Little Brain, Big Expectations
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. A Window to Connect the Whole Brain
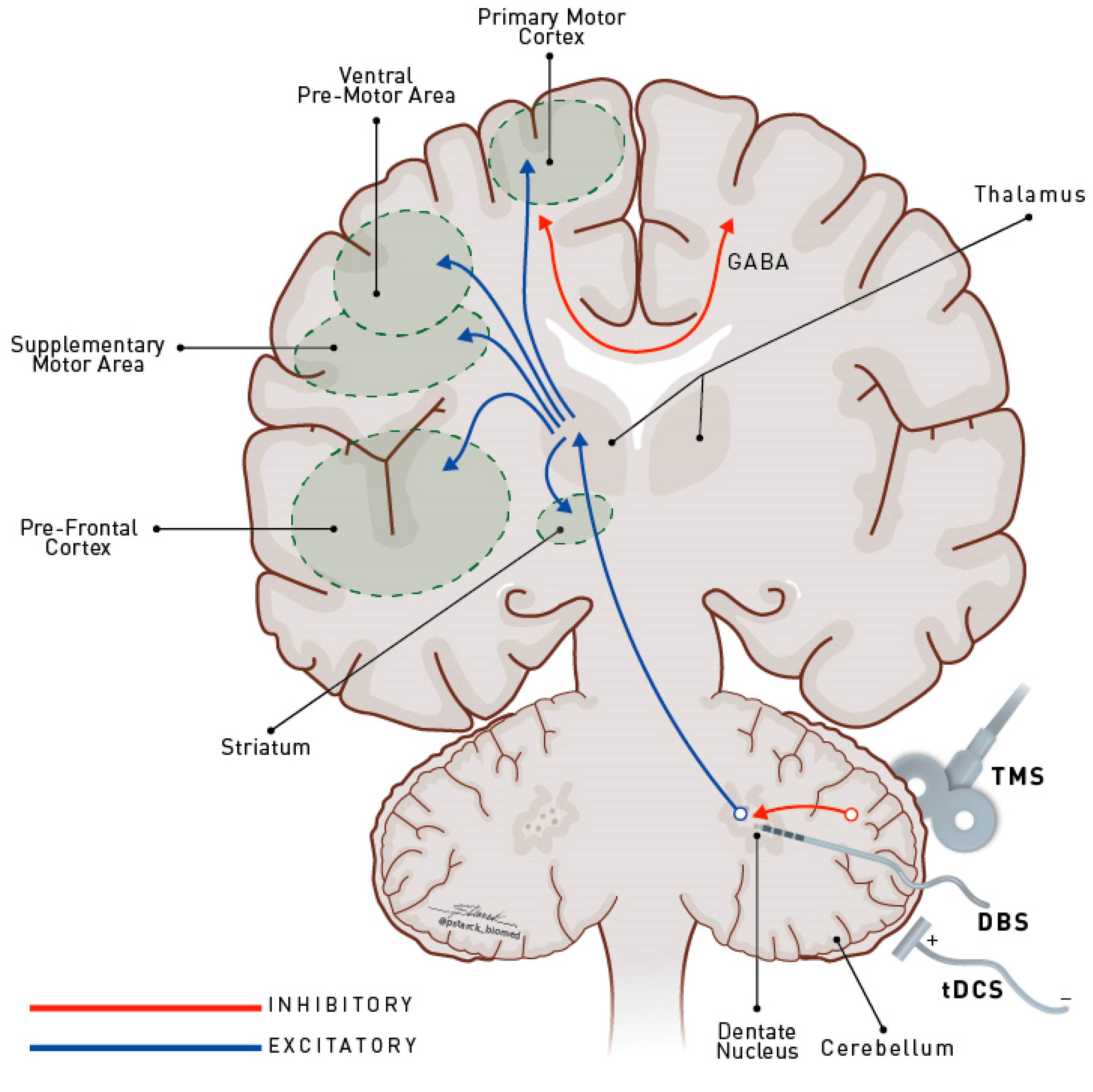
4. Why Target the Cerebellum in Movement Disorders?
5. What Recent Positive Studies Have Revealed
6. Playing Devil’s Advocate
7. So, What Is Next?
Author Contributions
Funding
Conflicts of Interest
References
- Bostan, A.C.; Strick, P.L. The basal ganglia and the cerebellum: Nodes in an integrated network. Nat. Rev. Neurosci. 2018, 19, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Reich, M.; Vorwerk, J.; Li, N.; Wenzel, G.; Fang, Q.; Schmitz-Hübsch, T.; Nickl, R.; Kupsch, A.; Volkmann, J.; et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann. Neurol. 2017, 82, 67–78. [Google Scholar] [CrossRef] [PubMed]
- França, C.; de Andrade, D.C.; Teixeira, M.J.; Galhardoni, R.; Silva, V.; Barbosa, E.R.; Cury, R.G. Effects of cerebellar neuromodulation in movement disorders: A systematic review. Brain Stimul. 2018, 11, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Figueroa, K.P.; Dorval, A.D.; Pulst, S.M. Deep cerebellar stimulation reduces ataxic motor symptoms in the shaker rat. Ann. Neurol. 2019, 85, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Miterko, L.N.; Baker, K.B.; Beckinghausen, J.; Bradnam, L.V.; Cheng, M.Y.; Cooperrider, J.; DeLong, M.R.; Gornati, S.V.; Hallett, M.; Heck, D.H.; et al. Consensus Paper: Experimental Neurostimulation of the Cerebellum. Cerebellum 2019, 18, 1064–1097. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.; Teixeira, M.J.; Mendes, M.M.; França, C.; Iglesio, R.; Barbosa, E.R.; Cury, R.G. Exploring the clinical outcomes after deep brain stimulation in Tourette syndrome. J. Neurol. Sci. 2019, 402, 48–51. [Google Scholar] [CrossRef]
- Da Guarda, S.N.F.; Cohen, L.G.; da Cunha Pinho, M.; Yamamoto, F.I.; Marchiori, P.E.; Scaff, M.; Conforto, A.B. Interhemispheric asymmetry of corticomotor excitability after chronic cerebellar infarcts. Cerebellum 2010, 9, 398–404. [Google Scholar] [CrossRef][Green Version]
- Cury, R.G.; Teixeira, M.J.; Galhardoni, R.; Barboza, V.R.; Alho, E.; Seixas, C.M.; Lepski, G.; de Andrade, D.C. Neuronavigation-guided transcranial magnetic stimulation of the dentate nucleus improves cerebellar ataxia: A sham-controlled, double-blind n = 1 study. Parkinsonism Relat. Disord. 2015. [Google Scholar] [CrossRef]
- França, C.; de Andrade, D.C.; Silva, V.; Galhardoni, R.; Barbosa, E.R.; Teixeira, M.J.; Cury, R.G. Effects of cerebellar transcranial magnetic stimulation on ataxias: A randomized trial. Parkinsonism Relat. Disord. 2020, 80, 1–6. [Google Scholar] [CrossRef]
- Hoshi, E.; Tremblay, L.; Féger, J.; Carras, P.L.; Strick, P.L. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 2005, 8, 1491–1493. [Google Scholar] [CrossRef]
- Pelzer, E.A.; Hintzen, A.; Goldau, M.; von Cramon, D.Y.; Timmermann, L.; Tittgemeyer, M. Cerebellar networks with basal ganglia: Feasibility for tracking cerebello-pallidal and subthalamo-cerebellar projections in the human brain. Eur. J. Neurosci. 2013, 38, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Fremont, R.; Khodakhah, K. It’s not just the basal ganglia: Cerebellum as a target for dystonia therapeutics. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Calderon, D.P.; Fremont, R.; Kraenzlin, F.; Khodakhah, K. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat. Neurosci. 2011, 14, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Argyelan, M.; Carbon, M.; Niethammer, M.; Ulug, A.M.; Voss, H.U.; Bressman, S.B.; Dhawan, V.; Eidelberg, D. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 9740–9747. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.G.; Kalia, S.K.; Shah, B.B.; Jimenez-Shahed, J.; Prashanth, L.K.; Moro, E. Surgical treatment of dystonia. Expert Rev. Neurother. 2018, 18, 477–492. [Google Scholar] [CrossRef]
- Ni, Z.; Pinto, A.D.; Lang, A.E.; Chen, R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann. Neurol. 2010, 68, 816–824. [Google Scholar] [CrossRef]
- White, J.J.; Sillitoe, R.V. Genetic silencing of olivocerebellar synapses causes dystonia-like behaviour in mice. Nat. Commun. 2017, 8, 14912. [Google Scholar] [CrossRef]
- Bradnam, L.V.; Graetz, L.J.; McDonnell, M.N.; Ridding, M.C. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front. Hum. Neurosci. 2015, 9, 286. [Google Scholar] [CrossRef]
- Sokal, P.; Rudaś, M.; Harat, M.; Szylberg, Ł.; Zieliński, P. Deep anterior cerebellar stimulation reduces symptoms of secondary dystonia in patients with cerebral palsy treated due to spasticity. Clin. Neurol. Neurosurg. 2015, 135, 62–68. [Google Scholar] [CrossRef]
- Horisawa, S.; Arai, T.; Suzuki, N.; Kawamata, T.; Taira, T. The striking effects of deep cerebellar stimulation on generalized fixed dystonia: Case report. J. Neurosurg. 2019, 132, 712–716. [Google Scholar] [CrossRef]
- Koch, G.; Brusa, L.; Carrillo, F.; Lo Gerfo, E.; Torriero, S.; Oliveri, M.; Mir, P.; Caltagirone, C.; Stanzione, P. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology 2009, 73, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Minks, E.; Mareček, R.; Pavlík, T.; Ovesná, P.; Bareš, M. Is the cerebellum a potential target for stimulation in Parkinson’s disease? Results of 1-Hz rTMS on upper limb motor tasks. Cerebellum 2011, 10, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Di Biasio, F.; Conte, A.; Iezzi, E.; Modugno, N.; Berardelli, A. Effects of cerebellar continuous theta burst stimulation on resting tremor in Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Cortese, F.; Bianchi, M.; Pittera, D.; Turrone, R.; Bocci, T.; Borroni, B.; Vergari, M.; Cogiamanian, F.; Ardolino, G.; et al. Cerebellar and Motor Cortical Transcranial Stimulation Decrease Levodopa-Induced Dyskinesias in Parkinson’s Disease. Cerebellum 2016, 15, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Follesa, P.; Puligheddu, M.; Cannas, A.; Serra, M.; Pisu, M.G.; Dagostino, S.; Solla, P.; Tacconi, P.; Marrosu, F. Cerebellar continuous theta burst stimulation reduces levodopa-induced dyskinesias and decreases serum BDNF levels. Neurosci. Lett. 2020, 716, 134653. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Uc, E.Y.; Rudroff, T. Cerebellar Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Pilot Study. Brain Sci. 2020, 10, 96. [Google Scholar] [CrossRef]
- Sadnicka, A.; Hamada, M.; Bhatia, K.P.; Rothwell, J.C.; Edwards, M.J. Cerebellar stimulation fails to modulate motor cortex plasticity in writing dystonia. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1304–1307. [Google Scholar] [CrossRef]
- Koch, G.; Porcacchia, P.; Ponzo, V.; Carrillo, F.; Cáceres-Redondo, M.T.; Brusa, L.; Desiato, M.T.; Arciprete, F.; Di Lorenzo, F.; Pisani, A.; et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 2014, 7, 564–572. [Google Scholar] [CrossRef]
- Shiga, Y. Transcranial magnetic stimulation alleviates truncal ataxia in spinocerebellar degeneration. J. Neurol. Neurosurg. Psychiatry 2002, 72, 124–126. [Google Scholar] [CrossRef]
- Ihara, Y.; Takata, H.; Tanabe, Y.; Nobukuni, K.; Hayabara, T. Influence of repetitive transcranial magnetic stimulation on disease severity and oxidative stress markers in the cerebrospinal fluid of patients with spinocerebellar degeneration. Neurol. Res. 2005, 27, 310–313. [Google Scholar] [CrossRef]
- Grimaldi, G.; Manto, M. Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann. Biomed. Eng. 2013, 41, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Bonnì, S.; Ponzo, V.; Caltagirone, C.; Koch, G. Cerebellar theta burst stimulation in stroke patients with ataxia. Funct. Neurol. 2014, 29, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Jung, S.H.; Oh, M.K.; Min, Y.S.; Lim, J.Y.; Paik, N.-J. Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: A pilot study. J. Rehabil. Med. 2014, 46, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Koch, G.; Cotelli, M.; Padovani, A.; Borroni, B. Cerebellar transcranial direct current stimulation in patients with ataxia: A double-blind, randomized, sham-controlled study. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Grecco, L.A.C.; Oliveira, C.S.; de Almeida Carvalho Duarte, N.; Lima, V.L.C.C.; Zanon, N.; Fregni, F. Cerebellar transcranial direct current stimulation in children with ataxic cerebral palsy: A sham-controlled, crossover, pilot study. Dev. Neurorehabilit. 2017, 20, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Dell’Era, V.; Cotelli, M.S.; Turla, M.; Casali, C.; Padovani, A.; Borroni, B. Long term clinical and neurophysiological effects of cerebellar transcranial direct current stimulation in patients with neurodegenerative ataxia. Brain Stimul. 2017, 10, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Bonetta, E.; Grasso, R.; Manenti, R.; Cotelli, M.; Padovani, A.; Borroni, B. Cerebello-spinal tDCS in ataxia: A randomized, double-blind, sham-controlled, crossover trial. Neurology 2018, 91, e1090–e1101. [Google Scholar] [CrossRef]
- Manor, B.; Greenstein, P.E.; Davila-Perez, P.; Wakefield, S.; Zhou, J.; Pascual-Leone, A. Repetitive Transcranial Magnetic Stimulation in Spinocerebellar Ataxia: A Pilot Randomized Controlled Trial. Front. Neurol. 2019, 10, 73. [Google Scholar] [CrossRef]
- Gironell, A.; Kulisevsky, J.; Lorenzo, J.; Barbanoj, M.; Pascual-Sedano, B.; Otermin, P. Transcranial magnetic stimulation of the cerebellum in essential tremor: A controlled study. Arch. Neurol. 2002, 59, 413–417. [Google Scholar] [CrossRef]
- Avanzino, L.; Bove, M.; Tacchino, A.; Ruggeri, P.; Giannini, A.; Trompetto, C.; Abbruzzese, G. Cerebellar involvement in timing accuracy of rhythmic finger movements in essential tremor. Eur. J. Neurosci. 2009, 30, 1971–1979. [Google Scholar] [CrossRef]
- Popa, T.; Russo, M.; Vidailhet, M.; Roze, E.; Lehéricy, S.; Bonnet, C.; Apartis, E.; Legrand, A.P.; Marais, L.; Meunier, S.; et al. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: An open label trial. Brain Stimul. 2013, 6, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Gironell, A.; Martínez-Horta, S.; Aguilar, S.; Torres, V.; Pagonabarraga, J.; Pascual-Sedano, B.; Ribosa-Nogué, R. Transcranial direct current stimulation of the cerebellum in essential tremor: A controlled study. Brain Stimul. 2014, 7, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Rocchi, L.; Leodori, G.; Paparella, G.; Conte, A.; Kahn, N.; Fabbrini, G.; Berardelli, A. Cerebellar continuous theta burst stimulation in essential tremor. Cerebellum 2015, 14, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-W.; Hallett, M.; Sohn, Y.H. Cerebellar repetitive transcranial magnetic stimulation for patients with essential tremor. Parkinsonism Relat. Disord. 2019, 64, 304–307. [Google Scholar] [CrossRef]
- Teixeira, M.J.; Cury, R.G.; Galhardoni, R.; Barboza, V.R.; Brunoni, A.R.; Alho, E.; Lepski, G.; de Andrade, D.C. Deep brain stimulation of the dentate nucleus improves cerebellar ataxia after cerebellar stroke. Neurology 2015, 85, 2075–2076. [Google Scholar] [CrossRef]
- Cury, R.G.; França, C.; Barbosa, E.R.; Galhardoni, R.; Lepski, G.; Teixeira, M.J.; Ciampi de Andrade, D. Dentate nucleus stimulation in a patient with cerebellar ataxia and tremor after cerebellar stroke: A long-term follow-up. Parkinsonism Relat. Disord. 2019, 60, 173–175. [Google Scholar] [CrossRef]
- Cury, R.G.; França, C.; Silva, V.; Barbosa, E.R.; Capato, T.T.C.; Lepski, G.; Duarte, K.P.; Teixeira, M.J.; Ciampi de Andrade, D. Effects of dentate nucleus stimulation in spinocerebellar ataxia type 3. Parkinsonism Relat. Disord. 2019, 69, 91–93. [Google Scholar] [CrossRef]
- Galea, J.M.; Jayaram, G.; Ajagbe, L.; Celnik, P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 9115–9122. [Google Scholar] [CrossRef]
- Bocci, T.; Santarcangelo, E.; Vannini, B.; Torzini, A.; Carli, G.; Ferrucci, R.; Priori, A.; Valeriani, M.; Sartucci, F. Cerebellar direct current stimulation modulates pain perception in humans. Restor. Neurol. Neurosci. 2015, 33, 597–609. [Google Scholar] [CrossRef]
- Panouillères, M.T.N.; Miall, R.C.; Jenkinson, N. The role of the posterior cerebellum in saccadic adaptation: A transcranial direct current stimulation study. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 5471–5479. [Google Scholar] [CrossRef]
- Sadnicka, A.; Kassavetis, P.; Saifee, T.A.; Pareés, I.; Rothwell, J.C.; Edwards, M.J. Cerebellar transcranial direct current stimulation does not alter motor surround inhibition. Int. J. Neurosci. 2013, 123, 425–432. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Study Design | Diagnosis, n | Intervention | Main Clinical Findings | Class of Evidence |
|---|---|---|---|---|---|
| Parkinson’s disease | |||||
| Koch et al., 2009 [21] | Double-blind, sham-controlled, crossover | PD with dyskinesias, 10 | rTMS (cTBS) single session with figure-of-eight coil | Decrease in waking time spent as ON with dyskinesias | III |
| Minks et al., 2011 [22] | Single-blind, sham-controlled, crossover | PD, 20 | One Hz rTMS, single session, with a double-cone coil | Improvement in gross upper limb movement; worsening in fine motor finger and hand function | III |
| Bologna et al., 2015 [23] | Double-blind, sham-controlled, crossover | PD, 13 + healthy controls, 10 | Unilateral TMS (cTBS) single session with figure-of-eight coil | No changes in tremor amplitude, frequency, or magnitude | III |
| Ferrucci et al., 2016 [24] | Double-blind, sham-controlled, crossover | PD with dyskinesias, 9 | Two mA anodal tDCS, five sessions | Improvement in UPDRS IV (dyskinesias section) | III |
| Sanna et al., 2020 [25] | Double-blind, sham-controlled, crossover | PD with dyskinesias, 11 | rTMS (cTBS) single session with circular coil | Decrease in dyskinesias and serum BDNF in active group | II |
| Workman et al., 2020 [26] | Double-blind, sham-controlled, crossover | PD, 7 | Two or 4 mA, unilateral or bilateral tDCS single session | Significant improvement in balance score in bilateral 4 mA group against sham; no gait improvement | II |
| Dystonia | |||||
| Sadnicka et al., 2014 [27] | Single-blinded, sham controlled with crossover | WC, 10 | Two mA ipsilateral anodal tDCS, single session | No subjective improvement or changes in the WCRS or timed writing assessment | III |
| Koch et al., 2014 [28] | Double-blind, sham-controlled | CD, 18 (9 active; 9 sham) | Bilateral rTMS (cTBS), 10 sessions | Small but significant clinical improvement as measured by the TWSTRS of approximately 15% | III |
| Bradnam et al., 2015 [18] | Double-blind, sham-controlled, crossover | FHD, 8 (WC = 5; MD = 3); healthy controls, 8 | Two mA anodal/cathodal tDCS, single session | No change in clinical outcomes | II |
| Cerebellar ataxia | |||||
| Shiga et al., 2002 [29] | Double-blind, sham-controlled | Spinocerebellar degeneration, 74 (39 active, 35 sham) | Single-pulse TMS, 21 sessions with circular coil | Improvement in 10 m time, 10 m steps, tandem steps. and standing capacities, especially in the cerebellar type | III |
| Ihara et al., 2005 [30] | Single-blind, uncontrolled | Spinocerebellar degeneration, 20 | Single-pulse TMS, 24 sessions with figure-of-eight coil | Improvement in ataxia (ICARS) | III |
| Grimaldi and Manto et al., 2013 [31] | Single-blind, sham-controlled, crossover | Varied cerebellar ataxias, 9 | One mA right anodal tDCS, single session | No change in posturography or upper limb dexterity | III |
| Bonnì et al., 2014 [32] | Open label | Posterior circulation stroke with ataxia, 6 | rTMS (iTBS, ipsilateral), 10 sessions with figure-of-eight coil + physical therapy | Ataxia improvement (MICARS), especially posture and gait subscales | IV |
| Kim et al., 2014 [33] | Double-blind, sham-controlled | Posterior circulation stroke with ataxia, 32 | One Hz ipsilateral rTMS, five sessions with figure-of-eight coil | Improvement in the 1 0m walk test 1 month after; balance improved after 5 days and after 1 month | III |
| Benussi et al., 2015 [34] | Double-blind, sham-controlled, crossover | Varied cerebellar ataxias, 19 | Two mA anodal tDCS, single session | Improvement in ataxia (SARA and ICARS), hand dexterity, and gait | III |
| Grecco et al., 2017 [35] | Single-blind, sham-controlled, crossover | Ataxic cerebral palsy, 6 | One mA anodal tDCS, 10 sessions + treadmill training | Improvement in hip oscillation during eyes-closed gait (stabilometric evaluation) | III |
| Benussi et al., 2017 [36] | Double-blind, sham-controlled | Varied neurodegenerative ataxias, 20; healthy controls, 10 | Two mA anodal tDCS, 10 sessions | Improvement lasting at least 3 months in SARA, ICARS, gait, and hand dexterity (in non-dominant hand) | III |
| Benussi et al., 2018 [37] | Double-blind, sham-controlled crossover | Varied neurodegenerative ataxias, 20 | Two mA anodal tDCS (cerebellum) and 2 mA cathodal tDCS (spinal cord), 10 sessions | Improvement lasting at least 3 months in SARA, ICARS, gait, hand dexterity, and quality of life | II |
| Manor et al., 2019 [38] | Double-blind, sham-controlled | Spinocerebellar ataxia, 20 | Single-pulse TMS, 20 sessions with circular coil | Improvement only in stance sub-score of SARA and standing postural sway metrics | II |
| França et al., 2020 [9] | Double-blind, sham-controlled, crossover | Spinocerebellar ataxia type 3, 9; multiple system atrophy cerebellar type, 8; post-lesion ataxia, 7 | One Hz unilateral rTMS, 10 sessions with double-cone coil | Improvement in SARA and ICARS | II |
| Essential tremor | |||||
| Gironell et al., 2002 [39] | Double-blind, sham-controlled, crossover (washout 1 week) | ET, 10 | One Hz rTMS, single session with butterfly coil | Tremor improvement according to the FTM (17%), and accelerometry evaluation on the 5 min assessment | II |
| Avanzino et al., 2009 [40] | Open label in five patients, and single-blind, sham-controlled, crossover in seven patients | ET, 10 + healthy controls, 11 | One Hz right rTMS, single session with figure-of-eight coil | Decrease of TD values; increase of ITI values and decrease of the coefficient of variation of ITI; no change in frequency or magnitude of accelerometer signal, and no change in tremor (FTM) | IV |
| Popa et al., 2013 [41] | Open label | ET, 11; healthy controls, 11 | One Hz rTMS, five sessions with figure-of-eight coil | Tremor improvement that built up until day 12 and persisted for 3 weeks (FTM); decrease in tremor amplitude. | IV |
| Gironell et al., 2014 [42] | Double-blind, sham-controlled crossover | ET, 10 | Two mA cathodal tDCS, 10 sessions | No acute or long-lasting benefit (FTM and accelerometric recordings) | III |
| Bologna et al., 2015 [43] | Double-blind, sham-controlled, crossover | ET, 16; healthy controls, 11 | rTMS (cTBS), single session with eight-shaped coil | No change in tremor severity and reaching movements (FTM and accelerometer) | III |
| Shin et al., 2019 [44] | Single-blind, sham-controlled | ET, 22 (12 active, 10 sham) | One Hz rTMS, five sessions with figure-of-eight coil | Improvement in tremor immediately after (33% active × 20% sham, according to FTM) and 4 weeks after (31% active × 17% sham); no significant difference between groups; no improvement in functions of daily lives | III |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cury, R.G.; França, C.; Reis Barbosa, E.; Jacobsen Teixeira, M.; Ciampi de Andrade, D. Little Brain, Big Expectations. Brain Sci. 2020, 10, 944. https://doi.org/10.3390/brainsci10120944
Cury RG, França C, Reis Barbosa E, Jacobsen Teixeira M, Ciampi de Andrade D. Little Brain, Big Expectations. Brain Sciences. 2020; 10(12):944. https://doi.org/10.3390/brainsci10120944
Chicago/Turabian StyleCury, Rubens Gisbert, Carina França, Egberto Reis Barbosa, Manoel Jacobsen Teixeira, and Daniel Ciampi de Andrade. 2020. "Little Brain, Big Expectations" Brain Sciences 10, no. 12: 944. https://doi.org/10.3390/brainsci10120944
APA StyleCury, R. G., França, C., Reis Barbosa, E., Jacobsen Teixeira, M., & Ciampi de Andrade, D. (2020). Little Brain, Big Expectations. Brain Sciences, 10(12), 944. https://doi.org/10.3390/brainsci10120944






