Effects of Blood Pressure on Cognitive Performance in Aging: A Systematic Review
Abstract
1. Introduction
Aims
2. Method
2.1. Research Strategies
2.2. Eligibility Criteria
2.3. Data Collection and Quality Assessment
| Study | Participants | Elevated Blood Pressure | Links to Cognitive Impairment | Follow-up (Years) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Group | N | Age M (SD) | Sex (% Men) a | SBPM (SD) a | DBPM (SD) a | ||||
| Cross-Sectional studies | |||||||||
| Farmer et al. [44] | 1382 | 55–94 | 55 | No Relationship | |||||
| Elias et al. [45] | 301 | 44.1 (12.8) | 45.1 | 135.0 (26.1) | 88.0 (15.5) | SBP/DBP | Positive | ||
| Starr et al. [46] | 900 | 75.7 | 160 | 86 | SBP/DBP | Positive | |||
| Cacciatore et al. [47] | 1106 | 73.9 | 55 | 145.3 (19.0) | 82.0 (9.2) | DBP | Positive | - | |
| Cerhan et al. [48] | 13,913 | 45–69 | 44 | SBP/DBP | Positive in women | ||||
| van Boxtel et al. [36] | 943 | 25–80 | 50.3 | SBP/DBP | Non-Linear | - | |||
| Di Carlo et al. [49] | 3134 | 74.0 (5.6) | SBP/DBP | No Relation\ | |||||
| Izquierdo-Porrera and Waldstein [50] | 43 | 59.0 (11.2) | 17.0 | 136.0 (21.0) | 78.0 (11.0) | DBP | Positive | - | |
| Morris et al. [37] | 5816 | 64–104 | 39 | SBP/DBP | U curve | ||||
| Pandav et al. [51] | 5446 | 74.1 (5.7) | 43 | 141.3 (18.4) | 76.2 (9.9) | Low DBP | Positive in Indian | - | |
| Kuo et al. [52] | 70 | 72.0 (4.0) | 55.7 | 134.4 (16.6) | - | SBP | Positive | - | |
| Wharton et al. [53] | 105 | 19.2 (1.1) | 43 | 114.1 (12.6) | 70.7 (9.3) | SBP/DBP | Positive | - | |
| Axelsson et al. [54] | 97 | 81 | 100 | SBP/DBP | Negative | - | |||
| Knecht et al. [55] | 377 | 64.0 (6.6) | 45.4 | 144.0 (18.8) | 85.0 (10.8) | SBP | Positive | - | |
| Gupta et al. [56] | 85 | 52.0 (7.5) | 69.4 | 137.4 (27.2) | 89.7 (18.3) | SBP/DBP | Positive | - | |
| Obisesan et al. [57] | 5724 | >60 | 42.9 | 139.8 | 74.7 | SBP/DBP | Positive | - | |
| Knecht et al. [58] | 377 | 64.0 (6.6) | 45.4 | 144.0 (18.8) | SBP | Positive | - | ||
| Gunstad et al. [59] | 99 | 69.2 (7.5) | 60.6 | SBP/DBP | Negative | - | |||
| Yeung and Thornton [60] | 74 | 66.2 (8.4) | 0 | 120.4 (17.2) | 75.1 (11.1) | SBP/DBP | Positive | - | |
| Richmond et al. [61] | 142 | >100 | 79 | SBP/DBP | Negative | ||||
| Peltz et al. [62] | 420 | 93.0 | 31 | SBP/DBP | No relationship | ||||
| Crichton et al. [63] | 972 | 23–98 | 41 | SBP/DBP | Positive | - | |||
| Conway et al. [64] | 319 | 72 | 34 | SBP/DBP | Positive SBP and Negative DBP | - | |||
| Waldstein et al. [65] | Young normotensive | 26 | 35.1 (3.8) | 100 | 117.9 (8.0) | 74.3 (7.9) | SBP/DBP | Positive in young | - |
| Young hypertensive | 59 | 35.15 (3.9) | 145.6 (13.5) | 97.2 (7.1) | |||||
| Middle-aged normotensive | 24 | 46.6 (4.5) | 118.3 (10.2) | 75.3 (5.2) | |||||
| Middle-aged hypertensive | 64 | 48.1 (4.7) | 146.9 (7.9) | 97.8 (7.9) | |||||
| Harrington et al. [66] | Hypertensive | 107 | 76.0 (4.0) | 45 | 164.0 (9.0) | 89.0 (7.0) | SBP/DBP | Positive | - |
| Normotensive | 116 | 76.0 (4.0) | 50 | 131.0 (10.0) | 74.0 (7.0) | ||||
| André-Petersson, et al. [67] | Normotensive | 72 | 68 | 100 | SBP/DBP | Positive | - | ||
| Hypertensive 1 | 166 | ||||||||
| Hypertensive 2 | 138 | ||||||||
| Hypertensive 3 | 88 | ||||||||
| Saxby et al. [68] | Hypertensive | 250 | 76.4 (4.0) | 47 | 165.0 (8.0) | 88.0 (7.0) | SBP/DBP | Positive | - |
| Normotensive | 256 | 56 | 131.0 (11.0) | 73.0 (7.0) | |||||
| Waldstein and Katzel [69] | Normotensive: | SBP/DBP | Positive | - | |||||
| Men | 30 | 66.8 (6.7) | 100 | 123.2(10.2) | 71.8 (6.7) | ||||
| Women | 26 | 65.1 (6.6) | 0 | 117.3(10.7) | 67.1 (6.9) | ||||
| Hypertensive | |||||||||
| Men | 31 | 68.9 (6.6) | 100 | 147.4(13.7) | 80.4(7.5) | ||||
| Women | 11 | 66.1 (5.6) | 0 | 146.2(13.5) | 81.4(6.9) | ||||
| Waldstein et al. [38] | Normotensive: | 101 | SBP/DBP | Positive | - | ||||
| Normal BP | 65.8(6.5) | 61 | 120.0(10.6) | 69.6(7.2) | |||||
| High BP | 67.0(6.0) | 65 | 145.5(7.8) | 80.9(5.4) | |||||
| Hypertensive: | |||||||||
| Normal BP | 68.4(9.8) | 69 | 132.7(5.4) | 76.5(7.9) | |||||
| High BP | 67.6 (5.0) | 72 | 159.3(8.8) | 84.8(6.5) | |||||
| Hannesdottir et al. [40] | Normotensive | 30 | 68.3(8.5) | 53.3 | 127.0(11.3) | 74.0(10.0) | SBP/DBP | Positive | - |
| Treated Hypertension | 40 | 69.3(11.3) | 60.0 | 152.0(19.4) | 85.0(11.0) | ||||
| Untreated Hypertension | 10 | 57.6(6.1) | 50.0 | 167.0(16.3) | 100.0(6.3) | ||||
| Huang et al. [39] | Hypertension | 446 | 93.6 (3.4) | 31.1 | 154.8 (17.4) | 77.0 (6.0) | SBP/DBP | No relation | - |
| Normotension | 336 | 93.7 (3.4) | 34.2 | 120.3 (12.3) | 66.9 (9.3) | ||||
| Yeung and Loken Thornton [70] | Hypertensive | 71 | 70.3 (6.5) | 50 | 136.3 (10.2) | 73.3 (8.6) | SBP | Positive | - |
| Normotensive | 62 | 70.2 (6.4) | 38 | 119.3 (12.8) | 71.2 (8.4) | ||||
| Longitudinal Studies | |||||||||
| Elias et al. [71] | 1702 | 55–88 | SBP/DBP | Positive | 20 | ||||
| Elias et al. [72] | 1695 | 67.2 (7.5) | 59.4 | 131.1 (17.6) | 82.1 (9.4) | SBP/DBP | Not direct | 28 | |
| Launer et al. [73] | 3735 | 52.7 (4.7) | 100 | 131.3 (16.6) | 82.9 (9.4) | SBP | Positive | 28 | |
| Guo et al. [74] | 1736 | 75–101 | 25 | SBP/DBP | Negative | 3 | |||
| Kilande et al. [75] | 999 | 100 | 82.0 (10.0) | DBP | Positive | 20 | |||
| Swan et al. [76] | 717 | 76.3 (4.1) | 134.2 (8.8) | 85.8 (5.9) | SBP | Positive | 25–30 | ||
| Glynn et al. [77] | 3809 | >65 | 38 | 145.6 (6.2) | SBP | J curve | 9 | ||
| Knopman et al. [78] | 10,882 | 56.8 (5.7) | 44 | SBP/DBP | Positive | 6 | |||
| Bohannon et al. [79] | 3202 | 73 (6.29) | 33 | 143.1 (20.3) | 79.2 (11.8) | SBP | J curve | 3 | |
| Elias et al. [80] | Men | 551 | 65.7 (6.9) | 100 | 131.4 (14.6) | 82.7 (8.3) | SBP/DBP | Positive only in men | 4–6 |
| Women | 872 | 67.2 (7.3) | 0 | 131.7 (16.9) | 80.1 (8.4) | ||||
| Reinprecht et al. [81] | 186 | 68.0 | DBP | Positive | 13 | ||||
| Kähönen-Väre et al. [82] | 650 | >75 | 26.3 | 157.9 (2.0) | 82.3 (1.0) | Lower DBP | Positive | 10 | |
| Hebert et al. [83] | 4284 | 74.0 (6.4) | 48 | 139.6 (19.6) | 77.3 (11.5) | DBP | U curve | 6 | |
| Waldstein et al. [84] | 847 | 70.6 (8.5) | 59 | 138.7 (20.0) | 82.0 (10.9) | SBP/DBP | U curve | 11 | |
| Kuo et al. [85] | 2802 | 73.6 (5.9) | 24.1 | SBP | Positive | 2 | |||
| Euser et al. [86] | 276 | >85 | 28 | SBP | Negative | ||||
| Singh-Manoux and Marmot [87] | Man | 5838 | 43.9 (6.0) | SBP/DBP | Positive in man | 2 | |||
| Woman | 44.4 (6.0) | ||||||||
| Gottesman et al. [88] | 13,476 | 57.0 (6.0) | SBP/DBP | Positive | 20 | ||||
| Yaffe et al. [89] | 3381 | 18–30 | SBP/DBP | Positive | 25 | ||||
| Van Vliet et al. [90] | 590 | >85 | 28 | 157.7 | SBP/DBP | No Association | 9 | ||
| Arntzen et al. [91] | Men | 2227 | 58.8 (9.2) | 143.3 (19.3) | SBP | Positive | 7 | ||
| Women | 2806 | 58.2 (9.7) | 141.6 (22.6) | ||||||
| Debette et al. [92] | 1352 | 54.0 (9.0) | 47 | SBP/DBP | Positive | 10 | |||
| Yasar et al. [93] | 336 | 76–80 | 0 | SBP/DBP | Positive | 9 | |||
| Sabayan et al. [94] | 572 | >85 | 33 | SBP | Negative | 5 | |||
| Dregan et al. [95] | 8780 | >50 | 45 | SBP/DBP | Positive | 6 | |||
| Goldstein et al. [96] | 1385 | 73.5 (8.9) | 48.7 | 134.1 (14.3) | 74.7 (9.0) | SBP/DBP | Positive | 3 | |
| Taylor et al. [97] | 1484 | 40–67 | 76 | DBP | U-Shaped | 20 | |||
| Kohler et al. [98] | 1805 | 60 | SBP/DBP | Positive | 6/12 | ||||
| Chen et al. [99] | 247 | 50.1 (2.58) | 0 | 123.3 (16.3) | SBP/DBP | Positive | 10 | ||
| Kesse-Guyot et al. [100] | 2788 | 40–67 | 76 | SBP/DBP | No Relationship | 10 | |||
| Harrison et al. [101] | 845 | >85 | DBP | Negative | 5 | ||||
| Goldstein et al. [102] | 844 | 74.7 | 36 | 140.9 | 76.2 | SBP/DBP | Positive | 4 | |
| Ferreira et al. [103] | 131 | 67.7 (5.3) | 48.1 | 136.7 (16.1) | 73.6 (9.4) | SBP | Indirect relation | 7 | |
| Levine et al. [104] | 22,164 | >45 | SBP/DBP | Positive | 8 | ||||
| André-Petersson et al. [105] | Normotensive | 24 | 68 | 100 | SBP/DBP | Positive | 13 | ||
| Hypertensive 1 | 73 | ||||||||
| Hypertensive 2 | 46 | ||||||||
| Hypertensive 3 | 25 | ||||||||
| Brady et al. [106] | Normal blood pressure: | SBP/DBP | No direct | 3 | |||||
| Normotensive | 203 | 66.0 (7.0) | 124.4 (9.4) | 78.5 (5.9) | |||||
| Controlled | 34 | 68.6 (6.0) | 127.2 (7.9) | 77.8 (8.1) | |||||
| Hypertensive: | |||||||||
| Untreated | 75 | 68.4 (7.5) | 156.8 (16.1) | 89.1 (11) | |||||
| Uncontrolled | 45 | 69.5 (6.1) | 15.2 (14.3) | 89.0 (9.4) | |||||
3. Results
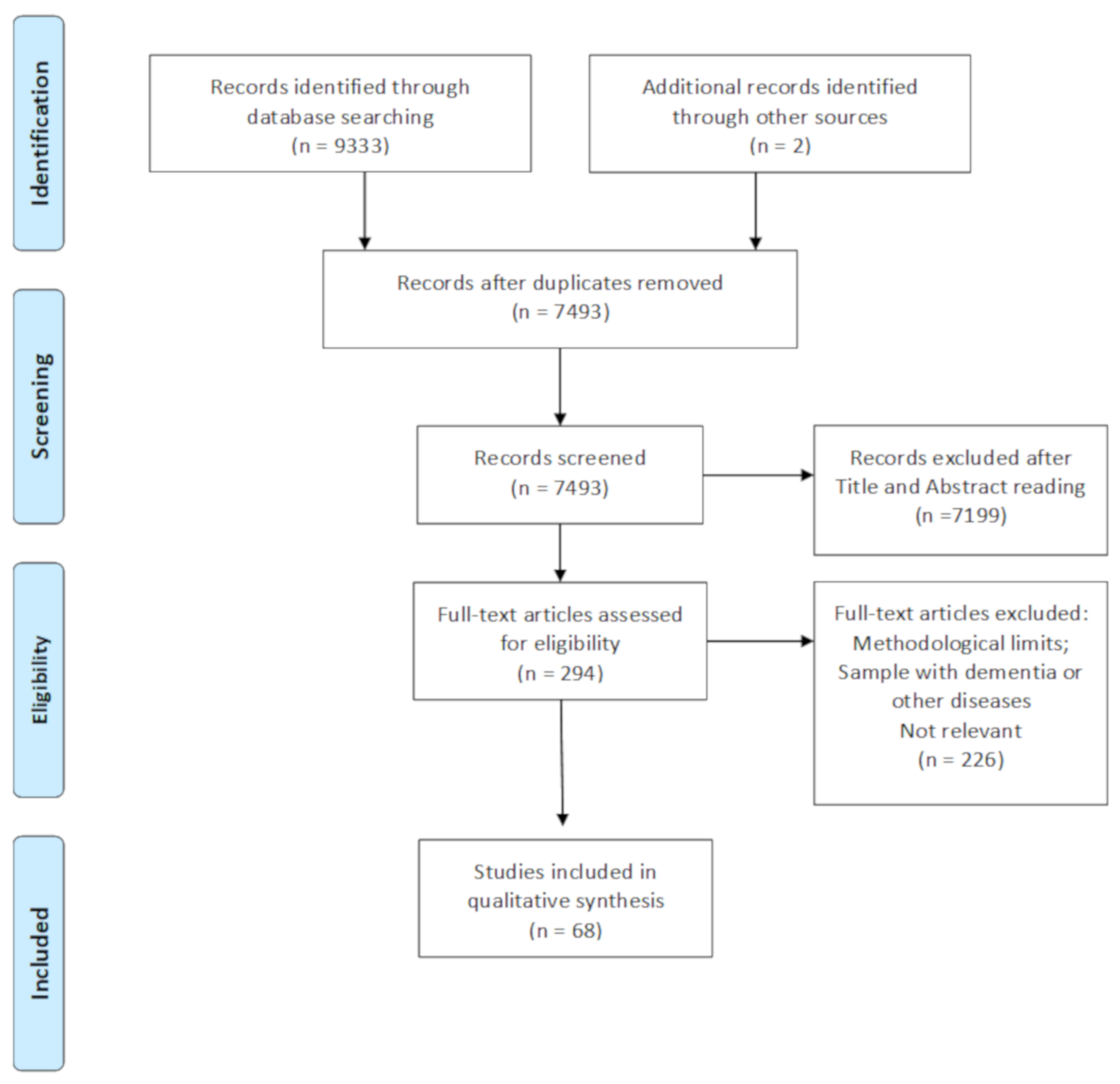
3.1. Studies Selection
3.2. Quality Assessment
3.3. Demographic Features
3.4. Blood Pressure Measurements
3.5. Summary of Evidence
3.5.1. Cross-Sectional Evidence of the Relation between Blood Pressure and Cognitive Functions
Young
Middle Aged
Elderly
Old Age
3.5.2. Longitudinal Evidence of the Relation between Blood Pressure and Cognitive Functions
Young
Middle Aged
Elderly
Old Age
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Domain | Support for Judgment |
|---|---|
| Selection Bias | |
| Use of international guidelines for the assessment and measurement of blood pressure | Describe the method used for the assessment of blood pressure and whether international guidelines have been used |
| Selection of Sample and control of confounding variables | Describe the method used to select the participant and to assess and control the confounding variables |
| Detection bias | |
| Use of appropriate tasks for the measurement of the cognitive domains considered | Describe all measures used to the assessment of cognitive domains |
| Attrition bias | |
| Incomplete outcome data | Describe the completeness of data for each main outcome, including attrition and exclusion from the analysis. State whether attrition and exclusion were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusion were reported. |
| Reporting bias | |
| Selective reporting | State how the possibility of selective outcome reporting was examined by the authors’ review and what was found. |
| Other bias | |
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre-specified in the review’s protocol, responses should be provided for each question/entry. |
References
- Harada, C.N.; Love, M.C.N.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Park, D.C.; Festini, S.B. Theories of memory and aging: A look at the past and a glimpse of the future. J. Gerontol. B Psychol. Sci. Soc. Sci. 2017, 72, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wecker, N.S.; Kramer, J.H.; Hallam, B.J.; Delis, D.C. Mental flexibility: Age effects on switching. Neuropsychology 2005, 19, 345. [Google Scholar] [CrossRef]
- Wecker, N.S.; Kramer, J.H.; Wisniewski, A.; Delis, D.C.; Kaplan, E. Age effects on executive ability. Neuropsychology 2000, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- DeCarli, C. Mild cognitive impairment: Prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003, 2, 15–21. [Google Scholar] [CrossRef]
- Murman, D.L. The impact of age on cognition. Semin. Hear. 2015, 36, 111. [Google Scholar] [CrossRef]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Deary, I.J. Introduction to the special issue on cognitive epidemiology. Intelligence 2009, 37, 517–519. [Google Scholar] [CrossRef]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Guarino, A.; Forte, G.; Giovannoli, J.; Casagrande, M. Executive functions in the elderly with mild cognitive impairment: A systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment. Health 2020, 24, 1028–1045. [Google Scholar] [CrossRef]
- Corley, J.; Gow, A.J.; Starr, J.M.; Deary, I.J. Smoking, childhood IQ, and cognitive function in old age. J. Psychosom. Res. 2012, 73, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Beresford, T.P.; Arciniegas, D.B.; Alfers, J.; Clapp, L.; Martin, B.; Du, Y.; Liu, D.; Shen, D.; Davatzikos, C. Hippocampus volume loss due to chronic heavy drinking. Alcohol. Clin. Exp. Res. 2006, 30, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Prickett, C.; Brennan, L.; Stolwysk, R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pract. 2015, 9, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Hay, P.; Campbell, L.; Trollor, J.N. A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obes. Rev. 2011, 12, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Forte, G.; Casagrande, M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front. Psychol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Dröge, W.; Schipper, H.M. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 2007, 6, 361–370. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Wei, J.; Liu, W.; Zhong, P.; Li, X.; Yan, Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012, 73, 962–977. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef]
- Jorm, A.F. Is depression a risk factor for dementia or cognitive decline? Gerontology 2000, 46, 219–227. [Google Scholar] [CrossRef]
- Leritz, E.C.; McGlinchey, R.E.; Kellison, I.; Rudolph, J.L.; Milberg, W.P. Cardiovascular disease risk factors and cognition in the elderly. Curr. Cardiovasc. Risk Rep. 2011, 5, 407. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Rodrigue, K.M. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci. Biobehav. Rev. 2006, 30, 730–748. [Google Scholar] [CrossRef] [PubMed]
- Flicker, L. Cardiovascular risk factors, cerebrovascular disease burden, and healthy brain aging. Clin. Geriatr. Med. 2010, 26, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Jennings, J.R.; Zanstra, Y. Is the brain the essential in hypertension? Neuroimage 2009, 47, 914–921. [Google Scholar] [CrossRef]
- Hayden, K.M.; Zandi, P.P.; Lyketsos, C.G.; Khachaturian, A.S.; Bastian, L.A.; Charoonruk, G.; JoAnn, T.T.; Maria, C.N.; Carl, F.P.; Ron, G.M.; et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: The Cache County study. Alzheimer Dis. Assoc. Disord. 2006, 20, 93–100. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Forte, G.; De Pascalis, V.; Favieri, F.; Casagrande, M. Effects of Blood Pressure on Cognitive Performance: A Systematic Review. J. Clin. Med. 2020, 9, 34. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.; Fullerton, H.J.; Howard, V.J.; et al. Executive summary: Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, 434–441. [Google Scholar] [CrossRef]
- Gabb, G.M.; Mangoni, A.A.; Anderson, C.S.; Cowley, D.; Dowden, J.S.; Golledge, J.; Hankey, G.J.; Howes, F.S.; Leckie, L.; Perkovic, V.; et al. Guideline for the diagnosis and management of hypertension in adults. Med. J. Aust. 2016, 205, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Davisson, R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A. Hypertension and the brain: A risk factor for more than heart disease. Cerebrovasc. Dis. 2016, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Duron, E.; Hanon, O. Vascular risk factors, cognitve decline, and dementia. Vasc. Health Risk Manag. 2008, 4, 363. [Google Scholar] [PubMed]
- Kennelly, S.P.; Lawlor, B.A.; Kenny, R.A. Blood pressure and dementia—A comprehensive review. Ther. Adv. Neurol. Disord. 2009, 2, 241–260. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, M.P.; Gaillard, C.; Houx, P.J.; Buntinx, F.; de Leeuw, P.W.; Jolles, J. Can the blood pressure predict cognitive task performance in a healthy population sample? J. Hypertens. 1997, 15, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Scherr, P.A.; Hebert, L.E.; Bennett, D.A.; Wilson, R.S.; Glynn, R.J.; Evans, D.A. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002, 21, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.R.; Brown, J.R.P.; Maier, K.J.; Katzel, L.I. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann. Behav. Med. 2005, 29, 174–180. [Google Scholar] [CrossRef]
- Huang, C.-Q.; Dong, B.-R.; Zhang, Y.-L.; Wu, H.-M.; Liu, Q.-X.; Flaherty, J.H. Cognitive impairment and hypertension among Chinese nonagenarians and centenarians. Hypertens. Res. 2009, 32, 554–558. [Google Scholar] [CrossRef]
- Hannesdottir, K.; Nitkunan, A.; Charlton, R.A.; Barrick, T.R.; MacGregor, G.A.; Markus, H.S. Cognitive impairment and white matter damage in hypertension: A pilot study. Acta Neurol. Scand. 2009, 119, 261–268. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4. [Google Scholar]
- Farmer, M.E.; White, L.R.; Abbott, R.D.; Kittner, S.J.; Kaplan, E.; Wolz, M.M.; Brody, J.A.; Wolf, P.A. Blood pressure and cognitive performance: The Framingham Study. Am. J. Epidemiol. 1987, 126, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.F.; Robbins, M.A.; Schultz, N.R.; Pierce, T.W. Is blood pressure an important variable in research on aging and neuropsychological test performance? J. Gerontol. 1990, 45, P128–P135. [Google Scholar] [CrossRef] [PubMed]
- Starr, J.M.; Deary, I.J.; Inch, S.; Cross, S.; MacLennan, W.J. Blood pressure and cognitive decline in healthy old people. J. Hum. Hypertens. 1997, 11, 777–781. [Google Scholar] [CrossRef]
- Cacciatore, F.; Abete, P.; Ferrara, N.; Paolisso, G.; Amato, L.; Canonico, S.; Maggi, S.; Varricchio, M.; Rengo, F. The role of blood pressure in cognitive impairment in an elderly population. J. Hypertens. 1997, 15, 135–142. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Folsom, A.R.; Mortimer, J.A.; Shahar, E.; Knopman, D.S.; McGovern, P.G.; Hays, M.A.; Crum, L.D.; Heiss, G. Correlates of Cognitive Function in Middle-Aged Adults. Gerontology 1998, 44, 95–105. [Google Scholar] [CrossRef]
- Di Carlo, A.; Baldereschi, M.; Amaducci, L.; Maggi, S.; Grigoletto, F.; Scarlato, G.; Inzitari, D.; For the Italian Longitudinal Study on Aging Working Group. Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J. Am. Geriatr. Soc. 2000, 48, 775–782. [Google Scholar] [CrossRef]
- Izquierdo-Porrera, A.M.; Waldstein, S.R. Cardiovascular risk factors and cognitive function in African Americans. Gerontol. B Psychol. Sci. Soc. Sci. 2002, 57, P377–P380. [Google Scholar] [CrossRef]
- Pandav, R.; Dodge, H.H.; DeKosky, S.T.; Ganguli, M. Blood Pressure and Cognitive Impairment in India and the United States: A Cross-National Epidemiological Study. Arch. Neurol. 2003, 60, 1123–1128. [Google Scholar] [CrossRef]
- Kuo, H.-K.; Sorond, F.; Iloputaife, I.; Gagnon, M.; Milberg, W.; Lipsitz, L.A. Effect of blood pressure on cognitive functions in elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Wharton, W.; Hirshman, E.; Merritt, P.; Stangl, B.; Scanlin, K.; Krieger, L. Lower blood pressure correlates with poorer performance on visuospatial attention tasks in younger individuals. Bio. Psychol. 2006, 73, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Reinprecht, F.; Siennicki-Lantz, A.; Elmstahl, S. Low ambulatory blood pressure is associated with lower cognitive function in healthy elderly men. Blood Press. Monit. 2008, 13, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Wersching, H.; Lohmann, H.; Bruchmann, M.; Duning, T.; Dziewas, R.; Berger, K.; Ringelstein, E.B. High-normal blood pressure is associated with poor cognitive performance. Hypertension 2008, 51, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Solanki, R.K.; Pathak, V. Blood pressure is associated with cognitive impairment in young hypertensives. World J. Biol. Psychiatry 2008, 9, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Obisesan, T.O.; Obisesan, O.A.; Martins, S.; Alamgir, L.; Bond, V.; Maxwell, C.; Gillum, R.F. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: The Third National Health and Nutrition Examination Survey. J. Am. Geriatr. Soc. 2008, 56, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Wersching, H.; Lohmann, H.; Berger, K.; Ringelstein, E.B. How much does hypertension affect cognition? Explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. J. Neurol. Sci. 2009, 283, 149–152. [Google Scholar] [CrossRef]
- Gunstad, J.; Keary, T.A.; Spitznagel, M.B.; Poppas, A.; Paul, R.H.; Sweet, L.H.; Hoth, K.F.; Haley, A.P.; Forman, D.E.; Cohen, R.A. Blood pressure and cognitive function in older adults with cardiovascular disease. Int. J. Neurol. 2009, 119, 2228–2242. [Google Scholar] [CrossRef]
- Yeung, S.E.; Thornton, W.L. Age-related effects of blood pressure on everyday cognitive function in community-dwelling women. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2011, 18, 733–755. [Google Scholar] [CrossRef]
- Richmond, R.; Law, J.; Kay-Lambkin, F. Higher blood pressure associated with higher cognition and functionality among centenarians in Australia. Am. J. Hypertens. 2011, 24, 299–303. [Google Scholar] [CrossRef]
- Peltz, C.B.; Corrada, M.M.; Berlau, D.J.; Kawas, C.H. Cognitive impairment in nondemented oldest-old: Prevalence and relationship to cardiovascular risk factors. Alzheimers Dement. 2012, 8, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Davey, A.; Alkerwi, A. Cardiovascular health and cognitive function: The Maine-Syracuse Longitudinal Study. PLoS ONE 2014, 9, e89317. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.S.; Forbang, N.; Beben, T.; Criqui, M.H.; Ix, J.H.; Rifkin, D.E. Relationship Between 24-Hour Ambulatory Blood Pressure and Cognitive Function in Community-Living Older Adults: The UCSD Ambulatory Blood Pressure Study. Am. J. Hypertens. 2015, 28, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.R.; Jennings, J.R.; Ryan, C.M.; Muldoon, M.F.; Shapiro, A.P.; Polefrone, J.M.; Fazzari, T.V.; Manuck, S. Hypertension and neuropsychological performance in men: Interactive effects of age. Health Psychol. 1996, 15, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Harrington, F.; Saxby, B.K.; McKeith, I.G.; Wesnes, K.; Ford, G.A. Cognitive performance in hypertensive and normotensive older subjects. Hypertension 2000, 36, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- André-Petersson, L.; Hagberg, B.; Janzon, L.; Steen, G. A comparison of cognitive ability in normotensive and hypertensive 68-year-old men: Results from population study “Men born in 1914,” in Malmö, Sweden. Exp. Aging Res. 2001, 27, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Saxby, B.K.; Harrington, F.; McKeith, I.G.; Wesnes, K.; Ford, G.A. Effects of hypertension on attention, memory, and executive function in older adults. Health Psychol. 2003, 22, 587–591. [Google Scholar] [CrossRef]
- Waldstein, S.R.; Katzel, L.I. Gender differences in the relation of hypertension to cognitive function in older adults. Neurol. Res. 2004, 26, 502–506. [Google Scholar] [CrossRef]
- Yeung, S.E.; Loken Thornton, W. “Do it-yourself”: Home blood pressure as a predictor of traditional and everyday cognition in older adults. PLoS ONE 2017, 12, e0177424. [Google Scholar] [CrossRef]
- Elias, M.F.; Wolf, P.A.; D’Agostino, R.B.; Cobb, J.; White, L.R. Untreated blood pressure level is inversely related to cognitive functioning: The Framingham Study. Am. J. Epidem. 1993, 138, 353–364. [Google Scholar] [CrossRef]
- Elias, M.F.; D’Agostino, R.B.; Elias, P.K.; Wolf, P.A. Neuropsychological test performance, cognitive functioning, blood pressure, and age: The Framingham Heart Study. Exp. Aging Res. 1995, 21, 369–391. [Google Scholar] [CrossRef]
- Launer, L.J.; Masaki, K.; Petrovitch, H.; Foley, D.; Havlik, R.J. The Association Between Midlife Blood Pressure Levels and Late-Life Cognitive Function: The Honolulu-Asia Aging Study. JAMA 1995, 274, 1846–1851. [Google Scholar] [CrossRef]
- Guo, Z.; Fratiglioni, L.; Winblad, B.; Viitanen, M. Blood pressure and performance on the Mini-Mental State Examination in the very old: Cross-sectional and longitudinal data from the Kungsholmen Project. Am. J. Epidemiol. 1997, 145, 1106–1113. [Google Scholar] [CrossRef]
- Kilander, L.; Nyman, H.; Boberg, M.; Hansson, L.; Lithell, H. Hypertension is related to cognitive impairment: A 20-year follow-up of 999 men. Hypertension 1998, 31, 780–786. [Google Scholar] [CrossRef]
- Swan, G.E.; Carmelli, D.; Larue, A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke 1998, 29, 2334–2340. [Google Scholar] [CrossRef]
- Glynn, R.J.; Beckett, L.A.; Hebert, L.E.; Morris, M.C.; Scherr, P.A.; Evans, D.A. Current and remote blood pressure and cognitive decline. JAMA 1999, 281, 438–445. [Google Scholar] [CrossRef]
- Knopman, D.; Boland, L.L.; Mosley, T.; Howard, G.; Liao, D.; Szklo, M.; McGovern, P.; Folsom, A.R. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001, 56, 42–48. [Google Scholar] [CrossRef]
- Bohannon, A.D.; Fillenbaum, G.G.; Pieper, C.F.; Hanlon, J.T.; Blazer, D.G. Relationship of race/ethnicity and blood pressure to change in cognitive function. J. Am. Geriatr. Soc. 2002, 50, 424–429. [Google Scholar] [CrossRef]
- Elias, M.F.; Elias, P.K.; Sullivan, L.M.; Wolf, P.A.; D’Agostino, R.B. Lower cognitive function in the presence of obesity and hypertension: The Framingham heart study. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 260–268. [Google Scholar] [CrossRef]
- Reinprecht, F.; Elmstahl, S.; Janzon, L.; Andre-Petersson, L. Hypertension and changes of cognitive function in 81-year-old men: A 13-year follow-up of the population study “Men born in 1914”, Sweden. J. Hypertens. 2003, 21, 57–66. [Google Scholar] [CrossRef]
- Kähönen-Väre, M.; Brunni-Hakala, S.; Lindroos, M.; Pitkala, K.; Strandberg, T.; Tilvis, R. Left ventricular hypertrophy and blood pressure as predictors of cognitive decline in old age. Aging Clin. Exp. Res. 2004, 16, 147–152. [Google Scholar] [CrossRef]
- Hebert, L.E.; Scherr, P.A.; Bennett, D.A.; Bienias, J.L.; Wilson, R.S.; Morris, M.C.; Evans, D.A. Blood pressure and late-life cognitive function change: A biracial longitudinal population study. Neurology 2004, 62, 2021–2024. [Google Scholar] [CrossRef]
- Waldstein, S.R.; Giggey, P.P.; Thayer, J.F.; Zonderman, A.B. Non-linear relations of blood pressure to cognitive function: The Baltimore Longitudinal Study of Aging. Hypertension 2005, 45, 374–379. [Google Scholar] [CrossRef]
- Kuo, H.-K.; Jones, R.N.; Milberg, W.P.; Tennstedt, S.; Talbot, L.; Morris, J.N.; Lipsitz, L.A. Effect of blood pressure and diabetes mellitus on cognitive and physical functions in older adults: A longitudinal analysis of the advanced cognitive training for independent and vital elderly cohort. J. Am. Geriatr. Soc. 2005, 53, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Euser, S.M.; Van Bemmel, T.; Schram, M.T.; Gussekloo, J.; Hofman, A.; Westendorp, R.G.J.; Breteler, M. The effect of age on the association between blood pressure and cognitive function later in life. J. Am. Geriatr. Soc. 2009, 57, 1232–1237. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Marmot, M. High blood pressure was associated with cognitive function in middle-age in the Whitehall II study. J. Clin. Epidemiol. 2005, 58, 1308–1315. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Schneider, A.L.C.; Albert, M.; Alonso, A.; Bandeen-Roche, K.; Coker, L.; Coresh, J.; Knopman, D.; Power, M.C.; Rawlings, A.; et al. Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014, 71, 1218–1227. [Google Scholar] [CrossRef]
- Yaffe, K.; Vittinghoff, E.; Pletcher, M.J.; Hoang, T.D.; Launer, L.J.; Whitmer, R.A.; Coker, L.H.; Sidney, S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014, 129, 1560–1567. [Google Scholar] [CrossRef]
- Van Vliet, P.; Westendorp, R.G.; Van Heemst, D.; de Craen, A.J.; Oleksik, A.M. Cognitive decline precedes late-life longitudinal changes in vascular risk factors. J. Neurol. Neurosur Psychiatry 2010, 81, 1028–1032. [Google Scholar] [CrossRef][Green Version]
- Arntzen, K.A.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B. Impact of cardiovascular risk factors on cognitive function: The Tromso study. Eur. J. Neurol. 2011, 18, 737–743. [Google Scholar] [CrossRef]
- Debette, S.; Seshadri, S.; Beiser, A.; Au, R.; Himali, J.J.; Palumbo, C.; Wolf, P.A.; DeCarli, C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011, 77, 461–468. [Google Scholar] [CrossRef]
- Yasar, S.; Ko, J.Y.; Nothelle, S.; Mielke, M.M.; Carlson, M.C. Evaluation of the effect of systolic blood pressure and pulse pressure on cognitive function: The Women’s Health and Aging Study II. PLoS ONE 2011, 6, e27976. [Google Scholar] [CrossRef]
- Sabayan, B.; Oleksik, A.M.; Maier, A.B.; Van Buchem, M.A.; Poortvliet, R.K.; De Ruijter, W.; Gussekloo, J.; De Craen, A.J.M.; Westendorp, R.G.J. High blood pressure and resilience to physical and cognitive decline in the oldest old: The Leiden 85-plus study. J. Am. Geriatr. Soc. 2012, 60, 2014–2019. [Google Scholar] [CrossRef]
- Dregan, A.; Stewart, R.; Gulliford, M.C. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: A population-based cohort study. Age Ageing 2013, 42, 338–345. [Google Scholar] [CrossRef]
- Goldstein, F.C.; Levey, A.I.; Steenland, N.K. High blood pressure and cognitive decline in mild cognitive impairment. J. Am. Geriatr. Soc. 2013, 61, 67–73. [Google Scholar] [CrossRef]
- Taylor, C.; Tillin, T.; Chaturvedi, N.; Dewey, M.; Ferri, C.P.; Hughes, A.; Prince, M.; Richards, M.; Shah, A.; Stewart, R. Midlife Hypertensive Status and Cognitive Function 20 Years Later: The S outhall and B rent Revisited Study. J. Am. Geriatr. Soc. 2013, 61, 1489–1498. [Google Scholar] [CrossRef]
- Köhler, S.; Baars, M.A.; Spauwen, P.; Schievink, S.; Verhey, F.R.; van Boxtel, M.J. Temporal evolution of cognitive changes in incident hypertension: Prospective cohort study across the adult age span. Hypertension 2014, 63, 245–251. [Google Scholar] [CrossRef]
- Chen, K.H.; Henderson, V.W.; Stolwyk, R.J.; Dennerstein, L.; Szoeke, C. Prehypertension in midlife is associated with worse cognition a decade later in middle-aged and older women. Age Ageing 2014, 44, 439–445. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Andreeva, V.A.; Lassale, C.; Hercberg, S.; Galan, P. Clustering of midlife lifestyle behaviors and subsequent cognitive function: A longitudinal study. Am. J. Public Health 2014, 104, e170–e177. [Google Scholar] [CrossRef]
- Harrison, S.L.; Stephan, B.C.; Siervo, M.; Granic, A.; Davies, K.; Wesnes, K.A.; Kirkwood, T.B.L.; Robinson, L.; Jagger, C. Is there an association between metabolic syndrome and cognitive function in very old adults? The Newcastle 85+ Study. J. Am. Geriatr. Soc. 2015, 63, 667–675. [Google Scholar] [CrossRef]
- Goldstein, F.C.; Hajjar, I.M.; Dunn, C.B.; Levey, A.I.; Wharton, W. The Relationship Between Cognitive Functioning and the JNC-8 Guidelines for Hypertension in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Kearney Schwartz, A.; Watfa, G.; Zohra, L.; Felblinger, J.; Boivin, J.-M.; Bracard, S.; Hossu, G.; Verger, A.; Joly, L.; et al. Memory Alterations and White Matter Hyperintensities in Elderly Patients With Hypertension: The ADELAHYDE-2 Study. J. Am. Med. Dir. Assoc. 2017, 18, 451.e13–451.e25. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Galecki, A.T.; Langa, K.M.; Unverzagt, F.W.; Kabeto, M.U.; Giordani, B.; Cushman, M.; McClure, L.A.; Safford, M.M.; Wadley, V.G. Blood pressure and cognitive decline over 8 years in middle-aged and older black and white americans. Hypertension 2019, 73, 310–318. [Google Scholar] [CrossRef] [PubMed]
- André-Petersson, L.; Elmståhl, S.; Hagberg, B.; Janzon, L.; Reinprecht, F.; Steen, G. Is blood pressure at 68 an independent predictor of cognitive decline at 81? Results from follow-up study “Men born in 1914”, Malmö, Sweden. Aging Ment. Health 2003, 7, 61–72. [Google Scholar]
- Brady, C.B.; Spiro A Gaziano, J.M., 3rd. Effects of age and hypertension status on cognition: The Veterans Affairs Normative Aging Study. Neuropsychology 2005, 19, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Guidelines Committee. 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J. Hypertens. 2003, 21, 1011–1053. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press. 2013, 22, 193–278. [Google Scholar] [CrossRef]
- Iadecola, C.; Yaffe, K.; Biller, J.; Bratzke, L.C.; Faraci, F.M.; Gorelick, P.B.; Gulati, M.; Kamel, H.; Knopman, D.S.; Launer, L.J.; et al. Impact of hypertension on cognitive function: A scientific statement from the American Heart Association. Hypertension 2016, 68, e67–e94. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurol. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Lacolley, P. Structural and genetic bases of arterial stiffness. Hypertension 2005, 45, 1050–1055. [Google Scholar] [CrossRef]
- Iadecola, C. Hypertension and dementia. Hypertension 2014, 64, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J. Cereb. Blood Flow Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef]
- Mathiesen, C.; Caesar, K.; Akgören, N.; Lauritzen, M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J. Physiol. 1998, 512, 555–566. [Google Scholar] [CrossRef]
- Jennings, J.R.; Muldoon, M.F.; Ryan, C.; Price, J.C.; Greer, P.; Sutton-Tyrrell, K.; Van Der Veen, F.M.; Meltzer, C.C. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology 2005, 64, 1358–1365. [Google Scholar] [CrossRef]
- Shekhar, S.; Liu, R.; Travis, O.K.; Roman, R.J.; Fan, F. Cerebral autoregulation in hypertension and ischemic stroke: A mini review. J. Pharm. Sci. Exp. Pharmacol. 2017, 2017, 21. [Google Scholar]
- Gottesman, R.F. Should hypertension be treated in late life to preserve cognitive function? Con side of the argument. Hypertension 2018, 71, 787–792. [Google Scholar] [CrossRef]
- Cherubini, A.; Lowenthal, D.T.; Paran, E.; Mecocci, P.; Williams, L.S.; Senin, U. Hypertension and cognitive function in the elderly. Dis. Mon. 2010, 56, 106–147. [Google Scholar] [CrossRef]
- Baron, J.C. Perfusion thresholds in human cerebral ischemia: Historical perspective and therapeutic implications. Cerebrovasc. Dis. 2001, 11 (Suppl. 1), 2–8. [Google Scholar] [CrossRef]
- Strandgaard, S.; Paulson, O.B. Regulation of cerebral blood flow in health and disease. J. Cardiovasc. Pharmacol. 1992, 19, S89–S93. [Google Scholar] [CrossRef]
- Roman, H.; Descargues, G.; Lopes, M.; Emery, E.; Clavier, E.; Diguet, A.; Proust, F. Subarachnoid hemorrhage due to cerebral aneurysmal rupture during pregnancy. Acta Obstet. Gynecol. Scand. 2004, 83, 330–334. [Google Scholar] [CrossRef]
- Lung, H.W.; Kim, K.I. Blood pressure variability and cognitive function in the elderly. Pulse 2013, 1, 29–34. [Google Scholar]
- Schroeder, E.B.; Liao, D.; Chambless, L.E.; Prineas, R.J.; Evans, G.W.; Heiss, G. Hypertension, blood pressure, and heart rate variability: The Atherosclerosis Risk in Communities (ARIC) study. Hypertension 2003, 42, 1106–1111. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neuros. 2019, 13, 710. [Google Scholar] [CrossRef]

| Issue | Database | Script |
|---|---|---|
| Blood Pressure | Pubmed | (cognit* or neuropsychology*) AND (blood pressure or hypertens* or high blood pressure) |
| PsychINFO | (cognit* or neuropsychology*) AND (blood pressure or hypertens* or high blood pressure) | |
| Medline | (cognit* or neuropsychology*) AND (blood pressure or hypertens* or high blood pressure) | |
| PsycArticles | (cognit* or neuropsychology*) AND (blood pressure or hypertens* or high blood pressure) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forte, G.; Casagrande, M. Effects of Blood Pressure on Cognitive Performance in Aging: A Systematic Review. Brain Sci. 2020, 10, 919. https://doi.org/10.3390/brainsci10120919
Forte G, Casagrande M. Effects of Blood Pressure on Cognitive Performance in Aging: A Systematic Review. Brain Sciences. 2020; 10(12):919. https://doi.org/10.3390/brainsci10120919
Chicago/Turabian StyleForte, Giuseppe, and Maria Casagrande. 2020. "Effects of Blood Pressure on Cognitive Performance in Aging: A Systematic Review" Brain Sciences 10, no. 12: 919. https://doi.org/10.3390/brainsci10120919
APA StyleForte, G., & Casagrande, M. (2020). Effects of Blood Pressure on Cognitive Performance in Aging: A Systematic Review. Brain Sciences, 10(12), 919. https://doi.org/10.3390/brainsci10120919





