Developmental Language Disorder: Wake and Sleep Epileptiform Discharges and Co-morbid Neurodevelopmental Disorders
Abstract
1. Introduction
2. Subjects and Methods
2.1. Phoniatric Examination
2.2. Psychological Tests
2.3. Neurological Examination
2.4. EEG and Nocturnal Video EEG or PSG Study
2.5. Statistical Analyses
3. Results
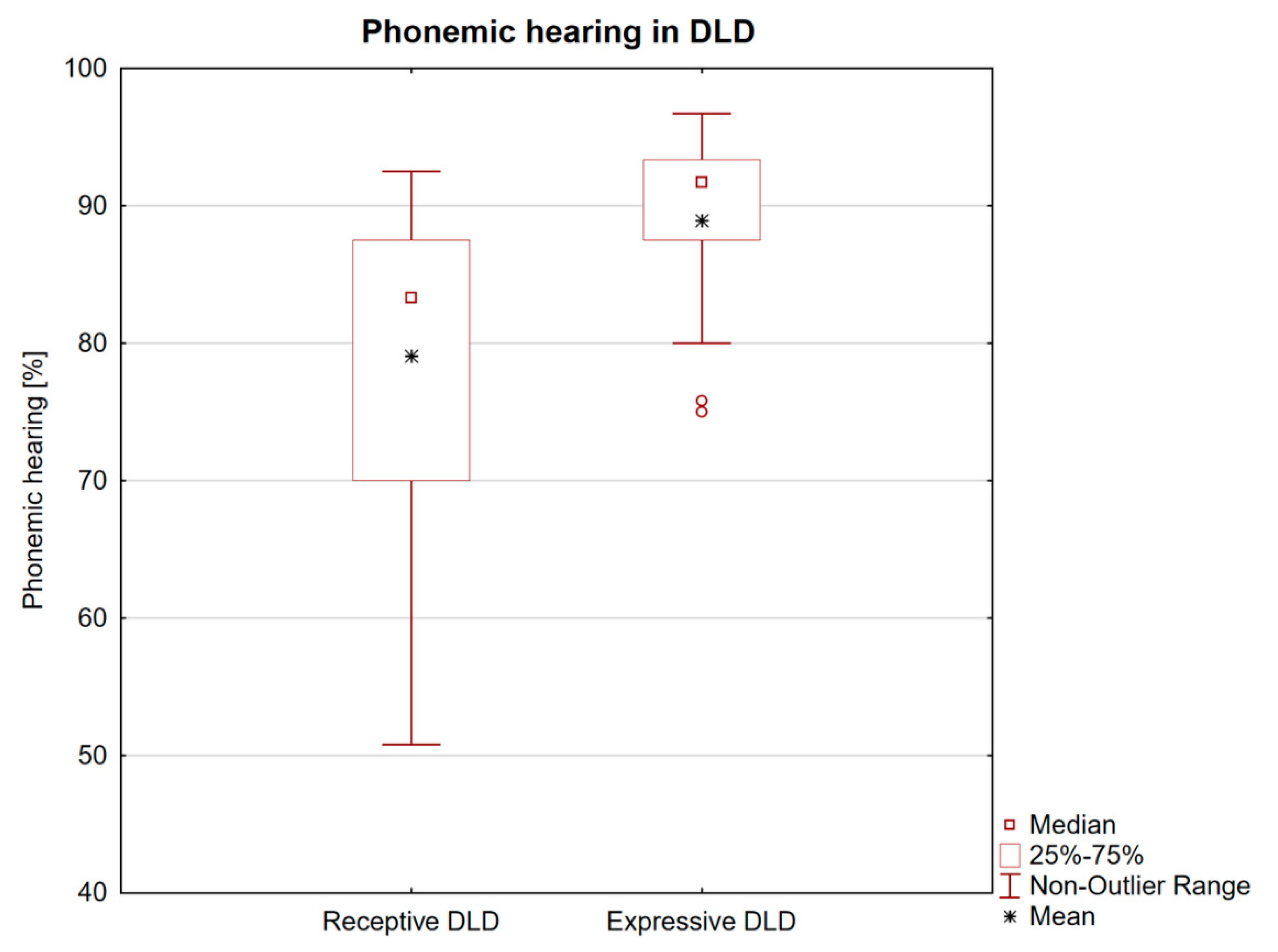
3.1. Clinical Data
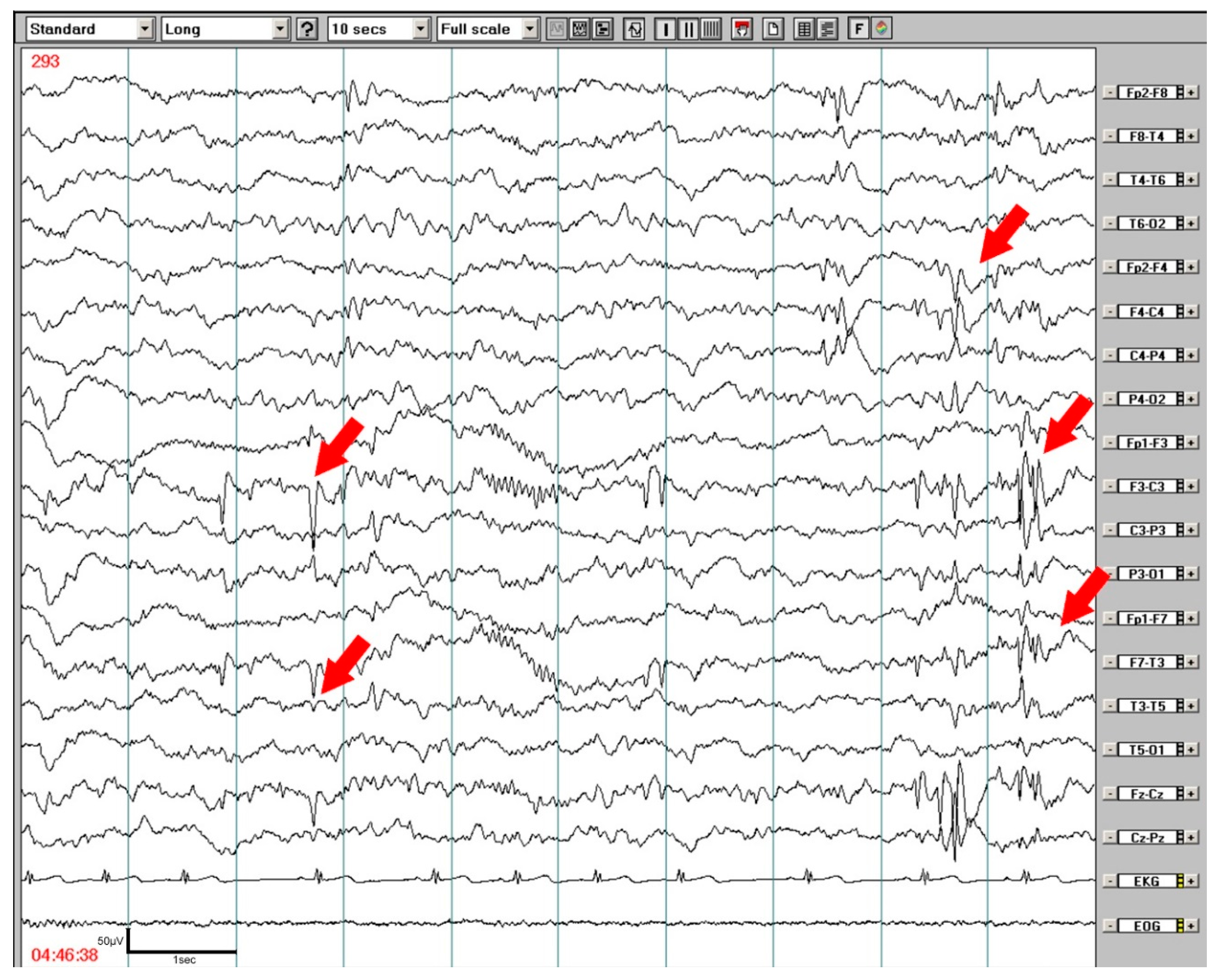
3.2. Wake EEG and Nocturnal EEG and PSG Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laasonen, M.; Smolander, S.; Lahti-Nuuttila, P.; Leminen, M.; Lajunen, H.R.; Heinonen, K.; Arkkila, A. Understanding developmental language disorder the Helsinki longitudinal SLI study (HelSLI): A study protocol. BMC Psychol. 2018, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.M. Uncommon Understanding; Psychology Press: East Sussex, UK, 1999; p. 277. [Google Scholar]
- Bishop, D.V.M. How does the brain learn language? Insights from the study of children with and without language impairment. Dev. Med. Child Neurol. 2000, 42, 133–142. [Google Scholar] [CrossRef]
- Rice, M.L. Language growth and genetics of specific language impairment. Int. J. Speech Lang. Pathol. 2013, 15, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.W.; Evans, J.L. Complex sentence comprehensionand working memoryin children with specific language impairment. J. Speech Lang. Hear. Res. 2009, 52, 269–288. [Google Scholar] [CrossRef]
- Nickisch, A.; Von Kries, R. Short-term memory (STM) constraints in children with SLI: Are there differences between receptive and expressive SLI? J. Speech Lang. Hear. 2009, 52, 578–595. [Google Scholar] [CrossRef]
- Tallal, P.; Miller, S.L.; Bedi, G.; Byma, G.; Wang, X.; Nagarajan, S.S.; Schreiner, C.; Jenkins, W.M.; Merzenich, M.M. Language comprehension in language-learning impaired children improved with acoustically modified speech. Science 1996, 271, 81–84. [Google Scholar] [CrossRef]
- Njiokiktjien, C. Developmental dysphasia: Clinical importance and underlying neurological causes. Acta Paedopsychiatr. (Ger.) 1990, 53, 126–137. [Google Scholar]
- Chermak, G.D.; Musiek, F. Central Auditory Processing Disorders; (New perspectives); Singular Publishing Group, Inc.: London, UK, 1997; 374p. [Google Scholar]
- Ferre, J.M. Understanding children´s central auditory disorders. In Abstracts, 25th World Cogress of the International Association of Logopedics and Phoniatrics; S Karger Ag: Montreal, QC, Canada, 2001; pp. 5–9. [Google Scholar]
- Fergusson, M.A.; Hall, R.L.; Riley, A.; Moore, D.R. Communication, listening, cognitive and speech perception skills in children with auditory processing disorder (APD) or specific language impairment (SLI). J. Speech Lang. Hear Res. 2011, 54, 211–227. [Google Scholar] [CrossRef]
- Diepeveen, F.B.; van Dommelen, P.; Oudesluys-Murphy, A.M.; Verkerk, P.H. Children with specific language impairment are more likely to reach motor milestones late. Child Care Health Dev. 2018, 44, 857–862. [Google Scholar] [CrossRef]
- Redmond, S.M. Markers, models, and measurement error: Exploring the link between attention deficits and language impairments. J. Speech Lang. Hear Res. 2016, 59, 62–71. [Google Scholar] [CrossRef]
- Bishop, D.V.M.; North, T.; Weir, F. Genetic basis of specific language impairment: Evidence from a twin study. Dev. Med. Child Neurol. 1995, 37, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bartlett, C.W. Defining the genetic architecture of human developmental language impairment. Life Sci. 2012, 90, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Badcock, N.A.; Bishop, D.V.; Hardiman, M.U.; Barry, J.G.; Watkins, K.E. Co-localization of abnormal brain structure and function in specific language impairment. Brain Lang. 2012, 120, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.L.; Lenck-Santini, P.P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006, 8, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.L. What is more harmful, seizure or epileptic EEG abnormalities? Is there any clinical data? Epileptic Disord. 2014, 16, S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Overvliet, G.M.; Besseling, R.M.; Vles, J.S.; Hofman, P.A.; van Hall, M.H.; Backes, W.H.; Aldenkamp, A.P. Association between frequency of nocturnal epilepsy and language disturbance in children. Pediatr. Neurol. 2011, 44, 333–339. [Google Scholar] [CrossRef]
- Systad, S.; Bjornvold, M.; Sorensen, C.; Halaas Lyster, S.A. The value of electroencephalogram in assessung children with speech and language impairments. J. Speech Lang. Hear Res. 2019, 62, 153–168. [Google Scholar] [CrossRef]
- Skodova, E.; Michek, F.; Moravcova, M. Evaluation of Fonemic Hearing in Pre-School Children; Realia: Prague, Czech Republic, 1995; 15p. (In Czech) [Google Scholar]
- Dlouha, O. (Ed.) Developmental Speech-language Disorders; Galen: Prague, Czech Republic, 2017; 254p. (In Czech) [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Bubrick, E.J.; Yazdani, S.; Pavlova, M.K. Beyond standard polysomnography: Advances and indications for use of extended 10–20 EEG montage during laboratory sleep study evaluation. Seizure 2014, 23, 699–702. [Google Scholar] [CrossRef]
- Bishop, D.V.M. (Ed.) Uncommon Understanding. Development and Disorders of Language Comprehension in Children; Reprinted Hove, Psychology Press: East Sussex, UK, 2001; 277p. [Google Scholar]
- Fraser, J.; Goswami, U.; Conti-Ramsden, G. Dyslexia and specific language impairment: The role of phonology and auditory processing. Sci. Stud. Read. 2013, 14, 8–29. [Google Scholar] [CrossRef]
- Kirby, A.; Sugden, D.; Purcell, C. Diagnosing developmental coordination disorders. Arch. Dis. Child 2014, 99, 292–296. [Google Scholar] [CrossRef]
- Keller, R.; Chieregato, S.; Bari, S.; Castaldo, R.; Rutto, F.; Chiocchetti, A.; Dianzani, U. Autism in adulthood: Clinical and demographic characteristics of a cohort of five hundred persons with autism analyzed by a novel multistep network model. Brain Sci. 2020, 10, 416. [Google Scholar] [CrossRef]
- Kaddah, F.A.; Abdel-Raouf, M. ADHD: Linguistic abilities and EEG findings compared to specific language impairment. Egypt. J. Ear Nose Throat Sci. 2011, 12, 53–59. [Google Scholar] [CrossRef]
- Gooch, D.; Sears, C.; Maydew, H.; Vamvakas, G.; Norbury, C.F. Does inattention and hyperactivity moderate the relation between speed of processing and language skills? Child Dev. 2019, 90, e565–e583. [Google Scholar] [CrossRef] [PubMed]
- Echenne, B.; Cheminai, R.; Rivier, F.; Negre, C.; Touchon, J.; Billard, M. Epileptic electroencephalographic abnormalities and developmental dysphasias: A study of 32 patients. Brain Dev. 1992, 14, 216–225. [Google Scholar] [CrossRef]
- Tuchman, R.F.; Rapin, I. Regression in pervasive developmental disorders: Seizures and epileptiform electroencephalogram correlates. Pediatrics 1997, 99, 560–566. [Google Scholar] [CrossRef]
- Dlouha, O.; Nevsimalova, S. EEG changes and epilepsy in developmental dysphasia. In Supplements to Clinical Neurophysiology; Ambler, Z., Nevsimalova, S., Rossini, P.M., Eds.; Elsevier: New York, NY, USA, 2000; Volume 53, pp. 271–274. [Google Scholar]
- Picard, A.; ChelioutHeraut, F.; Bouskraoui, M.; Lemoine, M.; Lacert, P.; Delattre, J. Sleep EEG and developmental dysphasia. Dev. Med. Child Neurol. 1998, 40, 595–599. [Google Scholar] [CrossRef]
- Binnie, C.D. Cognitive impairment during epileptiform discharges. Is it ever justifiable to treat the EEG? Lancet Neurol. 2003, 2, 725–730. [Google Scholar] [CrossRef]
- Van Bogaert, P.; Urbain, C.; Galer, S.; Ligot, N.; Peigneux, P.; De Tiege, X. Impact of focal interictal epileptiform discharges on behaviour and cognition in children. Clin. Neurophysiol. 2011, 42, 53–58. [Google Scholar] [CrossRef]
- Nenadovic, V.; Stokic, M.; Vukovic, M.; Dokovic, S.; Subotic, M. Cognitive and electrophysiological characteristics of children with specific language impairment and subclinical epileptiform electroencephalogram. J. Clin. Experneuropsychol. 2014, 36, 981–991. [Google Scholar] [CrossRef]
- Billard, C.; Fluss, J.; Pinton, F. Specific language impairment versus Landau-Kleffner syndrome. Epilepsia 2009, 50, 21–24. [Google Scholar] [CrossRef]
- Overvliet, G.M.; Besseling, R.M.H.; Vles, J.S.H.; Hofman, P.A.M.; Backes, W.H.; van Hall, M.H.J.A.; Aldenkamp, A.P. Nocturnal epileptiform EEG discharges, nocturnal epileptic seizures, and language impairments in children: Review of the literature. Epilepsy Behav. 2010, 19, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Lévy Rueff, M.; Bourgeois, M.; Assous, A.; Beauquier-Maccota, B.; Boucheron, E.; Clouard, C.; Robel, L. Abnormal electroencephalography results and specific language impairment: Towards a theoretical neurodevelopmental model? L´Encéphale 2012, 38, 318–328. [Google Scholar]
- Mehta, B.; Chawla, V.K.; Parakh, M.; Parakh, P.; Bhandari, B.; Gurjar, A.S. EEG abnormalities in children with speech and language impairment. J. Clin. Diagn. Res. 2015, 9, CC04–CC07. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.M.C.; Walker, M.P.; Stickgold, R. Sleep and memory consolidation. In Sleep Disorders Medicine. Basic Science, Technical Considerations and Clinical Aspects, 4th ed.; Chokroverty, S., Ed.; Springer: New York, NY, USA, 2017; Volume 1, pp. 205–223. [Google Scholar]
- Lai, C.S.; Fisher, S.E.; Hurst, J.A.; Vargha-Khadem, J.A.; Monaco, A.P. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 2001, 413, 519–523. [Google Scholar] [CrossRef]
- Fisher, S.E.; Scharff, C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009, 25, 166–177. [Google Scholar] [CrossRef]
| Children (n/%) | Additional Notes | |
|---|---|---|
| male | 42/75 | |
| age (years) 6.2 ± 1.4 | ||
| range 4–9 years | ||
| right-handedness | 45/81.8 | |
| DLD forms (n/%) | ||
| receptive | 34/63.0 | 1 child was not precisely classified due to limited exact cooperation. |
| expressive | 20/37.0 | |
| Family predisposition (n/%) | Data missing in one family. | |
| specific language impairment | 6/11.1 | |
| unspecified speech disorder | 11/20.4 | |
| Perinatal risk factors | ||
| (including neonatal icterus) | 28/50.9 | |
| Developmental comorbidities | ||
| DMC | 33/61.1 | Data missing in one child. |
| ADHD | 39/70.9 | |
| IQ 70–80 | 5/9.1 | |
| Epilepsy (clinical absences) | 9/16.4 |
| Epileptiform Discharges | Wake EEG Number (%) | Nocturnal EEG/PSG Number (%) | p-Value |
|---|---|---|---|
| Total | 20 (36.4) | 30 (55.6) | 0.0124 |
| Focal left-sided | 12 (21.8) | 23 (41.8) | 0.008 |
| Focal right-sided | 13 (23.6) | 21 (38.2) | 0.032 |
| Generalized | 4 (7.3) | 10 (18.2) | 0.058 |
| Epileptiform Discharges Wake EEG | Expressive DLD (n = 20) Number (%) | Receptive DLD (n = 34) Number (%) | p-Value |
|---|---|---|---|
| Total | 6 (30) | 14 (41) | NS (0.41) |
| Focal left-sided | 3 (15.0) | 9 (26.5) | NS |
| Focal right-sided | 4 (20.0) | 9 (26.5) | NS |
| Generalized | 2 (10.0) | 2 (5.9) | NS |
| Epileptiform discharges Nocturnal sleep recordings | |||
| Total | 9 (45) | 21 (62) | NS (0.23) |
| Focal left-sided | 7 (35.0) | 16 (47.1) | NS |
| Focal right-sided | 6 (30.0) | 15 (44.1) | NS |
| Generalized | 3 (15.0) | 7 (20.6) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlouha, O.; Prihodova, I.; Skibova, J.; Nevsimalova, S. Developmental Language Disorder: Wake and Sleep Epileptiform Discharges and Co-morbid Neurodevelopmental Disorders. Brain Sci. 2020, 10, 910. https://doi.org/10.3390/brainsci10120910
Dlouha O, Prihodova I, Skibova J, Nevsimalova S. Developmental Language Disorder: Wake and Sleep Epileptiform Discharges and Co-morbid Neurodevelopmental Disorders. Brain Sciences. 2020; 10(12):910. https://doi.org/10.3390/brainsci10120910
Chicago/Turabian StyleDlouha, Olga, Iva Prihodova, Jelena Skibova, and Sona Nevsimalova. 2020. "Developmental Language Disorder: Wake and Sleep Epileptiform Discharges and Co-morbid Neurodevelopmental Disorders" Brain Sciences 10, no. 12: 910. https://doi.org/10.3390/brainsci10120910
APA StyleDlouha, O., Prihodova, I., Skibova, J., & Nevsimalova, S. (2020). Developmental Language Disorder: Wake and Sleep Epileptiform Discharges and Co-morbid Neurodevelopmental Disorders. Brain Sciences, 10(12), 910. https://doi.org/10.3390/brainsci10120910





