of Desmodium gangeticum (L.) DC. and Desmodium adscendens (Sw.) DC. J. Ethnopharmacol. 2011, 136, 283–296. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Chandrasekharan, S.; Balakrishna, K.; Connolly, J.D. Gangetinin and desmodin, two minor pterocarpanoids of Desmodium gangeticum. Phytochemistry. 1975, 14, 1129–1130. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P. An ethnobotanical study of medicinal plants in Chandauli District of Uttar Pradesh, India. J. Ethnopharmacol. 2009, 121, 324–329. [Google Scholar] [CrossRef]
- Mishra, P.K.; Singh, N.; Ahmad, G.; Dube, A.; Maurya, R. Glycolipids and other constituents from Desmodium gangeticum with antileishmanial and immunomodulatory activities. Bioorganic Med. Chem. Lett. 2005, 15, 4543–4546. [Google Scholar] [CrossRef]
- Joshi, H.; Parle, M. Anti-amnesic effect of Desmodium gangeticum in mice. Yakugaku Zasshi 2006, 126, 795–804. [Google Scholar] [CrossRef]
- Joshi, H.; Parle, M. Pharmacological evidences for the antiamnesic effects of Desmodium gangeticum in mice. Iran. J. Pharm. Res. 2007, 6, 199–207. [Google Scholar]
- Mahajan, K.; Kumar, D.; Kumar, S. Antiamnesic Activity of Extracts and Fraction of Desmodium Gangeticum. J. Pharm. Technol. Res. Manag. 2015, 3, 67–77. [Google Scholar] [CrossRef]
- Govindarajan, R.; Rastogi, S.; Vijayakumar, M.; Shirwaikar, A.; Rawat, A.K.S.; Mehrotra, S.; Pushpangadan, P. Studies on the antioxidant activities of Desmodium gangeticum. Biol. Pharm. Bull. 2003, 26, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.; Rao, C.; Ravishankar, B.; De, S.; Mehrotra, S. Anti-inflammatory and anti-nociceptive activity of the water decoction Desmodium gangeticum. J. Ethnopharmacol. 2004, 95, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Puri, H.S. Rasayana: Ayurvedic Herbs for Longevity and Rejuvenation: Volume 2 of Traditional Herbal Medicines for Modern Times. J. Altern. Complement. Med. 2003, 9, 331–332. [Google Scholar] [CrossRef]
- Kapoor, L.D. Handbook of Ayurvedic Medicinal Plants: Herbal Reference Library; CRC Press: New Delhi, India, 2000; p. 169. [Google Scholar]
- Ashok, D.B. The status and scope of Indian Medicinal Plants acting on Central nervous system. Indian J. Pharmacol. 1997, 29, 340–343. [Google Scholar]
- Thakur, V.; Mengi, S. Neuropharmacological profile of Eclipta alba (Linn.) Hassk. J. Ethnopharmacol. 2005, 102, 23–31. [Google Scholar] [CrossRef]
- Rajani, G.P. Prasad KVSRG. Effect of Eclipta alba Linn on learning and memory in rats. Indian J. Pharm. Educ. Res. 2007, 41, 369–372. [Google Scholar]
- Choi, Y.H.; Kim, Y.S.; Yeo, S.J.; Roh, S.H.; Jeong, Y.C.; Kang, J.S.; Ryu, S.Y. Ameliorating effect of balloon flower saponin on the ethanol-induced memory impairment in ice. Phytother. Res. 2008, 22, 973–976. [Google Scholar] [CrossRef]
- Kim, D.-I.; Lee, S.-H.; Hong, J.-H.; Lillehoj, H.S.; Park, H.-J.; Rhie, S.-G.; Lee, G.-S. The butanol fraction of Eclipta prostrata (Linn) increases the formation of brain acetylcholine and decreases oxidative stress in the brain and serum of cesarean-derived rats. Nutr. Res. 2010, 30, 579–584. [Google Scholar] [CrossRef]
- Kim, D.-I.; Lee, S.-H.; Choi, J.-H.; Lillehoj, H.S.; Yu, M.-H.; Lee, G.-S. The butanol fraction of Eclipta prostrata (Linn) effectively reduces serum lipid levels and improves antioxidant activities in CD rats. Nutr. Res. 2008, 28, 550–554. [Google Scholar] [CrossRef]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; Jayyed Press: Delhi, India, 1975. [Google Scholar]
- Rajagopal, V. Standardization of Botanicals; Eastern Publishers: New Delhi, India, 2002; Volume 1. [Google Scholar]
- Banji, O.; Banji, D.; Annamalai, A.R.; Manavalan, R. Investigation on the effect of Eclipta alba on animal models of learning and memory. Indian J. Physiol. Pharmacol. 2007, 51, 274–278. [Google Scholar]
- Singh, B.; Saxena, A.K.; Chandan, B.K.; Agarwal, S.G.; Bhatia, M.S.; Anand, K.K. Hepatoprotective effect of ethanolic extract ofEclipta alba on experimental liver damage in rats and mice. Phytother. Res. 1993, 7, 154–158. [Google Scholar] [CrossRef]
- Dhongade, H.K.J.; Paikra, B.K.; Gidwani, B. Phytochemistry and Pharmacology of Moringa oleifera Lam. J. Pharm. 2017, 20, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharm. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Jimenez, M.; AlMatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Caceres, A.; Saravia, A.; Rizzo, S.; Zabala, L.; De Leon, E.; Nave, F. Pharmacologic properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity. J. Ethnopharmacol. 1992, 36, 233–237. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.-U.-H. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry 1995, 38, 957–963. [Google Scholar] [CrossRef]
- Ghasi, S.; Nwobodo, E.; Ofili, J. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed wistar rats. J. Ethnopharmacol. 2000, 69, 21–25. [Google Scholar] [CrossRef]
- Mohan, M.; Kaul, N.; Punekar, A.; Girnar, R.; Junnare, P.; Patil, L. Nootropic activity of Moringa oleifera leaves. J. Nat. Remed. 2005, 5, 59–62. [Google Scholar]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2196–2201. [Google Scholar] [CrossRef]
- Ganguly, R.; Guha, D. Protective role of an Indian herb, Moringa oleifera in memory impairment by high altitude hypoxic exposure, Possible role of monoamines. Biog. Amines. 2006, 20, 121–133. [Google Scholar]
- Ganguly, R.; Guha, D. Alteration of brain monoamines & EEG wave pattern in rat model of Alzheimer’s disease & protection by Moringa oleifera. Indian J. Med Res. 2008, 128, 744–751. [Google Scholar] [PubMed]
- Sutalangka, C.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Moringa oleiferaMitigates Memory Impairment and Neurodegeneration in Animal Model of Age-Related Dementia. Oxidative Med. Cell. Longev. 2013, 2013, 695936. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, W.-S.; Suo, D.-Q.; Li, Y.; Peng, L.; Xu, L.-X.; Zeng, K.-Y.; Ren, T.; Wang, Y.; Zhou, Y.; et al. Moringa oleifera Seed Extract Alleviates Scopolamine-Induced Learning and Memory Impairment in Mice. Front. Pharm. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Mahaman, Y.A.R.; Huang, F.; Wu, M.; Wang, Y.; Wei, Z.; Bao, J.; Salissou, M.T.M.; Ke, D.; Wang, Q.; Liu, R.; et al. Moringa Oleifera Alleviates Homocysteine-Induced Alzheimer’s Disease-Like Pathology and Cognitive Impairments. J. Alzheimer’s Dis. 2018, 63, 1141–1159. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Mogbojuri, O.M.; Emikpe, B.O. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. J. Med. Plants Res. 2009, 3, 586–591. [Google Scholar]
- Kasolo, J.N.; Bimenya, G.S.; Ojok, L.; Ogwal-okeng, J.W. Phytochemicals and acute toxicity of Moringa oleifera roots in mice. J. Pharmacog. Phytother. 2011, 3, 38–42. [Google Scholar]
- Adams, M.; Gmünder, F.; Hamburger, M. Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. J. Ethnopharmacol. 2007, 113, 363–381. [Google Scholar] [CrossRef]
- Singh, V.K.; Ali, Z.A.; Zaidi, S.T.H.; Siddiqui, M.K. Ethnomedicinal uses of plants of Gonda district forests of Uttar Pradesh, India. Fitoterapia. 1996, 2, 129–139. [Google Scholar]
- Sethiya, N.K. An update on Shankhpushpi, a cognition-boosting Ayurvedic medicine. J. Chin. Integr. Med. 2009, 7, 1001–1022. [Google Scholar] [CrossRef]
- Ahmad, S.; Zafar, R.-U.; Shahid, M. Anticonvulsant potential of callus cultures of Convolvulus microphyllus Sieb. Orient. Pharm. Exp. Med. 2007, 7, 46–50. [Google Scholar] [CrossRef]
- Dhingra, D.; Valecha, R. Evaluation of the antidepressant-like activity of Convolvulus pluricaulis choisy in the mouse forced swim and tail suspension tests. Med. Sci. Monit. 2007, 13, BR155–BR161. [Google Scholar] [PubMed]
- Dubey, G.P.; Pathak, S.R.; Gupta, B.S. Combined effect of Brahmi (Bacopa monniera) and Shankhpushpi (Convolvulus pluricaulis) on cognitive functions. Pharmacopsychoecol 1994, 3, 249–251. [Google Scholar]
- Sharma, K.; Arora, V.; Rana, A.C.; Bhatnagar, M. Anxiolytic effect of Convolvulus pluricaulis petals on elevated plus maze model of anxiety in mice. J. Herb. Med. Toxicol. 2009, 1, 41–46. [Google Scholar]
- Nahata, A.; Patil, U.K.; Dixit, V.K. Effect of Convulvulus pluricaulis Choisy on learning behavior and memory enhancement activity in rodents. Nat. Prod. Res. 2008, 22, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.; Greig, N.H.; Holloway, H.W.; Raffaele, K.C.; Berardi, A.; Schapiro, M.B.; Rapoport, S.I.; Soncrant, T.T. Clinical pharmacokinetics of arecoline in subjects with Alzheimer’s disease. Clin. Pharm. 1996, 60, 276–282. [Google Scholar] [CrossRef]
- Mirzaev, Y.R.; Aripova, S.F. Neuro- and psychopharmacological investigation of the alkaloids convolvine and atropine. Chem. Nat. Compd. 1998, 34, 56–58. [Google Scholar] [CrossRef]
- Sharma, K.; Bhatnagar, M.; Kulkarni, S.K. Effect of Convolvulus pluricaulis Choisy and Asparagus racemosus Willd on learning and memory in young and old mice: A comparative evaluation. Indian J. Exp. Boil. 2010, 48, 479–485. [Google Scholar]
- Chaturvedi, M.; Mali, P.C.; Dixit, V.P. Hypolipidaemic effect of Convolvulus microphyllus on cholesterol fed gerbils. J. Phytol. Res. 1997, 2, 153–155. [Google Scholar]
- Bihaqi, S.W.; Sharma, M.; Singh, A.P.; Tiwari, M. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J. Ethnopharmacol. 2009, 124, 409–415. [Google Scholar] [CrossRef]
- Liu, L.-F.; Durairajan, S.S.K.; Lu, J.-H.; Koo, I.; Li, M. In vitro screening on amyloid precursor protein modulation of plants used in Ayurvedic and Traditional Chinese medicine for memory improvement. J. Ethnopharmacol. 2012, 141, 754–760. [Google Scholar] [CrossRef]
- Malik, J.; Karan, M.; Vasisht, K. Attenuating effect of bioactive coumarins from Convolvulus pluricaulis on scopolamine-induced amnesia in mice. Nat. Prod. Res. 2016, 30, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.A.; Dhuley, J.; Naik, S. Neuropharmacology of an Extract derived from Convolvulus microphyllus. Pharm. Biol. 2001, 39, 253–258. [Google Scholar] [CrossRef][Green Version]
- Bhattacharya, S.K. Nootropic effect of BR-16A (Mentat), a psychotropic herbal formulation, on cognitive deficits induced by prenatal undernutrition, postnatal environmental impoverishment and hypoxia in rats. Indian J. Exp. Boil. 1994, 32, 31–36. [Google Scholar]
- Faruqi, S.; Andrade, C.; Ramteke, S.; Joseph, J.; Venkataraman, B.V.; Rani, M.A.N. Herbal pharmacotherapy for the attenuation of electroconvulsive shock-induced anterograde and retrograde amnestic deficits. Convuls. Ther. 1995, 11, 241–247. [Google Scholar] [PubMed]
- Handa, S.S.; Bhargava, V.K. Effect of BR-16A (MentatR) on cognitive deficits in aluminium-treated and aged rats. Indian J. Pharmacol. 1997, 29, 258–261. [Google Scholar]
- Ramteke, S.; Andrade, C.; Faruqi, S.; Joseph, J.; Venkataraman, B.V.; Naga Rani, M.A. BR-16A attenuates anterograde amnesia induced by electro-convulsive shocks in slow-learning rats. Indian J. Pharmacol. 1995, 27, 186–188. [Google Scholar]
- Verma, A.; Kulkarni, S.K. Effect of a herbal psychotropic preparation, BR-16A (Mentat), on performance of mice on elevated plus-maze. Indian J. Exp. Boil. 1991, 29, 1120–1123. [Google Scholar]
- Bhattacharya, S.K.; Kumar, A.; Jaiswal, A.K. Effect of Mentat, a Herbal Formulation, on Experimental Models of Alzheimer’s Disease and Central Cholinergic Markers in Rats. Fitoterapia 1995, 3, 216. [Google Scholar]
- Agarwal, A.; Dubey, M.; Dubey, G.P. Effect of Mentat on memory, anxiety scores and neuroticism index in normal subjectsin three age groups. Probe 1991, 3, 257–261. [Google Scholar]
- Koti, S.T. Effect of Mentat on school students performance. Probe 1991, 3, 250–252. [Google Scholar]
- Jagetia, G.C.; Baliga, M.S. Treatment of mice with a herbal preparation (Mentat) protects against radiation-induced mortality. Phytother. Res. 2003, 17, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Kumar, A. Effect of Trasina®, an Ayurvedic Herbal Formulation, on Experimental Models of Alzheimer’s Disease and Central Cholinergic Markers in Rats. J. Altern. Complement. Med. 1997, 3, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Gowda, S.; Chaturvedi, S. Treatment of Age-Related Cognitive Decline with a Herbal Formulation: A Double-Blind Study. Indian J. Psychiatry 1998, 40, 240–246. [Google Scholar] [PubMed]
- Vinekar, A.S.; Andrade, C.; Sriprada, V.T.; George, J.; Joseph, T.; Chandra, J.S. Attenuation of ECS-Induced Retrograde Amnesia by Using an Herbal Formulation. J. ECT 1998, 14, 83–88. [Google Scholar] [CrossRef]
- Tripathi, B. Caraka Samhita, 3rd ed.; Chaukhamba Surbharati Prakashan: Vanarasi, India, 1994; Volume 2. [Google Scholar]
- Achliya, G.; Barabde, U.; Wadodkar, S.; Dorle, A. Effect of Bramhi Ghrita, an polyherbal formulation on learning and memory paradigms in experimental animals. Indian J. Pharmacol. 2004, 36, 159–162. [Google Scholar]
- Reddy, K.R.C.; Kumar, V.; Yadav, K.D. Beneficial effect of Brahmi Ghrita on learning and memory in normal rat. Ayu (Int. Q. J. Res. Ayurveda) 2014, 35, 325–329. [Google Scholar] [CrossRef]
- Parle, M.; Vasudevan, M. Memory Enhancing Activity of Abana®: An Indian Ayurvedic Poly-Herbal Formulation. J. Health Sci. 2007, 53, 43–52. [Google Scholar] [CrossRef]
- USFDA. Botanical Drug Development: Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/botanical-drug-development-guidance-industry (accessed on 19 October 2020).
- EMA. Human Regulatory-Herbal Medicinal Products. Available online: https://www.ema.europa.eu/en/human-regulatory/herbal-medicinal-products (accessed on 19 October 2020).
- New Drugs and Clinical Trials Rules. Available online: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/NewDrugs_CTRules_2019.pdf (accessed on 29 October 2020).
- ASU Drug Industry. Good Manufacturing Practices for Ayurvedic, Siddha and Unani Medicines; Department of AYUSH, Ministry of Health & Family Welfare, Government of India: New Delhi, India, 2014. [Google Scholar]
- Department of AYUSH. Good Clinical Trial Practices for Clinical Trials in Ayurveda, Siddha and Unani Medicine (GCP-ASU); Department of AYUSH, Ministry of Health & Family Welfare, Government of India: New Delhi, India, 2013. [Google Scholar]
- Zhou, S.-F.; Zhou, Z.-W.; Li, C.G.; Chen, X.; Yu, X.; Xue, C.C.; Herington, A.C. Identification of drugs that interact with herbs in drug development. Drug Discov. Today 2007, 12, 664–673. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Seely, D.M.R. Clinically based evidence of drug-herb interactions: A systematic review. Expert Opin. Drug Saf. 2009, 9. [Google Scholar] [CrossRef]
- Izzo, A.A.; Ernst, E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs 2009, 69, 1777–1798. [Google Scholar] [CrossRef]
- Farlow, M.R. Clinical Pharmacokinetics of Galantamine. Clin. Pharm. 2003, 42, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Shintani, E.Y.; Uchida, K.M. Donepezil: An anticholinesterase inhibitor for Alzheimer’s disease. Am. J. Health Pharm. 1997, 54, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V. Herb-Drug Interactions in Neurological Disorders: A Critical Appraisal. Curr. Drug Metab. 2018, 19, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Oboh, G.; Oyeleye, S.I.; Ogunsuyi, O. Anti-amnestic Effect of Curcumin in Combination with Donepezil, an Anticholinesterase Drug: Involvement of Cholinergic System. Neurotox. Res. 2017, 31, 560–569. [Google Scholar] [CrossRef]
- Yan, J.; Hu, J.; Liu, A.; He, L.; Li, X.; Wei, H. Design, synthesis, and evaluation of multitarget-directed ligands against Alzheimer’s disease based on the fusion of donepezil and curcumin. Bioorganic Med. Chem. 2017, 25, 2946–2955. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehla, J.; Gupta, P.; Pahuja, M.; Diwan, D.; Diksha, D. Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment. Brain Sci. 2020, 10, 964. https://doi.org/10.3390/brainsci10120964
Mehla J, Gupta P, Pahuja M, Diwan D, Diksha D. Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment. Brain Sciences. 2020; 10(12):964. https://doi.org/10.3390/brainsci10120964
Chicago/Turabian StyleMehla, Jogender, Pooja Gupta, Monika Pahuja, Deepti Diwan, and Diksha Diksha. 2020. "Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment" Brain Sciences 10, no. 12: 964. https://doi.org/10.3390/brainsci10120964
APA StyleMehla, J., Gupta, P., Pahuja, M., Diwan, D., & Diksha, D. (2020). Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment. Brain Sciences, 10(12), 964. https://doi.org/10.3390/brainsci10120964






