Somatosensory Integration and Masking of Complex Tactile Information: Peripheral and Cortical Contributions
Abstract
1. Introduction
2. Experiment 1
2.1. Method
2.1.1. Participants
2.1.2. Design
2.1.3. Apparatus and Materials
2.1.4. Non-Task-Specific Perturbation (Induced Paresthesia)
2.1.5. Vibrotactile Task
2.1.6. SEPs Stimulation and Recording
2.1.7. Data Analysis
2.2. Results
2.2.1. Sensory Testing
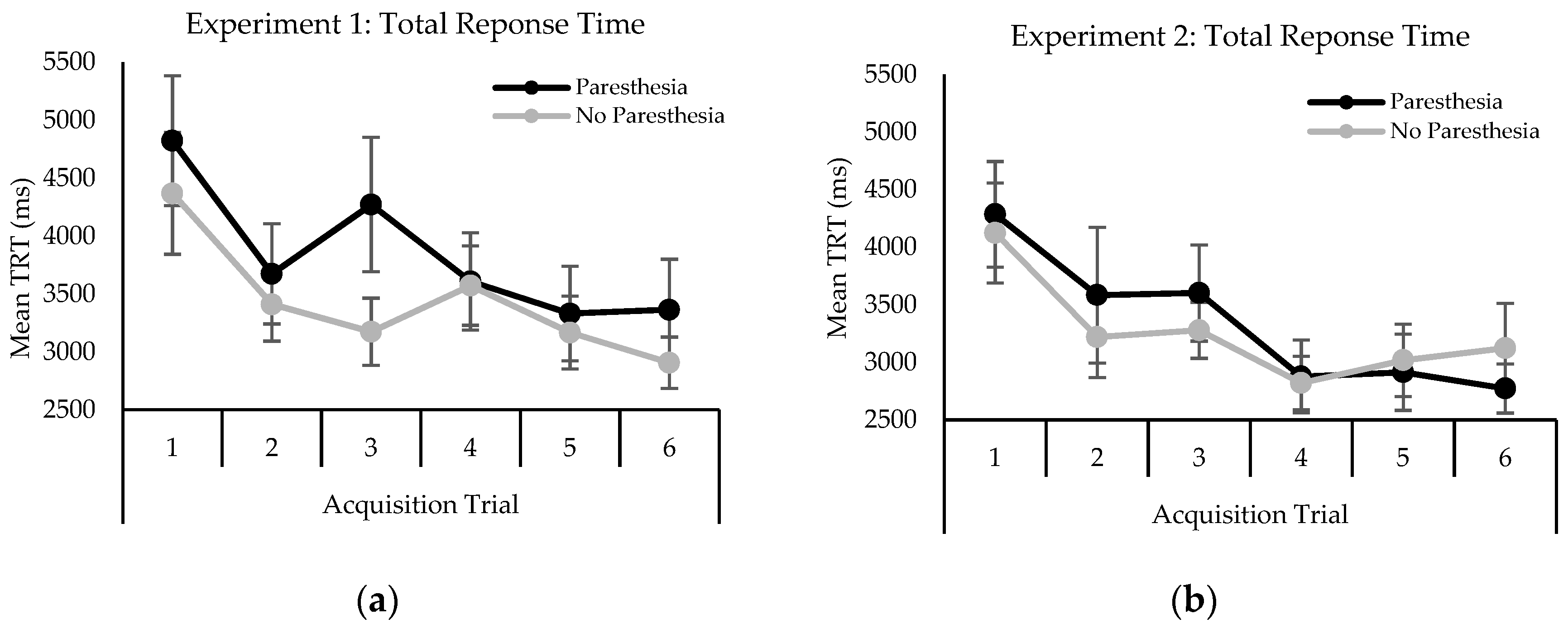
2.2.2. Behavioral Performance
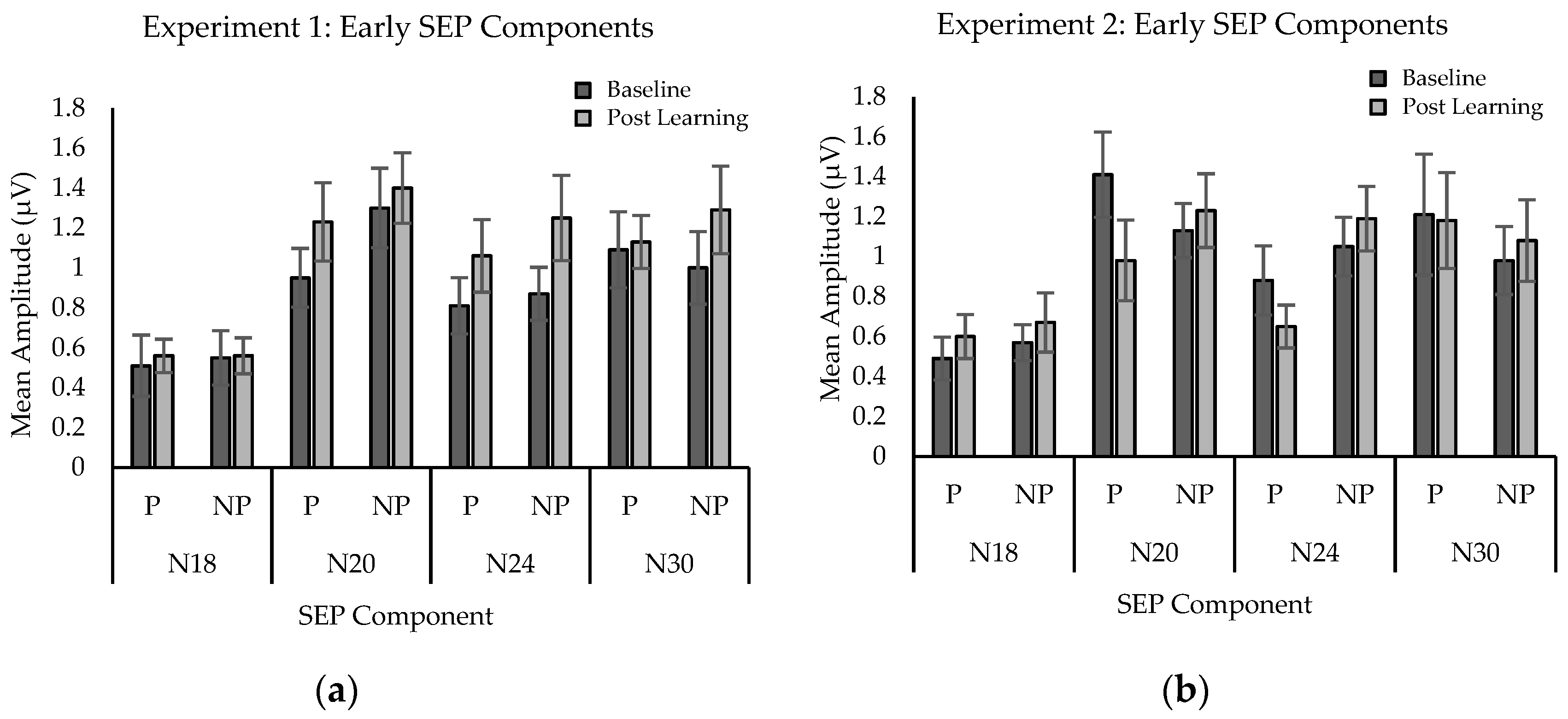
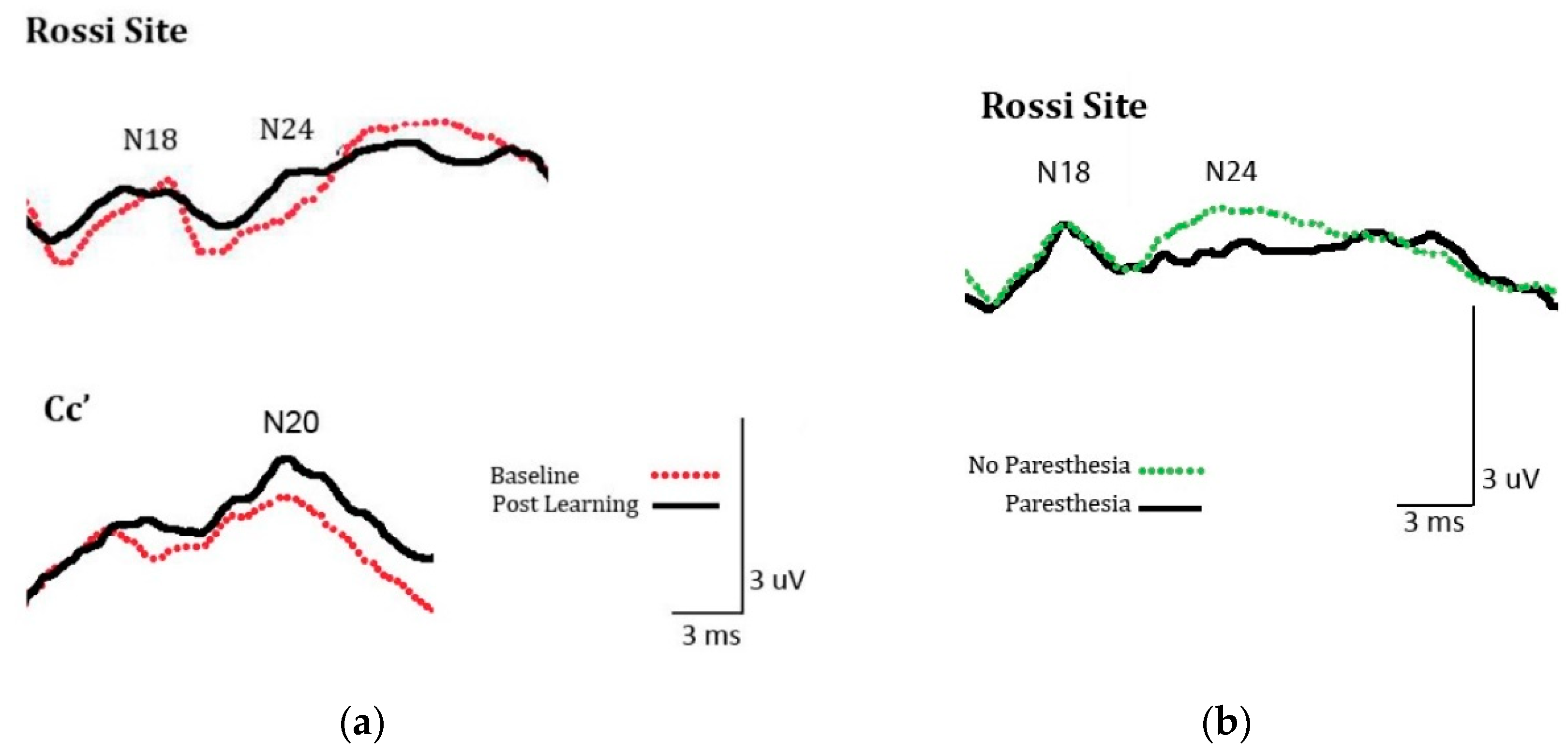
2.2.3. SEP Amplitude
2.3. Discussion
3. Experiment 2
3.1. Method
3.1.1. Participants
3.1.2. Apparatus, Materials, Design, Procedure, and Analysis
3.2. Results
3.2.1. Sensory Performance
3.2.2. Behavioral Performance
3.2.3. SEPs Amplitude
3.3. Discussion
4. General Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gallace, A.; Tan, H.Z.; Spence, C. The body surface as a communication system: The state of the art after 50 years. Presence Teleoper. Virtual Environ. 2007, 16, 655–676. [Google Scholar] [CrossRef]
- Persaud, B.N.; Retting, R.A.; Lyon, C.A. Crash reduction following installation of centerline rumble strips on rural two-lane roads. Accid. Anal. Prev. 2004, 36, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.C. Tactile pattern perception and its perturbations. J. Acoust. Soc. Am. 1985, 77, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Stea, R.A.; Bolanowski, S.J. Heat-induced pain diminishes vibrotactile perception: A touch gate. Somatosens. Mot. Res. 1994, 11, 259–267. [Google Scholar] [CrossRef]
- Spence, C.; Gallace, A. Recent developments in the study of tactile attention. Can. J. Exp. Psychol. 2007, 61, 196–207. [Google Scholar] [CrossRef]
- Craig, J.C. The role of onset in the perception of sequentially presented vibrotactile patterns. Percept. Psychophys. 1983, 34, 421–432. [Google Scholar] [CrossRef]
- Kekoni, J.; Tikkala, I.; Pertovaara, A.; Hämäläinen, H. Spatial features of vibrotactile masking effects on airpuff-elicited sensations in the human hand. Somatosens. Mot. Res. 1990, 7, 353–363. [Google Scholar] [CrossRef]
- Passmore, S.R.; Bosse, J.; Murphy, B.; Lee, T.D. The impact and specificity of nerve perturbation on novel vibrotactile sensory letter learning. Somatosens. Mot. Res. 2014, 31, 167–177. [Google Scholar] [CrossRef]
- Craig, J.C. Vibrotactile masking: The role of response competition. Percept. Psychophys. 1995, 57, 1190–1200. [Google Scholar] [CrossRef][Green Version]
- Pellicciari, M.C.; Miniussi, C.; Rossini, P.M.; De Gennaro, L. Increased cortical plasticity in the elderly: Changes in the somatosensory cortex after paired associative stimulation. Neuroscience 2009, 163, 266–276. [Google Scholar] [CrossRef]
- Murphy, B.A.; Taylor, H.H.; Wilson, S.A.; Oliphant, G.; Mathers, K.M. Rapid reversible changes to multiple levels of the human somatosensory system following the cessation of repetitive contractions: A somatosensory evoked potential study. Clin. Neurophysiol. 2003, 114, 1531–1537. [Google Scholar] [CrossRef]
- Andrew, D.; Haavik, H.; Dancey, E.; Yielder, P.; Murphy, B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin. Neurophysiol. 2015, 126, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Andrew, D.; Yielder, P.; Haavik, H.; Murphy, B. The effects of subclinical neck pain on sensorimotor integration following a complex motor pursuit task. Exp. Brain Res. 2018, 236, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Passmore, S.R.; Murphy, B.; Lee, T.D. The origin, and application of somatosensory evoked potentials as a neurophysiological technique to investigate neuroplasticity. J. Can. Chiropr. Assoc. 2014, 58, 170–183. [Google Scholar]
- Pascual-Leone, A.; Torres, F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain 1993, 116 Pt 1, 39–52. [Google Scholar] [CrossRef]
- Likert, R. A technique for the measurement of attitudes. Arch. Psychol. 1932, 22, 55. [Google Scholar]
- Zehr, E.P.; Chua, R. Modulation of human cutaneous reflexes during rhythmic cyclical arm movement. Exp. Brain Res. 2000, 135, 241–250. [Google Scholar] [CrossRef]
- Tang, K.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Novel vibrotactile discrimination task for investigating the neural correlates of short-term learning with fMRI. J. Neurosci. Methods 2009, 178, 65–74. [Google Scholar] [CrossRef]
- Patterson, J.T.; Lee, T.D. Learning a new human-computer alphabet: The role of similarity and practice. Acta. Psychol. 2005, 120, 267–287. [Google Scholar] [CrossRef]
- Fujii, M.; Yamada, T.; Aihara, M.; Kokubun, Y.; Noguchi, Y.; Matsubara, M. The effects of stimulus rates upon median, ulnar and radial nerve somatosensory evoked potentials. Evoked Potentials Electroencephalogr. Clin. Neurophysiol. 1994, 92, 518–526. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B.A. Selective changes in cerebellar-cortical processing following motor training. Exp. Brain Res. 2013, 231, 397–403. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Aminoff, M.; Desmedt, J.; Eisen, A.A.; Goodin, D.; Matsuoka, S.; Mauguière, F.; Shibasaki, H.; Sutherling, W.; Vibert, J.F. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 6–11. [Google Scholar] [CrossRef]
- Rossi, S.; della Volpe, R.; Ginanneschi, F.; Ulivelli, M.; Bartalini, S.; Spidalieri, R.; Rossi, A. Early somatosensory processing during tonic muscle pain in humans: Relation to loss of proprioception and motor ‘defensive’ strategies. Clin. Neurophysiol. 2003, 114, 1351–1358. [Google Scholar] [CrossRef]
- Cruccu, G.; Aminoff, M.J.; Curio, G.; Guerit, J.M.; Kakigi, R.; Mauguiere, F.; Rossini, P.M.; Treede, R.D.; Garcia-Larrea, L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Mauguiere, F.; Allison, T.; Babiloni, C.; Buchner, H.; Eisen, A.A.; Goodin, D.S.; Jones, S.J.; Kakigi, R.; Matsuoka, S.; Nuwer, M.; et al. Somatosensory evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 79–90. [Google Scholar] [PubMed]
- Tinazzi, M.; Fiaschi, A.; Rosso, T.; Faccioli, F.; Grosslercher, J.; Aglioti, S.M. Neuroplastic Changes Related to Pain Occur at Multiple Levels of the Human Somatosensory System: A Somatosensory-Evoked Potentials Study in Patients with Cervical Radicular Pain. J. Neurosci. 2000, 20, 9277–9283. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, J.E.; Ozaki, I. SEPs to finger joint input lack the N20-P20 response that is evoked by tactile inputs: Contrast between cortical generators in areas 3b and 2 in humans. Evoked Potentials Electroencephalogr. Clin. Neurophysiol. 1991, 80, 513–521. [Google Scholar] [CrossRef]
- Molinari, M.; Restuccia, D.; Leggio, M.G. State estimation, response prediction, and cerebellar sensory processing for behavioral control. Cerebellum 2009, 8, 399–402. [Google Scholar] [CrossRef]
- Foulke, E.; Brodbeck, A.A. Transmission of Morse Code by Electrocutaneous Stimulation. Psychol. Rec. 1968, 18, 617–622. [Google Scholar] [CrossRef]
- Craig, J.C. Processing of sequential tactile patterns: Effects of a neutral stimulus. Percept. Psychophys. 2000, 62, 596–606. [Google Scholar] [CrossRef]
- Hari, R.; Karhu, J.; Hämäläinen, M.; Knuutila, J.; Salonen, O.; Sams, M.; Vilkman, V. Functional organization of the human first and second somatosensory cortices: A neuromagnetic study. Eur. J. Neurosci. 1993, 5, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Kristeva-Feige, R.; Rossi, S.; Pizzella, V.; Tecchio, F.; Romani, G.L.; Erne, S.; Edrich, J.; Orlacchio, A.; Rossini, P.M. Neuromagnetic fields of the brain evoked by voluntary movement and electrical stimulation of the index finger. Brain. Res. 1995, 682, 22–28. [Google Scholar] [CrossRef]
- Rossini, P.M.; Babiloni, F.; Babiloni, C.; Ambrosini, A.; Onorati, P.; Urbano, A. Topography of spatially enhanced human shortlatency somatosensory evoked potentials. NeuroReport 1997, 8, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, J.E.; Cheron, G. Central somatosensory conduction in man: Neural generators and interpeak latencies of the far-field components recorded from neck and right or left scalp and earlobes. Electroencephalogr. Clin. Neurophysiol. 1980, 50, 382–403. [Google Scholar] [CrossRef]
- Hlushchuk, Y.; Hari, R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J. Neurosci. 2006, 26, 5819–5824. [Google Scholar] [CrossRef]
- Mauguiere, F. Somatosensory evoked potentials: Normal responses, abnormal waveforms, and clinical applications in neurological diseases. In Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Niedermeyer, E., Lopes da Silva, F., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 1067–1120. [Google Scholar]
- Garcia Larrea, L.; Bastuji, H.; Mauguiere, F. Unmasking of cortical SEP components by changes in stimulus rate: A topographic study. Electroencephalogr. Clin. Neurophysiol. 1992, 84, 71–83. [Google Scholar] [CrossRef]
- Cheron, G.; Borenstein, S. Gating of the early components of the frontal and parietal somatosensory evoked potentials in different sensory-motor interference modalities. Electroencephalogr. Clin. Neurophysiol. 1991, 80, 522–530. [Google Scholar] [CrossRef]
- Waberski, T.D.; Buchner, H.; Perkuhn, M.; Gobbelé, R.; Wagner, M.; Kücker, W.; Silny, J. N30 and the effect of explorative finger movements: A model of the contribution of the motor cortex to early somatosensory potentials. Clin. Neurophysiol. 1999, 110, 1589–1600. [Google Scholar] [CrossRef]
- Restuccia, D.; Valeriani, M.; Barba, C.; Le Pera, D.; Capecci, M.; Filippini, V.; Molinari, M. Functional changes of the primary somatosensory cortex in patients with unilateral cerebellar lesions. Brain 2001, 124, 757–768. [Google Scholar] [CrossRef]
- Gandevia, S.C.; Burke, D.; McKeon, B.B. Convergence in the somatosensory pathway between cutaneous afferents from the index and middle fingers in man. Exp. Brain Res. 1983, 50, 415–425. [Google Scholar] [CrossRef]
- Jones, S.J.; Halonen, J.P.; Shawkat, F. Centrifugal and centripetal mechanisms involved in the ‘gating’ of cortical SEPs during movement. Evoked Potentials Electroencephalogr. Clin. Neurophysiol. 1989, 74, 36–45. [Google Scholar] [CrossRef]
- Jones, S.J.; Power, C.N. Scalp topography of human somatosensory evoked potentials: The effect of interfering tactile stimulation applied to the hand. Electroencephalogr. Clin. Neurophysiol. 1984, 58, 25–36. [Google Scholar] [CrossRef]
- Rossini, P.M.; Babiloni, C.; Babiloni, F.; Ambrosini, A.; Onorati, P.; Carducci, F.; Urbano, A. “Gating” of human short-latency somatosensory evoked cortical responses during execution of movement. A high resolution electroencephalography study. Brain Res. 1999, 843, 161–170. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passmore, S.R.; Mortaza, N.; Glazebrook, C.M.; Murphy, B.; Lee, T.D. Somatosensory Integration and Masking of Complex Tactile Information: Peripheral and Cortical Contributions. Brain Sci. 2020, 10, 954. https://doi.org/10.3390/brainsci10120954
Passmore SR, Mortaza N, Glazebrook CM, Murphy B, Lee TD. Somatosensory Integration and Masking of Complex Tactile Information: Peripheral and Cortical Contributions. Brain Sciences. 2020; 10(12):954. https://doi.org/10.3390/brainsci10120954
Chicago/Turabian StylePassmore, Steven R., Niyousha Mortaza, Cheryl M. Glazebrook, Bernadette Murphy, and Timothy D. Lee. 2020. "Somatosensory Integration and Masking of Complex Tactile Information: Peripheral and Cortical Contributions" Brain Sciences 10, no. 12: 954. https://doi.org/10.3390/brainsci10120954
APA StylePassmore, S. R., Mortaza, N., Glazebrook, C. M., Murphy, B., & Lee, T. D. (2020). Somatosensory Integration and Masking of Complex Tactile Information: Peripheral and Cortical Contributions. Brain Sciences, 10(12), 954. https://doi.org/10.3390/brainsci10120954






