Decolorization of Orange-G Aqueous Solutions over C60/MCM-41 Photocatalysts
Abstract
:1. Introduction
2. Experimental
2.1. Photocatalysts: Preparation and Characterization
2.2. Photocatalytic Tests
3. Results and Discussion
3.1. Physicochemical Characteristics of the Photocatalysts
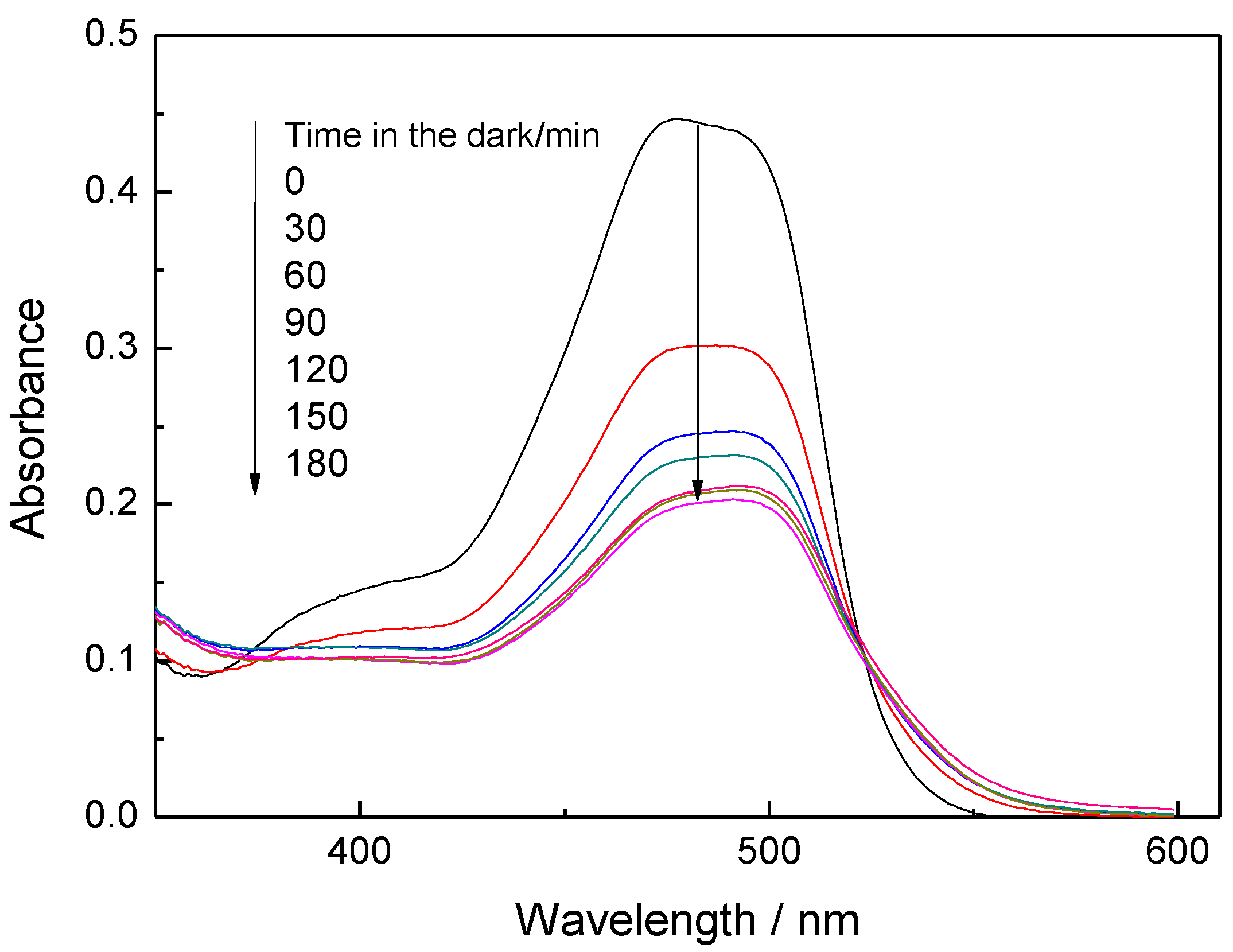
3.2. Decolorization by Adsorption
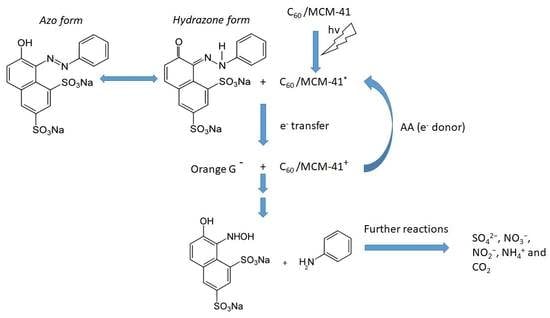
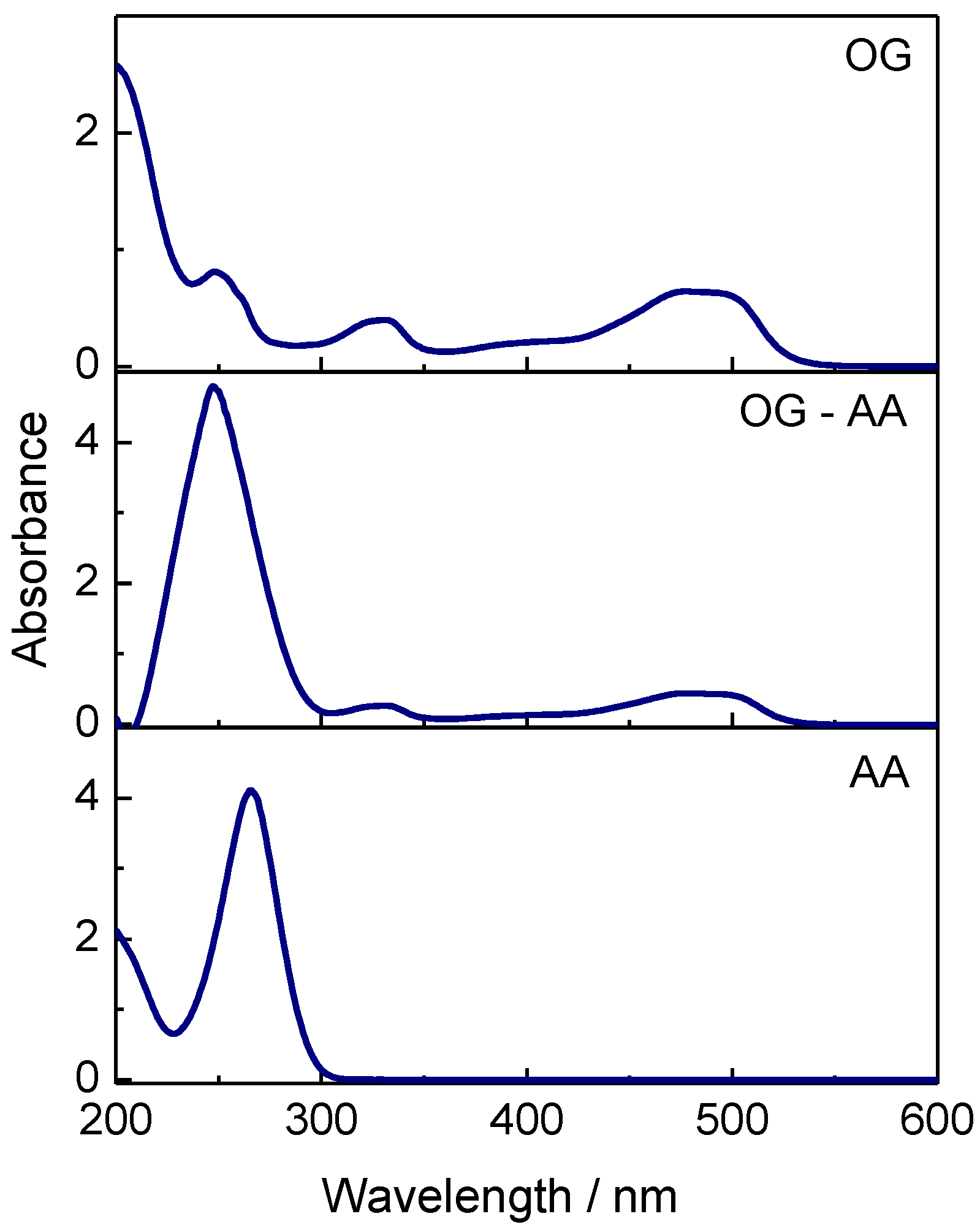
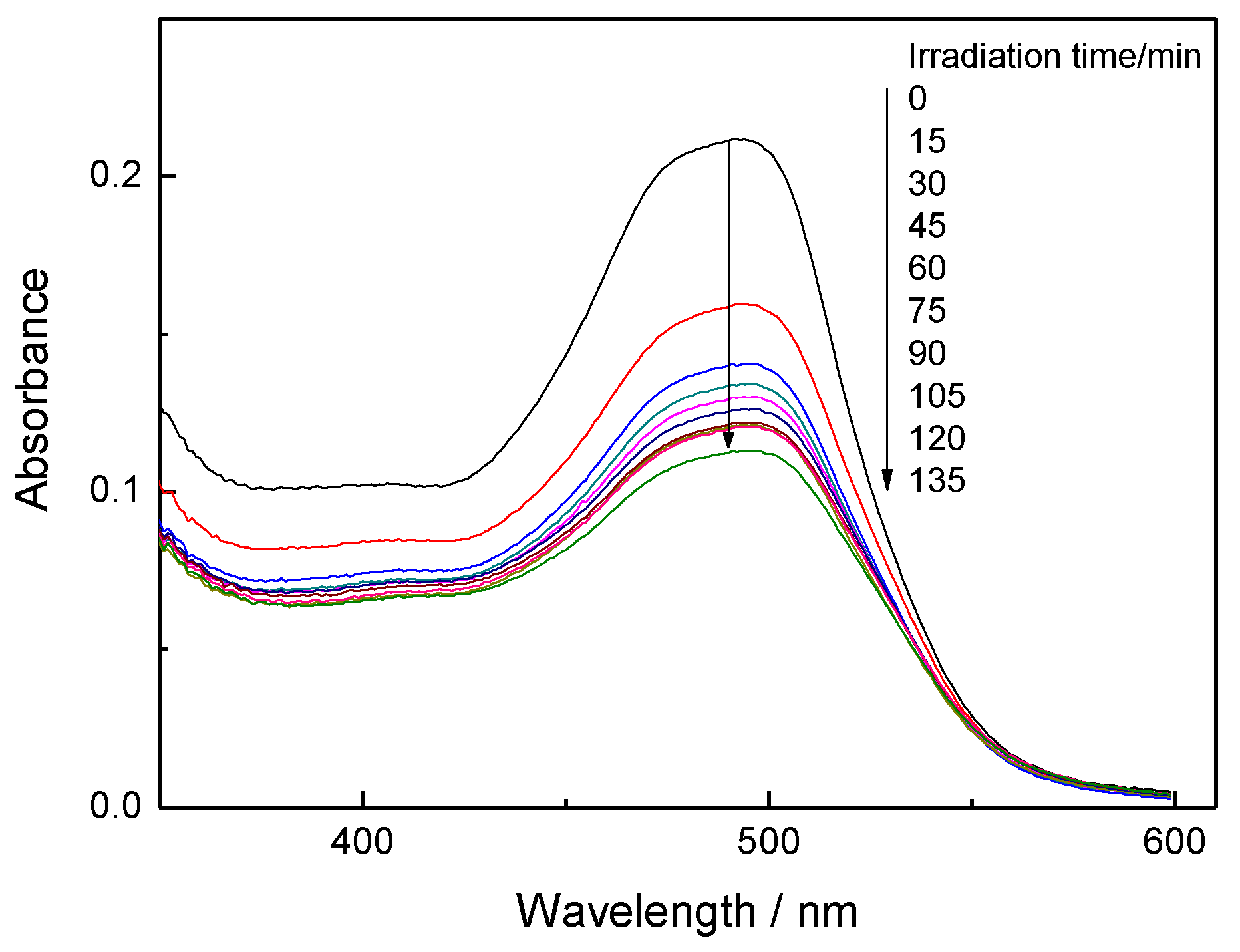
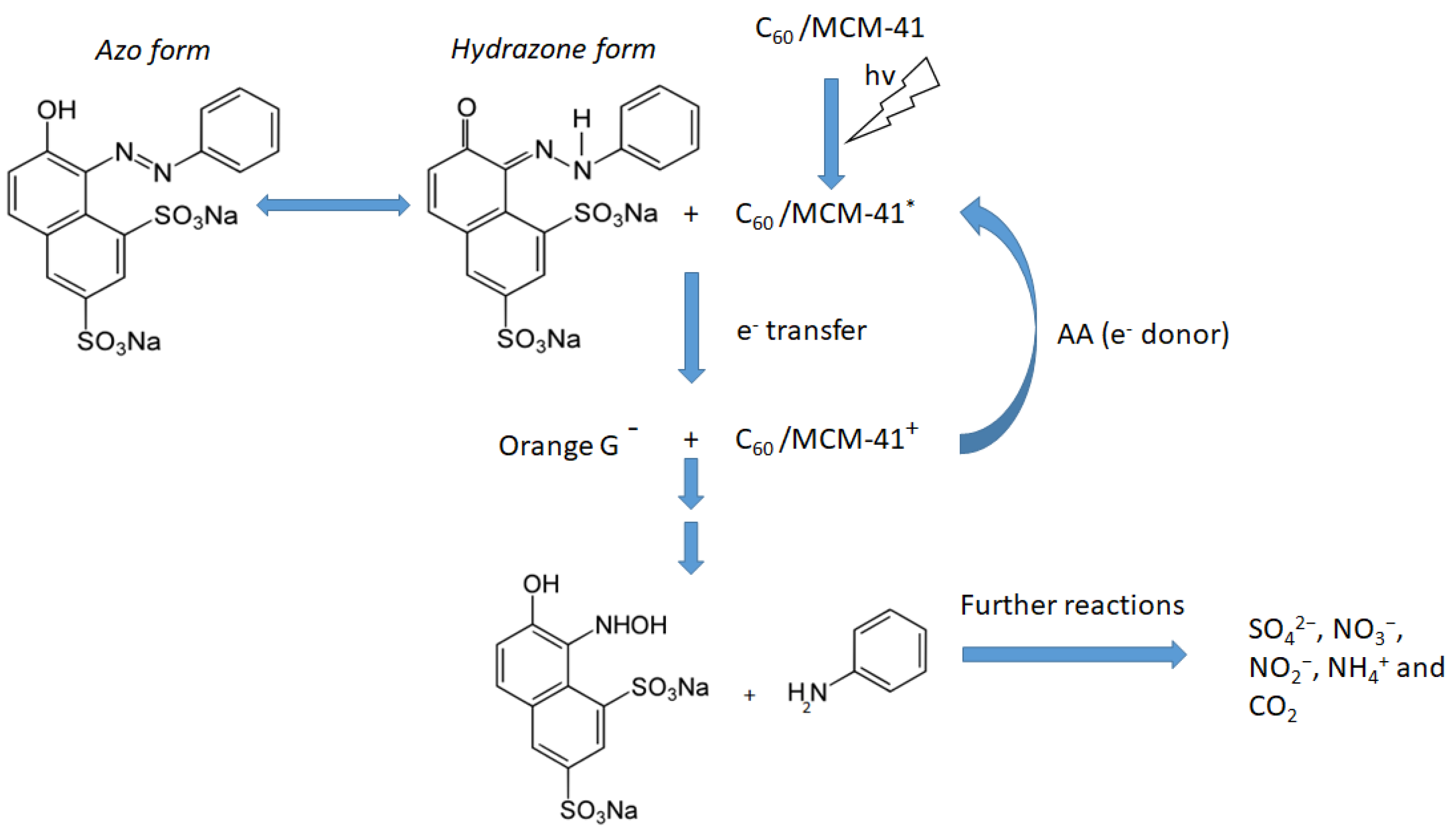
3.3. Decolorization through Photocatalytic Splitting of Azo-Bonds
3.4. Interesting Characteristics of Decolorization Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Saeed, A.; Sharif, M.; Iqbal, M. Application Potential of Grapefruit Peel as Dye Sorbent: Kinetics, Equilibrium and Mechanism of Crystal Violet Adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Monash, P.; Pugazhenthi, G. Adsorption of Crystal Violet Dye from Aqueous Solution Using Mesoporous Materials Synthesized at Room Temperature. Adsorption 2009, 15, 390–405. [Google Scholar] [CrossRef]
- Güzel, F.; Sayğılı, H.; Sayğılı, G.A.; Koyuncu, F. Decolorisation of Aqueous Crystal Violet Solution by a New Nanoporous Carbon: Equilibrium and Kinetic Approach. J. Ind. Eng. Chem. 2014, 20, 3375–3386. [Google Scholar] [CrossRef]
- Güzel, F.; Sayğılı, H.; Sayğılı, G.A.; Koyuncu, F. Elimination of Anionic Dye by Using Nanoporous Carbon Prepared from an Industrial Biowaste. J. Mol. Liq. 2014, 194, 130–140. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Kalaamani, P.; Subburaam, C.V. Liquid Phase Adsorption of Crystal Violet onto Activated Carbons Derived from Male Flowers of Coconut Tree. J. Hazard. Mater. B 2006, 136, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R. Studies on Adsorption of Crystal Violet Dye from Aqueous Solution onto Coniferous Pinus Bark Powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef]
- Chen, L.C.; Chou, T.C. Photodecolorization of Methyl Orange Using Silver Ion Modified TiO2 as Photocatalyst. Ind. Eng. Chem. Res. 1994, 33, 1436–1443. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Li, J.; Yin, J. Photocatalytic Degradation of Methyl Orange by TiO2-Coated Activated Carbon and Kinetic Study. Water Res. 2006, 40, 1119–1126. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, J.G.; Kamiyama, H.; Moriyoshi, Y.; Ishigaki, T. Wavelength-Sensitive Photocatalytic Degradation of Methyl Orange in Aqueous Suspension over Iron(III)-Doped TiO2 Nanopowders under UV and Visible Light Irradiation. J. Phys. Chem. B 2006, 110, 6804–6809. [Google Scholar] [CrossRef] [PubMed]
- Kordouli, E.; Bourikas, K.; Lycourghiotis, A.; Kordulis, C. The Mechanism of Azo-Dyes Adsorption on the Titanium Dioxide Surface and Their Photocatalytic Degradation over Samples with Various Anatase/Rutile Ratios. Catal. Today 2015, 252, 128–135. [Google Scholar] [CrossRef]
- Batista, L.M.B.; Dos Santos, A.J.; Da Silva, D.R.; Alves, A.P.D.; Garcia-Segura, S.; Martinez-Huitle, C.A. Solar Photocatalytic Application of NbO2OH as Alternative Photocatalyst for Water Treatment. Sci. Total Environ. 2017, 596, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kim, E.E.; Tsang, Y.F. Occurrences and Removal of Pharmaceuticals and Personal Care Products (PPCPs) in Drinking Water and Water/Sewage Treatment Plants: A Review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of Doped Photocatalysts for Organic Pollutant Degradation—A Review. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Uyguner-Demirel, C.S.; Birben, N.C.; Bekbolet, M. Elucidation of Background Organic Matter Matrix Effect on Photocatalytic Treatment of Contaminants Using TiO2: A Review. Catal. Today 2017, 284, 202–214. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, S.L.; Li, L.; Wang, T.; Liao, X.L.; Ye, Y.B. An Overview of Advanced Reduction Processes for Bromate Removal from Drinking Water: Reducing Agents, Activation Methods, Applications and Mechanisms. J. Hazard. Mater. 2017, 324, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Iervolino, G.; Rizzo, L.; Sannino, D. Advanced Oxidation Processes for the Removal of Food Dyes in Wastewater. Curr. Org. Chem. 2017, 21, 1068–1073. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Recent Advancements in the Sonophotocatalysis (SPC) and Doped-Sonophotocatalysis (DSPC) for the Treatment of Recalcitrant Hazardous Organic Water Pollutants. Ultrason. Sonochem. 2017, 36, 481–496. [Google Scholar] [CrossRef]
- Al-Hamdi, A.M.; Rinner, U.; Sillanpaa, M. Tin Dioxide as a Photocatalyst for Water Treatment: A Review. Process Saf. Environ. Prot. 2017, 107, 190–205. [Google Scholar] [CrossRef]
- Wakimoto, R.; Kitamura, T.; Ito, F.; Usami, H.; Moriwaki, H. Decomposition of Methyl Orange Using C60 Fullerene Adsorbed on Silica Gel as a Photocatalyst Via Visible-Light Induced Electron Transfer. Appl. Catal. B 2015, 166–167, 544–550. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Lyth, S.M.; Nabae, Y.; Moriya, S.; Kuroki, S.; Kakimoto, M.; Ozaki, J.; Miyata, S. Carbon Nitride as a Nonprecious Catalyst for Electrochemical Oxygen Reduction. J. Phys. Chem. C 2009, 113, 20148–20151. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Talukdar, S.; Dutta, R.K. A Mechanistic Approach for Superoxide Radicals and Singlet Oxygen Mediated Enhanced Photocatalytic Dye Degradation by Selenium Doped ZnS Nanoparticles. RSC Adv. 2016, 6, 928–936. [Google Scholar] [CrossRef]
- Kroto, H.W.; O’Brien, J.R.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Chai, Y.; Guo, T.; Jin, C.; Haufler, R.E.; Felipe, L.P.; Fure, J.; Wang, L.; Alford, J.M.; Smalley, R.E. Fullerenes with Metals Inside. J. Phys. Chem. 1991, 95, 7564–7568. [Google Scholar] [CrossRef]
- Panagiotou, G.D.; Tzirakis, M.D.; Vakros, J.; Loukatzikou, L.; Orfanopoulos, M.; Kordulis, C.; Lycourghiotis, A. Development of [60] Fullerene Supported on Silica Catalysts for the Photo-Oxidation of Alkenes. Appl. Catal. A 2010, 372, 16–25. [Google Scholar] [CrossRef]
- Apostolopoulou, V.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Preparation and Characterization of [60] Fullerene Nanoparticles Supported on Titania Used as a Photocatalyst. Colloids Surf. A Phys. Eng. Asp. 2009, 349, 189–194. [Google Scholar] [CrossRef]
- Tzirakis, M.D.; Vakros, J.; Loukatzikou, L.; Amargianitakis, V.; Orfanopoulos, M.; Kordulis, C.; Lycourghiotis, A. γ-Alumina-Supported [60] Fullerene Catalysts: Synthesis, Properties and Applications in the Photooxidation of Alkenes. J. Mol. Catal. A Chem. 2010, 316, 65–74. [Google Scholar] [CrossRef]
- Vakros, J.; Panagiotou, G.; Kordulis, C.; Lycourghiotis, A.; Vougioukalakis, G.C.; Angelis, Y.; Orfanopoulos, M. Fullerene C60 Supported on Silica and γ-Alumina Catalyzed Photooxidations of Alkenes. Catal. Lett. 2003, 89, 269–273. [Google Scholar] [CrossRef]
- Kyriakopoulos, J.; Tzirakis, M.D.; Panagiotou, G.D.; Alberti, M.N.; Triantafyllidis, K.S.; Giannakaki, S.; Bourikas, K.; Kordulis, C.; Orfanopoulos, M.; Lycourghiotis, A. Highly Active Catalysts for the Photooxidation of Organic Compounds by Deposition of [60] Fullerene onto the MCM-41 Surface: A Green Approach for the Synthesis of Fine Chemicals. Appl. Catal. B 2012, 117–118, 36–48. [Google Scholar] [CrossRef]
- Kyriakopoulos, J.; Papastavrou, A.T.; Panagiotou, G.D.; Tzirakis, M.D.; Triantafyllidis, K.S.; Alberti, M.N.; Bourikas, K.; Kordulis, C.; Orfanopoulos, M.; Lycourghiotis, A. Deposition of Fullerene C60 on the Surface of MCM-41 Via the One-Step Wet Impregnation Method: Active Catalysts for the Singlet Oxygen Mediated Photooxidation of Alkenes. J. Mol. Catal. A Chem. 2014, 381, 9–15. [Google Scholar] [CrossRef]
- Lalwani, G.; Sitharaman, B. Multifunctional Fullerene and Metallofullerene Based Nanobiomaterials. Nano LIFE 2013, 3, 1342003. [Google Scholar] [CrossRef]
- Moor, K.J.; Kim, J.-H. Simple Synthetic Method Toward Solid Supported C60 Visible Light-Activated Photocatalysts. Environ. Sci. Technol. 2014, 48, 2785–2791. [Google Scholar] [CrossRef]
- Boumaza, S.; Bellal, B.; Boudjemaa, A.; Trari, M. Photodegradation of Orange G by the Hetero-Junction x%Bi2S3/TiO2 under Solar Light. Sol. Energy. 2016, 139, 444–451. [Google Scholar] [CrossRef]
- Sehati, S.; Entezari, M.H. Sono-Incorporation of CuO Nanoparticles on the Surface and into the Mesoporous Hexatitanate Layers: Enhanced Fenton-Like Activity in Degradation of Orange-G at Its Neutral pH. Appl. Surf. Sci. 2017, 399, 732–741. [Google Scholar] [CrossRef]
- Tang, L.; Wang, J.; Wang, L.; Jia, C.; Lv, G.; Liu, N.; Wu, M. Facile Synthesis of Silver Bromide-Based Nanomaterials and Their Efficient and Rapid Selective Adsorption Mechanisms Toward Anionic Dyes. ACS Sustain. Chem. Eng. 2016, 4, 4617–4625. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Differential Potentiometric Titration: Development of a Methodology for Determining the Point of Zero Charge of Metal (Hydr) Oxides by One Titration Curve. Environ. Sci. Technol. 2005, 39, 4100–4108. [Google Scholar] [CrossRef]
- Liu, C.-F.; Huang, C.P.; Hu, C.-C.; Juang, Y.; Huang, C. Photoelectrochemical Degradation of Dye Wastewater on TiO2-Coated Titanium Electrode Prepared by Electrophoretic Deposition. Sep. Purif. Technol. 2016, 165, 145–153. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Ali, A.A.; Amin, A.S. A Facile Pechini Sol–Gel Synthesis of TiO2/Zn2TiO2/ZnO/C Nanocomposite: An Efficient Catalyst for the Photocatalytic Degradation of Orange G Textile Dye. RSC Adv. 2017, 7, 30411–30421. [Google Scholar] [CrossRef]
- Hernandez-Uresti, D.B.; Martinez-de la Cruz, A.; Torres-Martinez, L.M. Photocatalytic Degradation of Organic Compounds by PbMoO4 Synthesized by a Microwave-Assisted Solvothermal Method. Ceram. Int. 2016, 42, 3096–3103. [Google Scholar] [CrossRef]
- Barzgari, Z.; Askari, S.Z.; Ghazizadeh, A. Fabrication of Nanostructured CuWO4 for Photocatalytic Degradation of Organic Pollutants in Aqueous Solution. J. Mater. Sci. Mater. Electron. 2017, 28, 3293–3298. [Google Scholar] [CrossRef]
- Roumila, Y.; Abdmeziem, K.; Rekhila, G.; Trari, M. Semiconducting Properties of Hydrothermally Synthesized Libethenite Application to Orange G Photodegradation. Mater. Sci. Semicond. Process. 2016, 41, 470–479. [Google Scholar] [CrossRef]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Pathways of Solar Light-Induced Photocatalytic Degradation of Azo Dyes in Aqueous TiO2 Suspensions. Appl. Catal. B Environ. 2003, 40, 271–286. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Degradation of Orange-G by Advanced Oxidation Processes. Ultrason. Sonochem. 2010, 17, 338–343. [Google Scholar] [CrossRef]
- Arbogast, J.W.; Foote, C.S.; Kao, M. Electron Transfer to Triplet Fullerene C60. J. Am. Chem. Soc. 1992, 114, 2277–2279. [Google Scholar] [CrossRef]



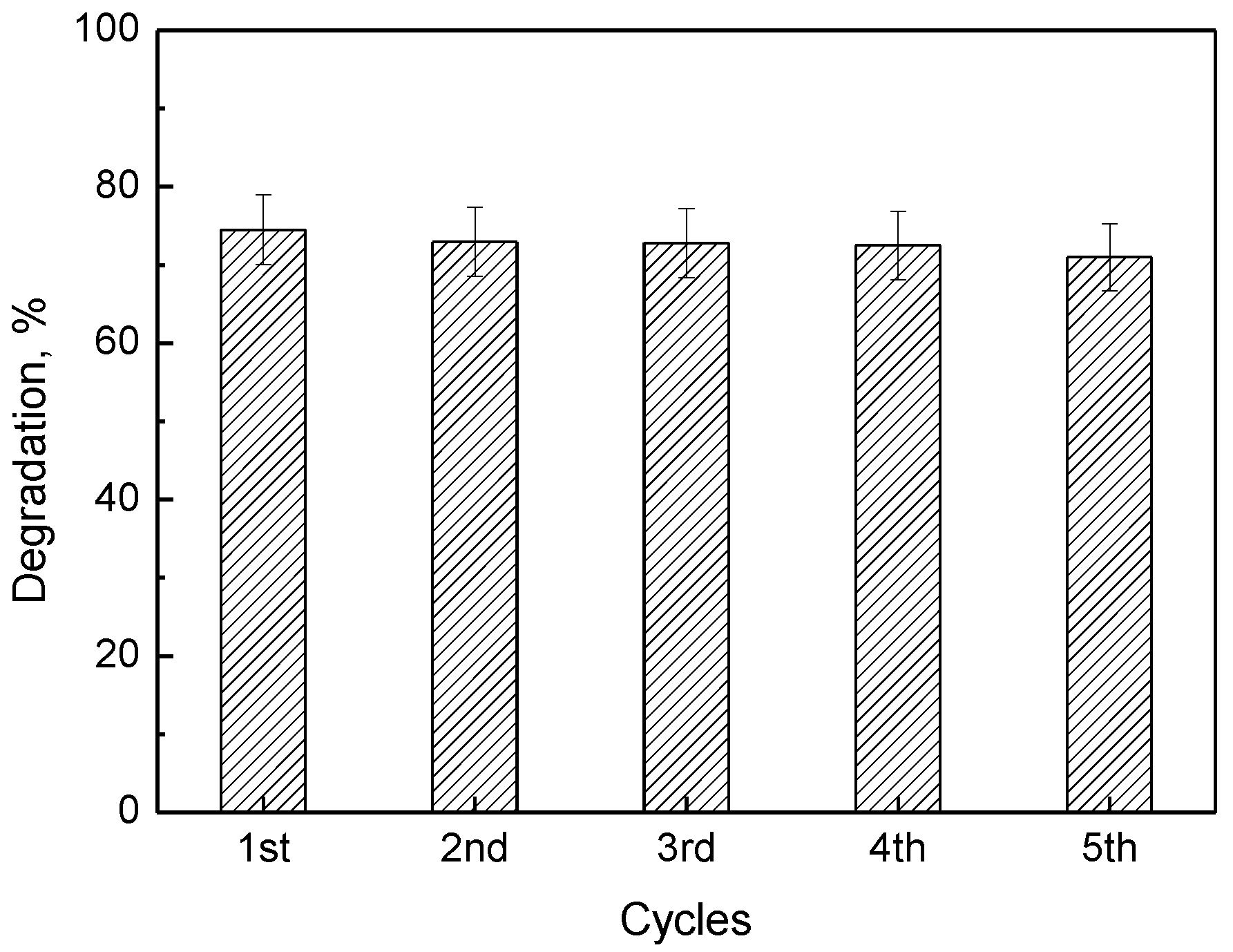
| Sample | C60 wt % | SSABET (m2g−1) | SA (μmol g−1) | kA2 × 103 (g min−1 mg−1) | kR × 102 (min−1g−1) | Decolorization (%)* |
|---|---|---|---|---|---|---|
| 1C60/MCM-41 | 1 | 851 | 37.2 | 6.1 | 0.56 | 41 |
| 3C60/MCM-41 | 3 | 831 | 36.5 | 5.1 | 1.38 | 74.9 |
| 6C60/MCM-41 | 6 | 813 | 11.2 | 0.5 | 0.63 | 39.5 |
| 9C60/MCM-41 | 9 | 777 | 10.4 | 0.2 | 0.54 | 40.8 |
| 12C60/MCM-41 | 12 | 760 | 9.6 | 0.2 | 0.48 | 44.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakopoulos, J.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Decolorization of Orange-G Aqueous Solutions over C60/MCM-41 Photocatalysts. Appl. Sci. 2019, 9, 1958. https://doi.org/10.3390/app9091958
Kyriakopoulos J, Kordouli E, Bourikas K, Kordulis C, Lycourghiotis A. Decolorization of Orange-G Aqueous Solutions over C60/MCM-41 Photocatalysts. Applied Sciences. 2019; 9(9):1958. https://doi.org/10.3390/app9091958
Chicago/Turabian StyleKyriakopoulos, John, Eleana Kordouli, Kyriakos Bourikas, Christos Kordulis, and Alexis Lycourghiotis. 2019. "Decolorization of Orange-G Aqueous Solutions over C60/MCM-41 Photocatalysts" Applied Sciences 9, no. 9: 1958. https://doi.org/10.3390/app9091958
APA StyleKyriakopoulos, J., Kordouli, E., Bourikas, K., Kordulis, C., & Lycourghiotis, A. (2019). Decolorization of Orange-G Aqueous Solutions over C60/MCM-41 Photocatalysts. Applied Sciences, 9(9), 1958. https://doi.org/10.3390/app9091958