Selecting the Most Effective DBS Contact in Essential Tremor Patients Based on Individual Tractography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Outcome and Lead Reconstruction
2.3. Probabilistic Tracking of the DRTT
2.4. Statistical Analysis
2.5. Data Availability
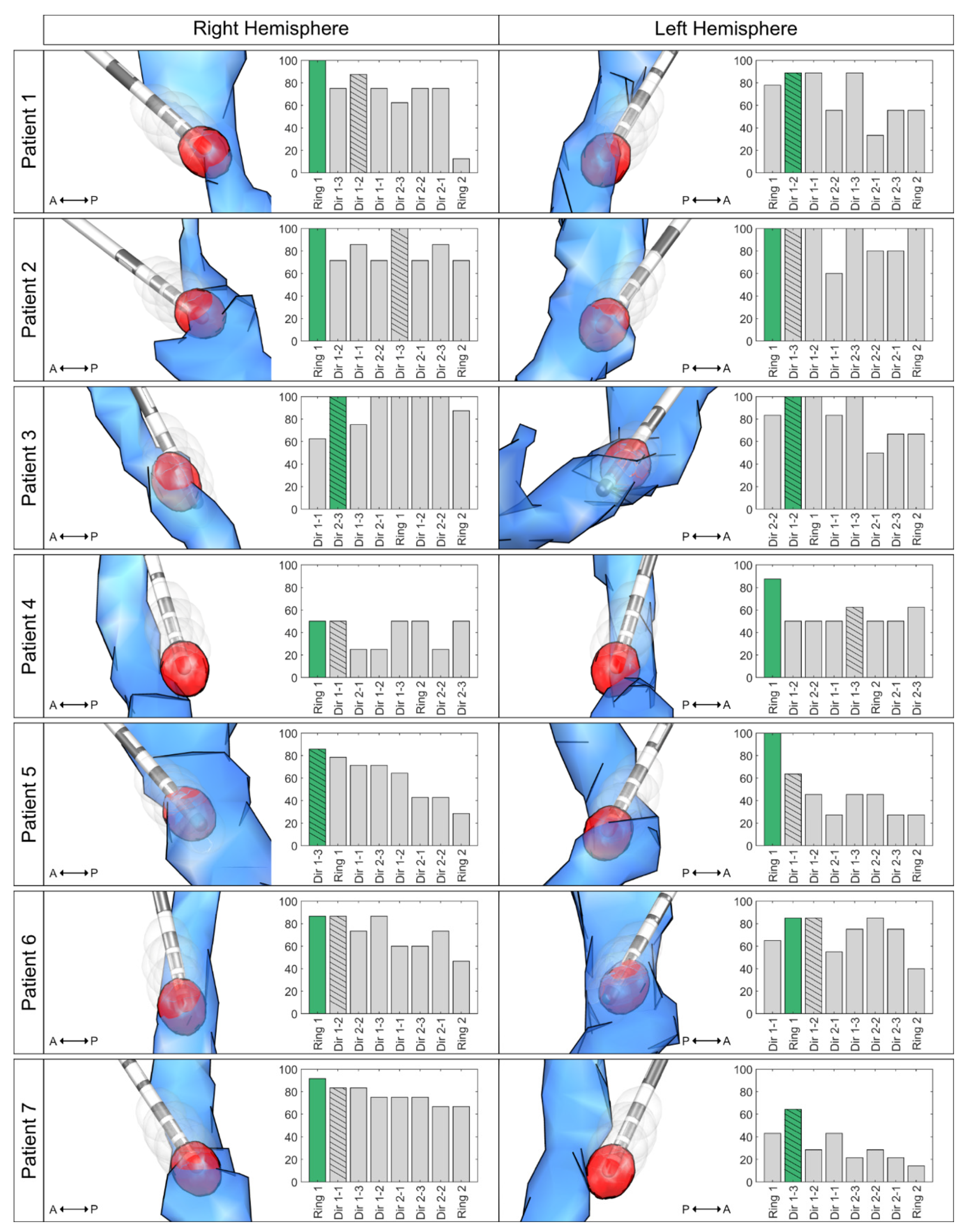
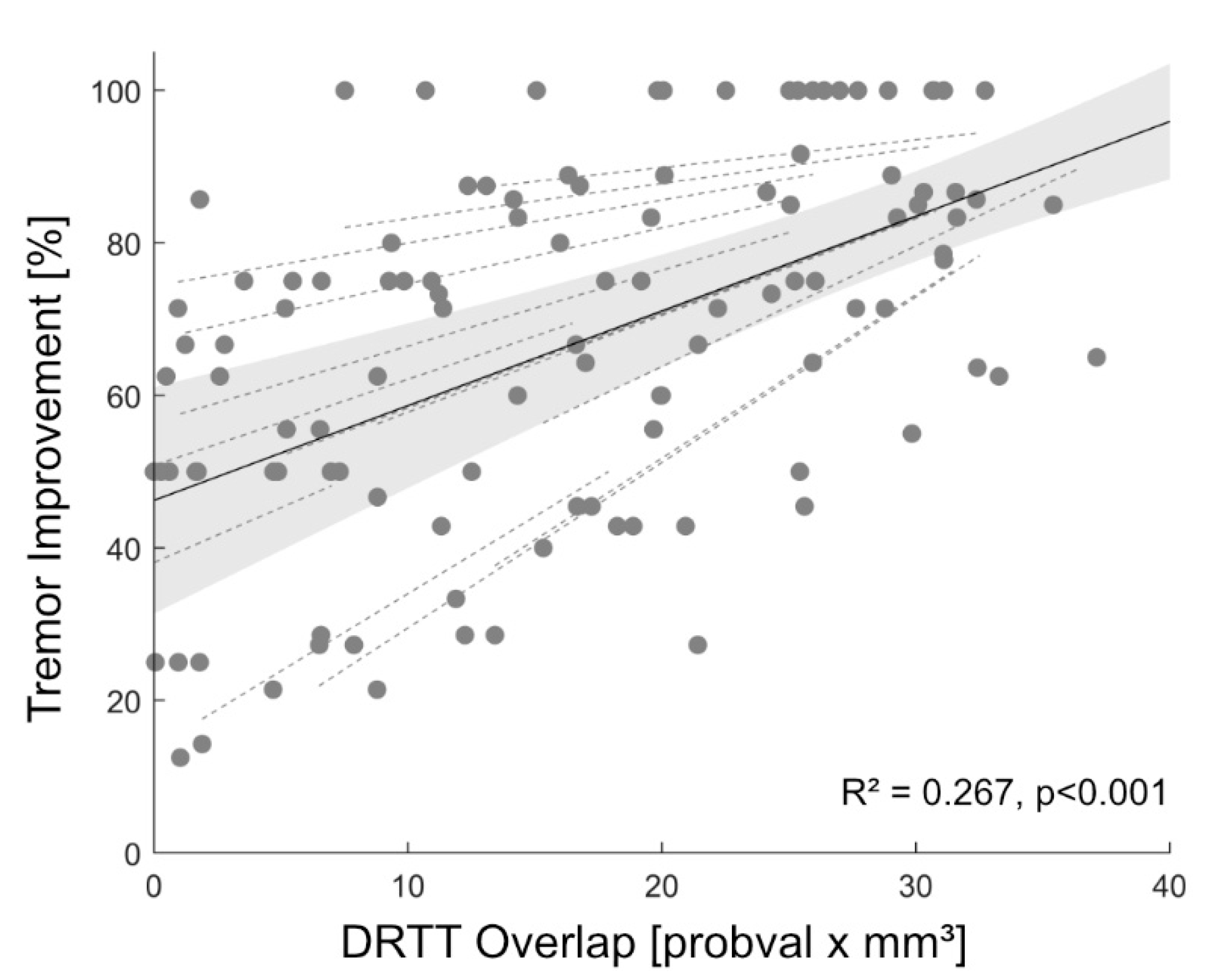
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schuurman, P.R.; Bosch, D.A.; Bossuyt, P.M.; Bonsel, G.J.; Van Someren, E.J.; De Bie, R.M.; Merkus, M.P.; Speelman, J.D. A Comparison of Continuous Thalamic Stimulation and Thalamotomy for Suppression of Severe Tremor. N. Engl. J. Med. 2000, 342, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.T.; Reker, P.; Hamacher, S.; Franklin, J.; Kraus, D.; Dembek, T.A.; Becker, J.; Steffen, J.K.; Allert, N.; Wirths, J.; et al. DBS of the PSA and the VIM in essential tremor: A randomized, double-blind, crossover trial. Neurology 2018, 91, e543–e550. [Google Scholar] [CrossRef] [PubMed]
- Coenen, V.A.; Allert, N.; Paus, S.; Kronenbürger, M.; Urbach, H.; Mädler, B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: A diffusion tensor imaging study. Neurosurgery 2014, 75, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, A.J.; Schiess, M.C. Deep Brain Stimulation of the Dentato-Rubro-Thalamic Tract: Outcomes of Direct Targeting for Tremor. Neuromodulation 2017, 20, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Dembek, T.A.; Petry-Schmelzer, J.N.; Reker, P.; Wirths, J.; Hamacher, S.; Steffen, J.; Dafsari, H.S.; Hövels, M.; Fink, G.R.; Visser-Vandewalle, V.; et al. PSA and VIM DBS efficiency in essential tremor depends on distance to the dentatorubrothalamic tract. Neuroimage Clin. 2020, 26, 102235. [Google Scholar] [CrossRef] [PubMed]
- Chazen, J.L.; Sarva, H.; Stieg, P.E.; Min, R.J.; Ballon, D.J.; Pryor, K.O.; Riegelhaupt, P.M.; Kaplitt, M.G. Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. J. Neurosurg. 2018, 129, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Tolosa, E.; Concepcion, M. Clinical Rating Scale for Tremor. In Parkinson’s Disease and Movement Disorders; Jankovic, J., Tolosa, E., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1993. [Google Scholar]
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.-J.; et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019, 184, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Dembek, T.A.; Hoevels, M.; Hellerbach, A.; Horn, A.; Petry-Schmelzer, J.N.; Borggrefe, J.; Wirths, J.; Dafsari, H.S.; Barbe, M.T.; Visser-Vandewalle, V.; et al. Directional DBS leads show large deviations from their intended implantation orientation. Park. Relat. Disord. 2019, 67, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Husch, A.; Petersen, M.V.; Gemmar, P.; Gonçalves, J.; Hertel, F. PaCER—A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation. Neuroimage Clin. 2018, 17, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, M.; Proverbio, D.; Gonçalves, J.; Hertel, F.; Husch, A. FastField: An open-source toolbox for efficient approximation of deep brain stimulation electric fields. Neuroimage 2020, 223, 117330. [Google Scholar] [CrossRef] [PubMed]
- Astrom, M.; Diczfalusy, E.; Martens, H.; Wardell, K. Relationship between Neural Activation and Electric Field Distribution during Deep Brain Stimulation. IEEE Trans. Biomed. Eng. 2015, 62, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Schlaier, J.R.; Beer, A.L.; Faltermeier, R.; Fellner, C.; Steib, K.; Lange, M.; Greenlee, M.W.; Brawanski, A.; Anthofer, J.M. Probabilistic vs. deterministic fiber tracking and the influence of different seed regions to delineate cerebellar-thalamic fibers in deep brain stimulation. Eur. J. Neurosci. 2017, 45, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Dembek, T.; Roediger, J.; Horn, A.; Reker, P.; Oehrn, C.; Dafsari, H.; Li, N.; Kühn, A.A.; Fink, G.R.; Visser-Vandewalle, V.; et al. Probabilistic sweet spots predict motor outcome for deep brain stimulation in Parkinson disease. Ann. Neurol. 2019, 86, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Dembek, T.A.; Reker, P.; Visser-Vandewalle, V.; Wirths, J.; Treuer, H.; Klehr, M.; Roediger, J.; Dafsari, H.S.; Barbe, M.T.; Timmermann, L. Directional DBS increases side-effect thresholds-A prospective, double-blind trial. Mov. Disord. 2017, 32, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Akram, H.; Dayal, V.; Mahlknecht, P.; Georgiev, D.; Hyam, J.; Foltynie, T.; Limousin, P.; De Vita, E.; Jahanshahi, M.; Ashburner, J.; et al. Connectivity derived thalamic segmentation in deep brain stimulation for tremor. Neuroimage Clin. 2018, 18, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatly, B.; Ewert, S.; Kübler, D.; Horn, A.; Horn, A.; Kühn, A.A. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain 2019, 142, 3086–3098. [Google Scholar] [CrossRef] [PubMed]
- Dembek, T.A.; Barbe, M.T.; Åström, M.; Hoevels, M.; Visser-Vandewalle, V.; Fink, G.R.; Timmermann, L. Probabilistic mapping of deep brain stimulation effects in essential tremor. Neuroimage Clin. 2017, 13, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Aström, M.; Samuelsson, J.; Roothans, J.; Fytagoridis, A.; Ryzhkov, M.; Nijlunsing, R.; Blomstedt, P. Prediction of Electrode Contacts for Clinically Effective Deep Brain Stimulation in Essential Tremor. Stereotact. Funct. Neurosurg. 2018, 96, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Aström, M.; Kibleur, A.; Bouthour, W.; Chaoui, E.T.; Maling, N.; Nguyen, T.A.K.; Momjian, S.; Gomez, M.I.V.; Zacharia, A.; Bally, J.F.; et al. Modeling of Electric Fields in Individual Imaging Atlas for Capsular Threshold Prediction of Deep Brain Stimulation in Parkinson’s Disease: A Pilot Study. Front. Neurol. 2020, 11, 532. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petry-Schmelzer, J.N.; Dembek, T.A.; Steffen, J.K.; Jergas, H.; Dafsari, H.S.; Fink, G.R.; Visser-Vandewalle, V.; Barbe, M.T. Selecting the Most Effective DBS Contact in Essential Tremor Patients Based on Individual Tractography. Brain Sci. 2020, 10, 1015. https://doi.org/10.3390/brainsci10121015
Petry-Schmelzer JN, Dembek TA, Steffen JK, Jergas H, Dafsari HS, Fink GR, Visser-Vandewalle V, Barbe MT. Selecting the Most Effective DBS Contact in Essential Tremor Patients Based on Individual Tractography. Brain Sciences. 2020; 10(12):1015. https://doi.org/10.3390/brainsci10121015
Chicago/Turabian StylePetry-Schmelzer, Jan Niklas, Till A. Dembek, Julia K. Steffen, Hannah Jergas, Haidar S. Dafsari, Gereon R. Fink, Veerle Visser-Vandewalle, and Michael T. Barbe. 2020. "Selecting the Most Effective DBS Contact in Essential Tremor Patients Based on Individual Tractography" Brain Sciences 10, no. 12: 1015. https://doi.org/10.3390/brainsci10121015
APA StylePetry-Schmelzer, J. N., Dembek, T. A., Steffen, J. K., Jergas, H., Dafsari, H. S., Fink, G. R., Visser-Vandewalle, V., & Barbe, M. T. (2020). Selecting the Most Effective DBS Contact in Essential Tremor Patients Based on Individual Tractography. Brain Sciences, 10(12), 1015. https://doi.org/10.3390/brainsci10121015





