An Exploratory Study Testing Autonomic Reactivity to Pain in Women with Sensory Over-Responsiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Pain Assessment
2.3. Heart Rate Variability (HRV) Assessment
2.4. Self-Report Questionnaires
2.4.1. The Sensory Responsiveness Questionnaire-Intensity Scale
2.4.2. The Spielberger State-Trait Anxiety Inventory Questionnaire
2.4.3. The Short Version of the Brief Symptom Inventory
2.5. Procedure
2.6. Data Analysis
3. Results
3.1. Pain Psychophysics
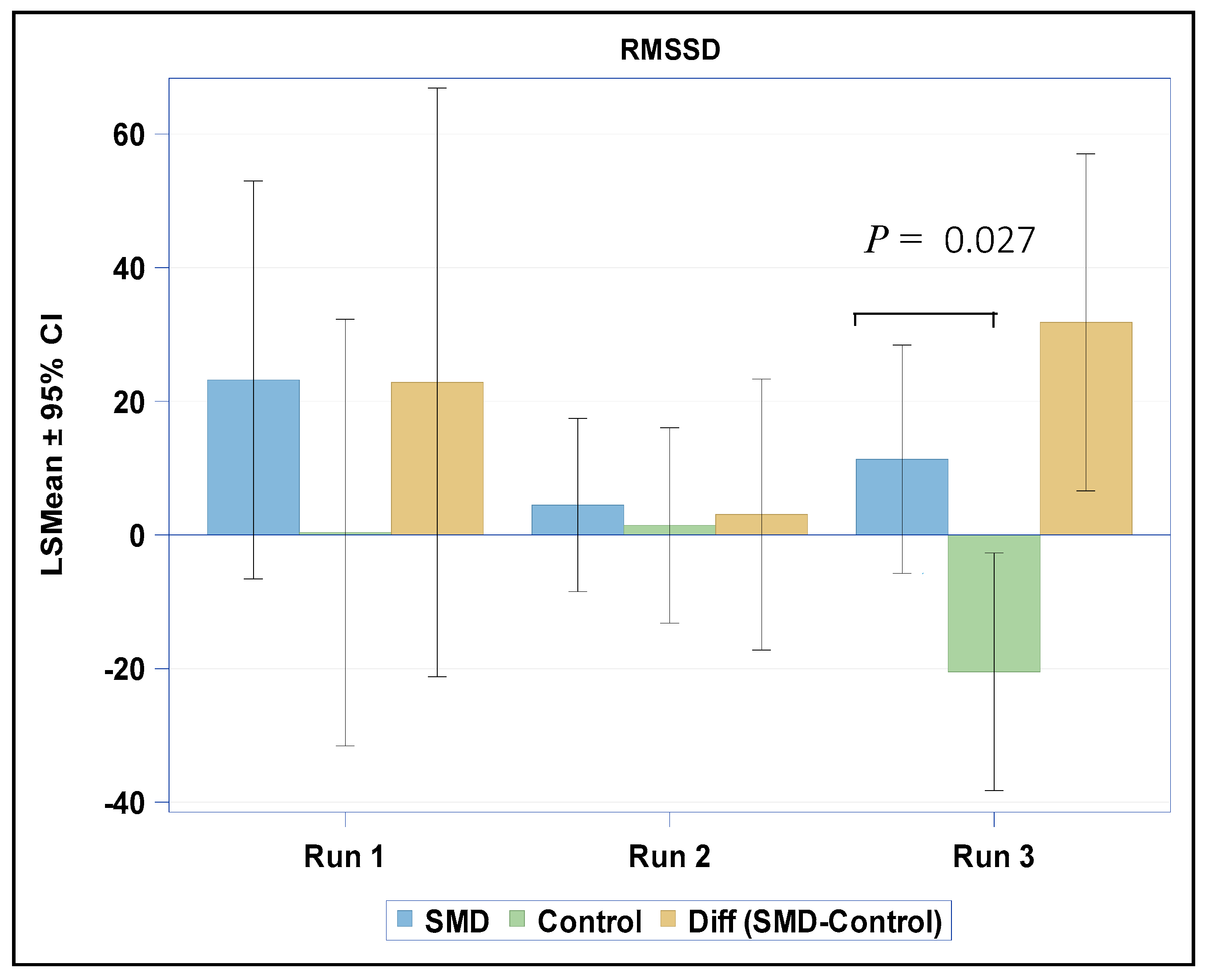
3.2. HRV Assessments
3.3. Self-Report Measures
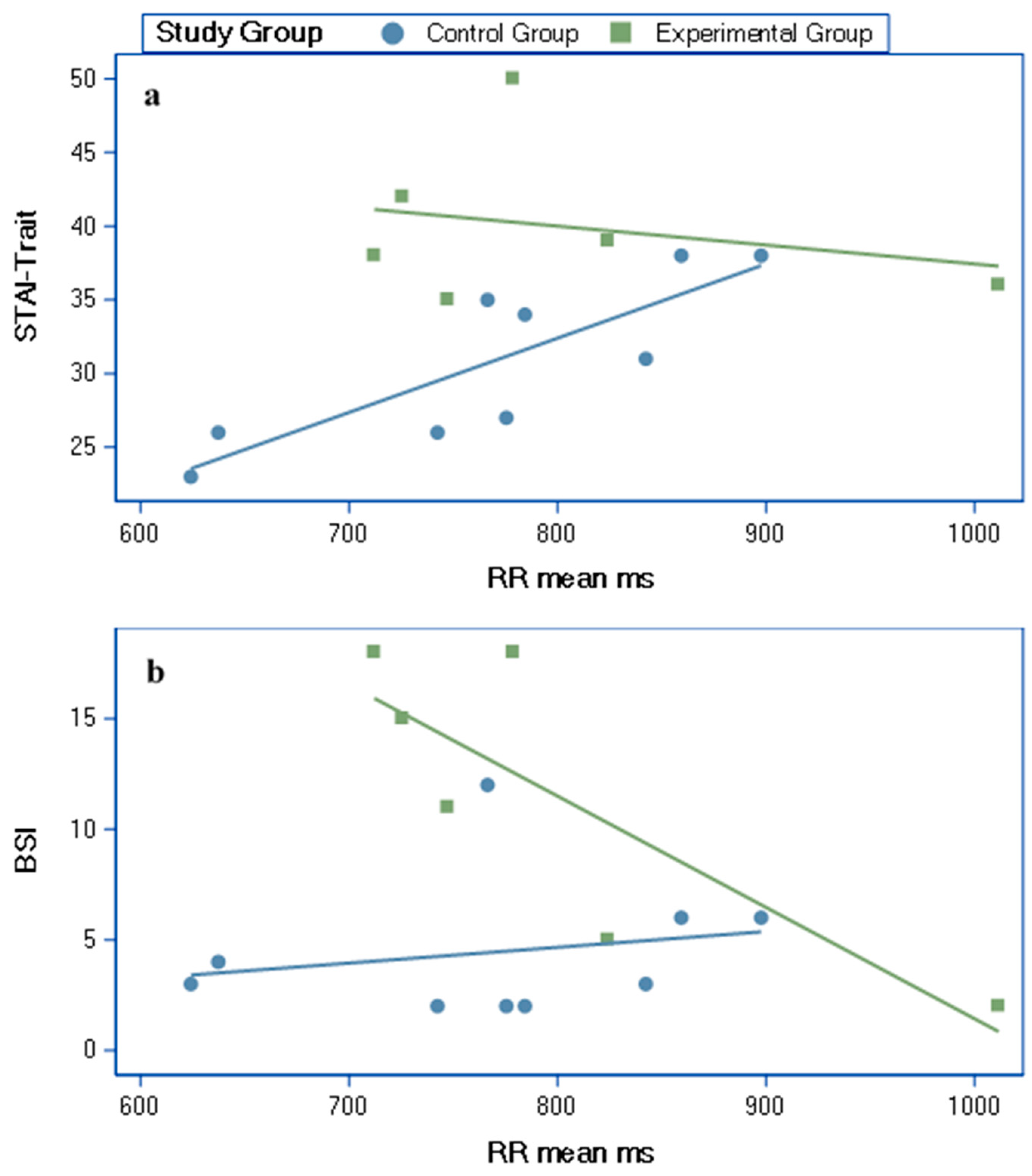
3.4. Partial Correlations (Age Adjusted) between HRV Variables and Self-Report Measures
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Davies, P.; Gavin, W. Validating the diagnosis of sensory processing disorders using EEG technology. Am. J. Occup. Ther. 2007, 61, 176–189. [Google Scholar] [CrossRef]
- Brett-Green, B.A.; Miller, L.J.; Gavin, W.J.; Davies, P.L. Multisensory integration in children: A preliminary ERP study. Brain Res. 2008, 1242, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Brett-Green, B.A.; Miller, L.J.; Schoen, S.A.; Nielsen, D.M. An exploratory event-related potential study of multisensory integration in sensory over-responsive children. Brain Res. 2010, 1321, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.L.; Chang, W.P.; Gavin, W.J. Middle and Late Latency ERP Components Discriminate between Adults, Typical Children, and Children with Sensory Processing Disorders. Front. Integr. Neurosci. 2010, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Gavin, W.J.; Dotseth, A.; Roush, K.K.; Smith, C.A.; Spain, H.D.; Davies, P.L. Electroencephalography in children with and without sensory processing disorders during auditory perception. Am. J. Occup. Ther. 2011, 65, 370–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zlotnik, S.; Attias, J.; Pratt, H.; Engel-Yeger, B. Neurophysiological Manifestations of Auditory Hypersensitivity Correlate with Daily Life Experiences. Neurosci. Med. 2018, 9, 29. [Google Scholar] [CrossRef]
- Kinnealey, M.; Koenig, K.P.; Smith, S. Relationships between sensory modulation and social supports and health-related quality of life. Am. J. Occup. Ther. 2011, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W.; Doussard-Roosevelt, J.A.; Maiti, A.K. Vagal tone and the physiological regulation of emotion. Monogr. Soc. Res. Child Dev. 1994, 59, 167–186. [Google Scholar] [CrossRef]
- Fox, N.A. The Development of Emotion Regulation: Biological and Behavioral Considerations; University of Chicago Press: Chicago, IL, USA, 1994; Volume 59. [Google Scholar]
- McIntosh, D.N.; Miller, L.J.; Shyu, V.; Hagerman, R.J. Sensory-modulation disruption, electrodermal responses, and functional behaviors. Dev. Med. Child Neurol. 1999, 41, 608–615. [Google Scholar] [CrossRef]
- Schaaf, R.C.; Benevides, T.W.; Blanche, E.; Brett-Green, B.A.; Burke, J.; Cohn, E.; Koomar, J.; Lane, S.J.; Miller, L.J.; May-Benson, T.A. Parasympathetic functions in children with sensory processing disorder. Front. Integr. Neurosci. 2010, 4, 4. [Google Scholar] [CrossRef]
- Schaaf, R.C.; Miller, L.J.; Seawell, D.; O’Keefe, S. Children with disturbances in sensory processing: A pilot study examining the role of the parasympathetic nervous system. Am. J. Occup. Ther. 2003, 57, 442–449. [Google Scholar] [CrossRef]
- Meredith, P.J.; Rappel, G.; Strong, J.; Bailey, K.J. Sensory sensitivity and strategies for coping with pain. Am. J. Occup. Ther. 2015, 69. [Google Scholar] [CrossRef][Green Version]
- Kinnealey, M.; Oliver, B.; Wilbarger, P. A phenomenological study of sensory defensiveness in adults. Am. J. Occup. Ther. 1995, 49, 444–451. [Google Scholar] [CrossRef]
- Bar-Shalita, T.; Deutsch, L.; Honigman, L.; Weissman-Fogel, I. Ecological aspects of pain in sensory modulation disorder. Res. Dev. Disabil. 2015, 45, 157–167. [Google Scholar] [CrossRef]
- Bar-Shalita, T.; Vatine, J.J.; Seltzer, Z.; Parush, S. Psychophysical correlates in adults with sensory modulation disorder. Disabil. Rehabil. 2011, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shalita, T.; Vatine, J.J.; Yarnitsky, D.; Parush, S.; Weissman-Fogel, I. Atypical central pain processing in sensory modulation disorder: Absence of temporal summation and higher after-sensation. Exp. Brain Res. 2014, 232, 587–595. [Google Scholar] [CrossRef]
- Weissman-Fogel, I.; Granovsky, Y.; Bar-Shalita, T. Sensory over-responsiveness among healthy subjects is associated with a pronociceptive state. Pain Pract. 2018, 18, 473–486. [Google Scholar] [CrossRef]
- Bruehl, S.; Chung, O.Y. Interactions between the cardiovascular and pain regulatory systems: An updated review of mechanisms and possible alterations in chronic pain. Neurosci. Biobehav. Rev. 2004, 28, 395–414. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Schlereth, T.; Birklein, F. The sympathetic nervous system and pain. Neuromol. Med. 2008, 10, 141–147. [Google Scholar] [CrossRef]
- Bar-Shalita, T.; Cermak, S.A. Atypical sensory modulation and psychological distress in the general population. Am. J. Occup. Ther. 2016, 70. [Google Scholar] [CrossRef]
- Leone, M.; Cecchini, A.P.; Mea, E.; Tullo, V.; Curone, M.; Bussone, G. Neuroimaging and pain: A window on the autonomic nervous system. Neurol. Sci. 2006, 27, s134–s137. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Umetani, K.; Singer, D.H.; McCraty, R.; Atkinson, M. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J. Am. Coll. Cardiol. 1998, 31, 593–601. [Google Scholar] [CrossRef]
- Bar-Shalita, T.; Seltzer, Z.; Vatine, J.J.; Yochman, A.; Parush, S. Development and psychometric properties of the Sensory Responsiveness Questionnaire (SRQ). Disabil. Rehabil. 2009, 31, 189–201. [Google Scholar] [CrossRef]
- Granot, M.; Weissman-Fogel, I.; Crispel, Y.; Pud, D.; Granovsky, Y.; Sprecher, E.; Yarnitsky, D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain 2008, 136, 142–149. [Google Scholar] [CrossRef]
- Quintana, D.; Alvares, G.A.; Heathers, J. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, D.L. Sympathovagal balance: A critical appraisal. Circulation 1997, 96, 3224–3232. [Google Scholar] [CrossRef]
- Spielberger, C.D. Anxiety: Current Trends in Theory and Research; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Derogatis, L.R.; Cleary, P.A. Confirmation of the dimensional structure of the SCL-90: A study in construct validation. J. Clin. Psychol. 1977, 33, 981–989. [Google Scholar] [CrossRef]
- Movius, H.L.; Allen, J.J. Cardiac vagal tone, defensiveness, and motivational style. Biol. Psychol. 2005, 68, 147–162. [Google Scholar] [CrossRef]
- Porges, S.W. Vagal tone: A physiologic marker of stress vulnerability. Pediatrics 1992, 90, 498–504. [Google Scholar]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Chalaye, P.; Devoize, L.; Lafrenaye, S.; Dallel, R.; Marchand, S. Cardiovascular influences on conditioned pain modulation. PAIN 2013, 154, 1377–1382. [Google Scholar] [CrossRef]
- McMahon, K.; Anand, D.; Morris-Jones, M.; Rosenthal, M.Z. A Path From Childhood Sensory Processing Disorder to Anxiety Disorders: The Mediating Role of Emotion Dysregulation and Adult Sensory Processing Disorder Symptoms. Front. Integr. Neurosci. 2019, 13, 22. [Google Scholar] [CrossRef]
- Kinnealey, M.; Fuiek, M. The relationship between sensory defensiveness, anxiety, depression and perception of pain in adults. Occup. Ther. Int. 1999, 6, 195–206. [Google Scholar] [CrossRef]
- Engel-Yeger, B.; Dunn, W. The relationship between sensory processing difficulties and anxiety level of healthy adults. Br. J. Occup. Ther. 2011, 74, 210–216. [Google Scholar] [CrossRef]
- Friedman, B.H. An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biol. Psychol. 2007, 74, 185–199. [Google Scholar] [CrossRef]

| HRV Variables | Experimental Group | Control Group | Between Group Comparison | |
|---|---|---|---|---|
| r | r | p | ||
| STAI-Trait | RR mean ms | −0.59 | 0.84 * | 0.006 |
| BSI | RR mean ms | −0.98 * | 0.22 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar-Shalita, T.; Ben-Ziv, N.; Granovsky, Y.; Weissman-Fogel, I. An Exploratory Study Testing Autonomic Reactivity to Pain in Women with Sensory Over-Responsiveness. Brain Sci. 2020, 10, 819. https://doi.org/10.3390/brainsci10110819
Bar-Shalita T, Ben-Ziv N, Granovsky Y, Weissman-Fogel I. An Exploratory Study Testing Autonomic Reactivity to Pain in Women with Sensory Over-Responsiveness. Brain Sciences. 2020; 10(11):819. https://doi.org/10.3390/brainsci10110819
Chicago/Turabian StyleBar-Shalita, Tami, Nurit Ben-Ziv, Yelena Granovsky, and Irit Weissman-Fogel. 2020. "An Exploratory Study Testing Autonomic Reactivity to Pain in Women with Sensory Over-Responsiveness" Brain Sciences 10, no. 11: 819. https://doi.org/10.3390/brainsci10110819
APA StyleBar-Shalita, T., Ben-Ziv, N., Granovsky, Y., & Weissman-Fogel, I. (2020). An Exploratory Study Testing Autonomic Reactivity to Pain in Women with Sensory Over-Responsiveness. Brain Sciences, 10(11), 819. https://doi.org/10.3390/brainsci10110819




