Mast Cells in the Auditory Periphery of Rodents
Abstract
1. Introduction
2. Materials and Methods
2.1. The Animals
2.2. Cryosections
2.3. Explant Cultures and Flat Preparations of the Cochleae
2.4. Exposure to Cisplatin
2.5. Paraffin Embedding
2.6. Immunofluorescent Staining
2.7. Light, Epifluorescent and Confocal Microscopy
2.8. Western Blot
2.9. Statistical Analyses
3. Results
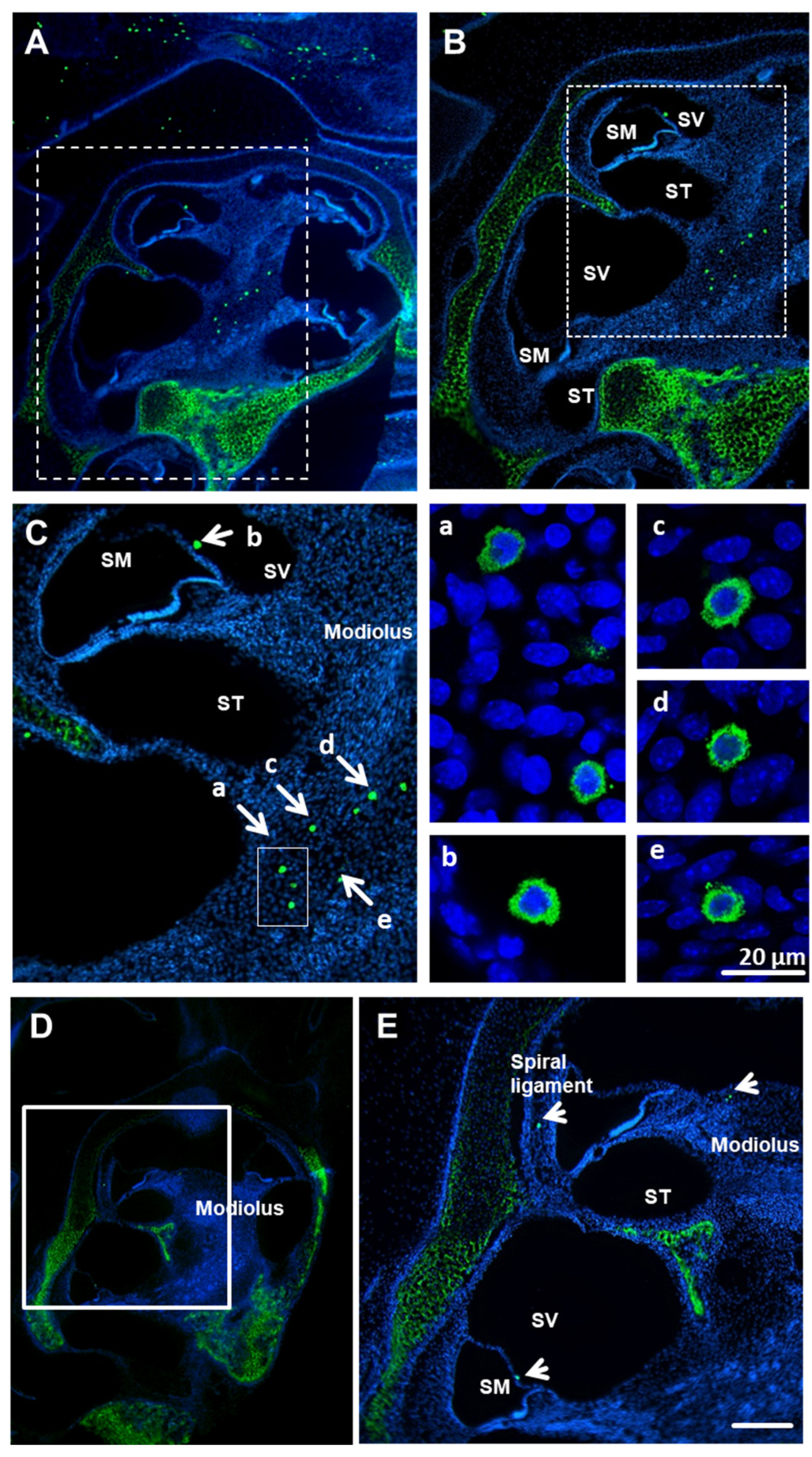
3.1. Avidin-Positive Cells Are Present in the Cochleae of Mice and Rats
3.2. The Avidin-Positive Cochlear Cells Express CD117
3.3. Cochlear MCs Express Tryptase, Chymase, and the High-Affinity Immunoglobulin Epsilon Receptor Subunit Alpha
3.4. Levels of c-Kit/CD117 Protein in the Cochlea Change During the First Nine Postnatal Days
3.5. The Number of MCs in Cochlear Explants Decreases During Postnatal Maturation of Rat
3.6. The Number of MCs in Cochlear Explants Changes upon Exposure of Cochlear Explants to Cisplatin
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.F.; The Immunological Genome Project Consortium; Barrett, N.A.; Austen, K.F.; Immunological Genome Project Consortium. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F.; Macniven, I.; Dyck, N.; Denburg, J.A.; Bienenstock, J.; Befus, D. Human Lung Mast Cells: Distribution and Abundance of Histochemically Distinct Subpopulations. Int. Arch. Allergy Immunol. 1987, 83, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Befus, A.D.; Pearce, F.; Gauldie, J.; Horsewood, P.; Bienenstock, J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J. Immunol. 1982, 128, 2475–2480. [Google Scholar] [PubMed]
- Ali, H.; Pearce, F.L. Isolation and properties of cardiac and other mast cells from the rat and guinea-pig. Inflamm. Res. 1985, 16, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Smelser, G.K.; Silver, S. The distribution of mast cells in the normal eye. Exp. Eye Res. 1963, 2, 134-IN7. [Google Scholar] [CrossRef]
- Dropp, J.J. Mast cells in mammalian brain. Cells Tissues Organs 1976, 94, 1–21. [Google Scholar] [CrossRef]
- Da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Passante, E. Mast cell and basophil cell lines: A compendium. In Basophils and Mast Cells; Springer: Berlin/Heidelberg, Germany, 2014; pp. 101–113. [Google Scholar]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Boil. 2018, 149, 461–477. [Google Scholar] [CrossRef]
- Frossi, B.; Mion, F.; Sibilano, R.; Danelli, L.; Pucillo, C. Is it time for a new classification of mast cells? What do we know about mast cell heterogeneity? Immunol. Rev. 2018, 282, 35–46. [Google Scholar] [CrossRef]
- Kubo, M. Mast cells and basophils in allergic inflammation. Curr. Opin. Immunol. 2018, 54, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.J.; Enciso, A.; Bissonnette, E.Y.; Szczepek, A.; Befus, A.D. Cytokine and drug modulation of TNF alpha in mast cells. Adv. Exp. Med. Biol. 1996, 409, 279–285. [Google Scholar] [PubMed]
- Naumova, E.M.; Sergeeva, V.E. Histochemical study of mast cells from the thymus of mice receiving ACTH1-24. Bull. Exp. Biol. Med. 2004, 138, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Caslin, H.L.; Kiwanuka, K.N.; Haque, T.T.; Taruselli, M.T.; Macknight, H.P.; Paranjape, A.; Ryan, J.J. Controlling mast cell activation and homeostasis: Work influenced by bill paul that continues today. Front. Immunol. 2018, 9, 868. [Google Scholar] [CrossRef]
- Hendriksen, E.; Van Bergeijk, D.A.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Weinberg, R.B. Mast cells and innate lymphoid cells: Underappreciated players in CNS autoimmune demyelinating disease. Front. Immunol. 2018, 9, 514. [Google Scholar] [CrossRef]
- Georgin-Lavialle, S.; The French Mast Cell Study Group; Moura, D.S.; Salvador, A.; Chauvet-Gelinier, J.-C.; Launay, J.-M.; Damaj, G.; Côté, F.; Soucié, E.; Chandesris, M.-O.; et al. Mast cells’ involvement in inflammation pathways linked to depression: Evidence in mastocytosis. Mol. Psychiatry 2016, 21, 1511–1516. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Thangavel, R.; Ahmed, M.E.; Zaheer, S.; Raikwar, S.P.; Iyer, S.S.; Bhagavan, S.M.; Beladakere-Ramaswamy, S.; Zaheer, A. Mast cell activation in brain injury, stress, and post-traumatic stress disorder and Alzheimer’s disease pathogenesis. Front. Neurosci. 2017, 11, 703. [Google Scholar] [CrossRef]
- Hershko, A.Y.; Rivera, J. Mast cell and T cell communication; amplification and control of adaptive immunity. Immunol. Lett. 2010, 128, 98–104. [Google Scholar] [CrossRef]
- Silver, R.; Curley, J.P. Mast cells on the mind: New insights and opportunities. Trends Neurosci. 2013, 36, 513–521. [Google Scholar] [CrossRef]
- Skaper, S.D.; Giusti, P.; Facci, L. Microglia and mast cells: Two tracks on the road to neuroinflammation. FASEB J. 2012, 26, 3103–3117. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.A.; Walls, A.F. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J. Immunol. 1996, 156, 275–283. [Google Scholar] [PubMed]
- Blom, H.M.; Godthelp, T.; Fokkens, W.J.; Jan, A.K.; Holm, A.F.; Vroom, T.M.; Rijntjes, E. Mast cells, eosinophils and IgE-positive cells in the nasal mucosa of patients with vasomotor rhinitis An immunohistochemical study. Eur. Arch. Oto-Rhino-Laryngol. 1995, 252, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Bentley, A.; Jacobson, M.; Cumberworth, V.; Barkans, J.; Moqbel, R.; Schwartz, L.; Irani, A.; Kay, A.; Durham, S. Immunohistology of the nasal mucosa in seasonal allergic rhinitis: Increases in activated eosinophils and epithelial mast cells. J. Allergy Clin. Immunol. 1992, 89, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Levene, R.Z. Mast cells and amines in normal ocular tissues. Investig. Ophthalmol. Vis. Sci. 1962, 1, 531–543. [Google Scholar]
- Kim, D.-J.; Roper, S.D. Localization of serotonin in taste buds: A comparative study in four vertebrates. J. Comp. Neurol. 1995, 353, 364–370. [Google Scholar] [CrossRef]
- Sleeckx, J.P.; Shea, J.J.; Peremans, J.M. The mast cells of the inner ear. Acta Otorhinolaryngol. Belg. 1976, 30, 443–449. [Google Scholar]
- Miyamura, K.; Kanzaki, Y.; Nagata, M.; Ishikawa, T. Provocation of nystagmus and deviation by type I allergy in the inner ear of the guinea pig. Ann. Allergy 1987, 58, 36–40. [Google Scholar]
- Takeda, T.; Takeda, S.; Egami, N.; Kakigi, A.; Nishioka, R.; Yamasoba, T. Type 1 allergy-induced endolymphatic hydrops and the suppressive effect of leukotriene receptor antagonist. Otol. Neurotol. 2012, 33, 886–890. [Google Scholar] [CrossRef]
- Yu, Y.; Szczepek, A.J.; Haupt, H.; Mazurek, B. Geldanamycin induces production of heat shock protein 70 and partially attenuates ototoxicity caused by gentamicin in the organ of Corti explants. J. Biomed. Sci. 2009, 16, 79. [Google Scholar] [CrossRef]
- Smorodchenko, A.; Rupprecht, A.; Fuchs, J.; Gross, J.; Pohl, E.E. Role of mitochondrial uncoupling protein 4 in rat inner ear. Mol. Cell. Neurosci. 2011, 47, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Tharp, M.D.; Seelig, L.L.; E Tigelaar, R.; Bergstresser, P.R. Conjugated avidin binds to mast cell granules. J. Histochem. Cytochem. 1985, 33, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bajpayee, A.G.; Wong, C.R.; Bawendi, M.G.; Frank, E.H.; Grodzinsky, A.J. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials 2013, 35, 538–549. [Google Scholar] [CrossRef]
- Cai, J.; Wu, X.; Li, X.; Ma, C.; Xu, L.; Guo, X.; Li, J.; Wang, H.; Han, Y. Allicin protects against cisplatin-induced stria vascularis damage: Possible relation to inhibition of Caspase-3 and PARP-1-AIF-mediated apoptotic pathways. ORL J. Otorhinolaryngol. Relat. Spec. 2019, 81, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Kett, W.C.; Osmond, R.I.W.; Moe, L.; E Skett, S.; Kinnear, B.F.; Coombe, D.R. Avidin is a heparin-binding protein. Affinity, specificity and structural analysis. Biochim. Biophys. Acta (BBA) Bioenergy 2003, 1620, 225–234. [Google Scholar] [CrossRef]
- Mulloy, B.; Lever, R.; Page, C.P. Mast cell glycosaminoglycans. Glycoconj. J. 2016, 34, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Wershil, B.K. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am. J. Pathol. 1993, 142, 965–974. [Google Scholar]
- Steel, K.P.; Barkway, C. Another role for melanocytes: Their importance for normal stria vascularis development in the mammalian inner ear. Development 1989, 107, 453–463. [Google Scholar]
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Chapter six—Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 211–252. [Google Scholar]
- Frossi, B.; Mion, F.; Tripodo, C.; Colombo, M.P.; Pucillo, C. Rheostatic functions of mast cells in the control of innate and adaptive immune responses. Trends Immunol. 2017, 38, 648–656. [Google Scholar] [CrossRef]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef]
- John, A.L.S.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Wedemeyer, J.; Metz, M.; Piliponsky, A.M.; Weller, K.; Chatterjea, D.; Clouthier, D.E.; Yanagisawa, M.M.; Tsai, M.; Galli, S.J. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature 2004, 432, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef] [PubMed]
- Minoda, R.; Toriya, T.; Masuyama, K.; Yumoto, E. The effects of histamine and its antagonists on the cochlear microphonic and the compound action potential of the guinea pig. Auris Nasus Larynx 2001, 28, 219–222. [Google Scholar] [CrossRef]
- Azuma, H.; Sawada, S.; Takeuchi, S.; Takeuchi, S.; Kakigi, A.; Takeda, T. Immunohistochemical localization of histamine receptors in rat cochlea. Laryngoscope 2004, 114, 2249–2251. [Google Scholar] [CrossRef]
- Takumida, M.; Takumida, H.; Anniko, M. Localization of histamine (H1, H2, H3 and H4) receptors in mouse inner ear. Acta Oto-Laryngol. 2016, 136, 537–544. [Google Scholar] [CrossRef]
- Moller, M.N.; Kirkeby, S.; Vikeså, J.; Nielsen, F.C.; Caye-Thomasen, P. Expression of histamine receptors in the human endolymphatic sac: The molecular rationale for betahistine use in Menieres disease. Eur. Arch. Otorhinolaryngol. 2015, 273, 1705–1710. [Google Scholar] [CrossRef]
- Housley, G.D.; Norris, C.H.; Guth, P.S. Histamine and related substances influence neurotransmission in the semicircular canal. Hear Res. 1988, 35, 87–97. [Google Scholar] [CrossRef]
- Liu, J.; Fu, T.; Song, F.; Xue, Y.; Xia, C.; Liu, P.; Wang, H.; Zhong, J.; Li, Q.; Chen, J.; et al. Mast cells participate in corneal development in mice. Sci. Rep. 2015, 5, 17569. [Google Scholar] [CrossRef]
- Leon, A.; Buriani, A.; Toso, R.D.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef]
- Staecker, H.; Galinovic-Schwartz, V.; Liu, W.; Lefebvre, P.; Kopke, R.D.; Malgrange, B.; Moonen, G.; Van De Water, T.R. The role of the neurotrophins in maturation and maintenance of postnatal auditory innervation. Am. J. Otol. 1996, 17, 486–492. [Google Scholar]
- Nautiyal, K.M.; Dailey, C.A.; Jahn, J.L.; Rodriquez, E.; Son, N.H.; Sweedler, J.V.; Silver, R. Serotonin of mast cell origin contributes to hippocampal function. Eur. J. Neurosci. 2012, 36, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. The role of mast cell in tissue morphogenesis. Thymus, duodenum, and mammary gland as examples. Exp. Cell Res. 2016, 341, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef]
- Brzezińska-Błaszczyk, E.; Mińcikiewicz, M.; Ochocki, J. Effect of cisplatin and cis-platinum (II) phosphonate complex on murine mast cells. Eur. J. Pharmacol. 1996, 298, 155–158. [Google Scholar] [CrossRef]
- Samorapoompichit, P.; Steiner, M.; Lucas, T.; Wachtler, F.; Schedle, A.; Sperr, W.R.; Valent, P. Induction of apoptosis in the human mast cell leukemia cell line HMC-1 by various antineoplastic drugs. Leuk. Lymphoma 2003, 44, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Perin, P.; Voigt, F.F.; Bethge, P.; Helmchen, F.; Pizzala, R. iDISCO+ for the study of neuroimmune architecture of the rat auditory brainstem. Front. Neuroanat. 2019, 13, 15. [Google Scholar] [CrossRef]






| Target Molecule | Description/Isotype/Conjugate | Company | Catalog # | Working Dilution | |
|---|---|---|---|---|---|
| Primary antibodies | c-Kit/CD117 | Rabbit polyclonal/IgG | Thermo Scientific | PA5-16770 | 1:100 |
| MC chymase | Rabbit polyclonal/IgG | Cusabio | CSB-PA005599GA01HU | 1:1000 | |
| MC tryptase | Mouse monoclonal/ IgG1 (kappa light chain) | Santa Cruz | sc-59587 | 1:200 | |
| High-affinity immunoglobulin epsilon Receptor subunit alpha (FcεRIα) | Rat polyclonal/IgG (whole molecule) | Cusabio | CSB-PA008532LA01HU-50 | 1:50 | |
| Secondary antibodies | Goat anti-Rat | Texas Red™-x (Ex = 595, Em = 615) | Thermo Scientific | T-6392 | 1:400 |
| Goat anti-Rabbit | Alexa Fluor® 594 (Ex = 590, Em = 617) | Thermo Scientific | R 37117 | 1:300 | |
| Goat anti-Rabbit IgG | Alexa Fluor® 488 (Ex = 459, Em = 519) | Thermo Scientific | A 11001 | 1:400 | |
| Goat anti-Mouse IgG | Alexa Fluor® 594 (Ex = 590, Em = 617) | Thermo Scientific | A11005 | 1:400 | |
| Goat anti-Mouse | Alexa Fluor® 633 (Ex = 632, Em = 647) | Thermo Scientific | A 21050 | 1:1000 | |
| Fluorochromes & other reagents | Avidin | Alexa Fluor® 488 (Ex = 459, Em = 519) | Invitrogen | A 21370 | 1:400 |
| DAPI (4‘,6 Diamidino-2-Phenylindole-Dihydrochloride) | (Ex = 364, Em = 454) | Sigma Aldrich | D 9542-5M6 | 1:10000 | |
| Phalloidin | iFluor 594 (Ex = 590, Em = 617) | CytoPainter | ab176757 | 1:1500 | |
| ProLong® Gold (Antifade reagent) | Mounting solution | Invitrogen | P 36930 |
| Inner Hair Cells | Outer Hair Cells | |||||
|---|---|---|---|---|---|---|
| Conditions | Apical | Medial | Basal | Apical | Medial | Basal |
| Control (n = 6) | 9.56 ± 0.58 | 0.28 ± 1.02 | 10.00 ± 0.69 | 12.33 ± 0.54 | 11.72 ± 0.54 | 11.37 ± 1.33 |
| 10 µM cisplatin (n = 6) | 8.78 ± 1.10 (n.s.) | 9.11 ± 1.04 (n.s.) | 9.83 ± 0.81 (n.s.) | 12.20 ± 0.93 (n.s.) | 11.56 ± 0.58 (n.s.) | 11.02 ± 0.46 (n.s.) |
| 20 µM cisplatin (n = 6) | 6.17 ± 0.35 ** | 7.61 ± 0.92 ** | 4.94 ± 1.71 ** | 8.54 ± 1.32 ** | 9.31 ± 1.32 ** | 5.74 ± 0.31 ** |
| 40 µM cisplatin (n = 6) | 4.78 ± 0.40 ** | 4.89 ± 2.74 ** | 3.83 ± 0.86 ** | 3.69 ± 1.84 ** | 4.87 ± 2.99 ** | 3.35 ± 1.02 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepek, A.J.; Dudnik, T.; Karayay, B.; Sergeeva, V.; Olze, H.; Smorodchenko, A. Mast Cells in the Auditory Periphery of Rodents. Brain Sci. 2020, 10, 697. https://doi.org/10.3390/brainsci10100697
Szczepek AJ, Dudnik T, Karayay B, Sergeeva V, Olze H, Smorodchenko A. Mast Cells in the Auditory Periphery of Rodents. Brain Sciences. 2020; 10(10):697. https://doi.org/10.3390/brainsci10100697
Chicago/Turabian StyleSzczepek, Agnieszka J., Tatyana Dudnik, Betül Karayay, Valentina Sergeeva, Heidi Olze, and Alina Smorodchenko. 2020. "Mast Cells in the Auditory Periphery of Rodents" Brain Sciences 10, no. 10: 697. https://doi.org/10.3390/brainsci10100697
APA StyleSzczepek, A. J., Dudnik, T., Karayay, B., Sergeeva, V., Olze, H., & Smorodchenko, A. (2020). Mast Cells in the Auditory Periphery of Rodents. Brain Sciences, 10(10), 697. https://doi.org/10.3390/brainsci10100697






