Enhancement of Methane Production in Thermophilic Anaerobic Co-Digestion of Exhausted Sugar Beet Pulp and Pig Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Experimental Design
2.3. Analytical Methods
2.4. Indirect Parameters
3. Results and Discussion
3.1. Waste Characteristics
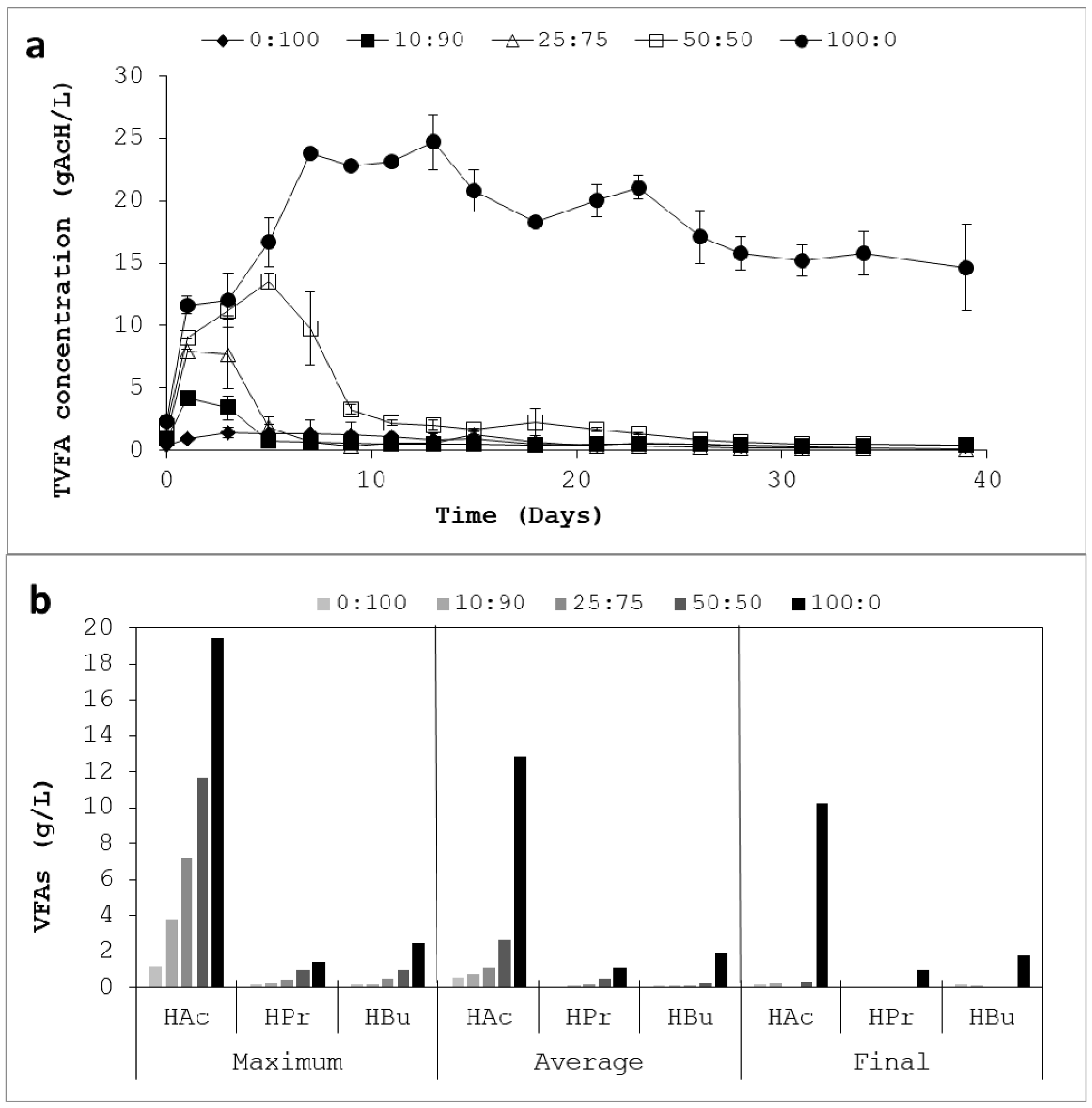
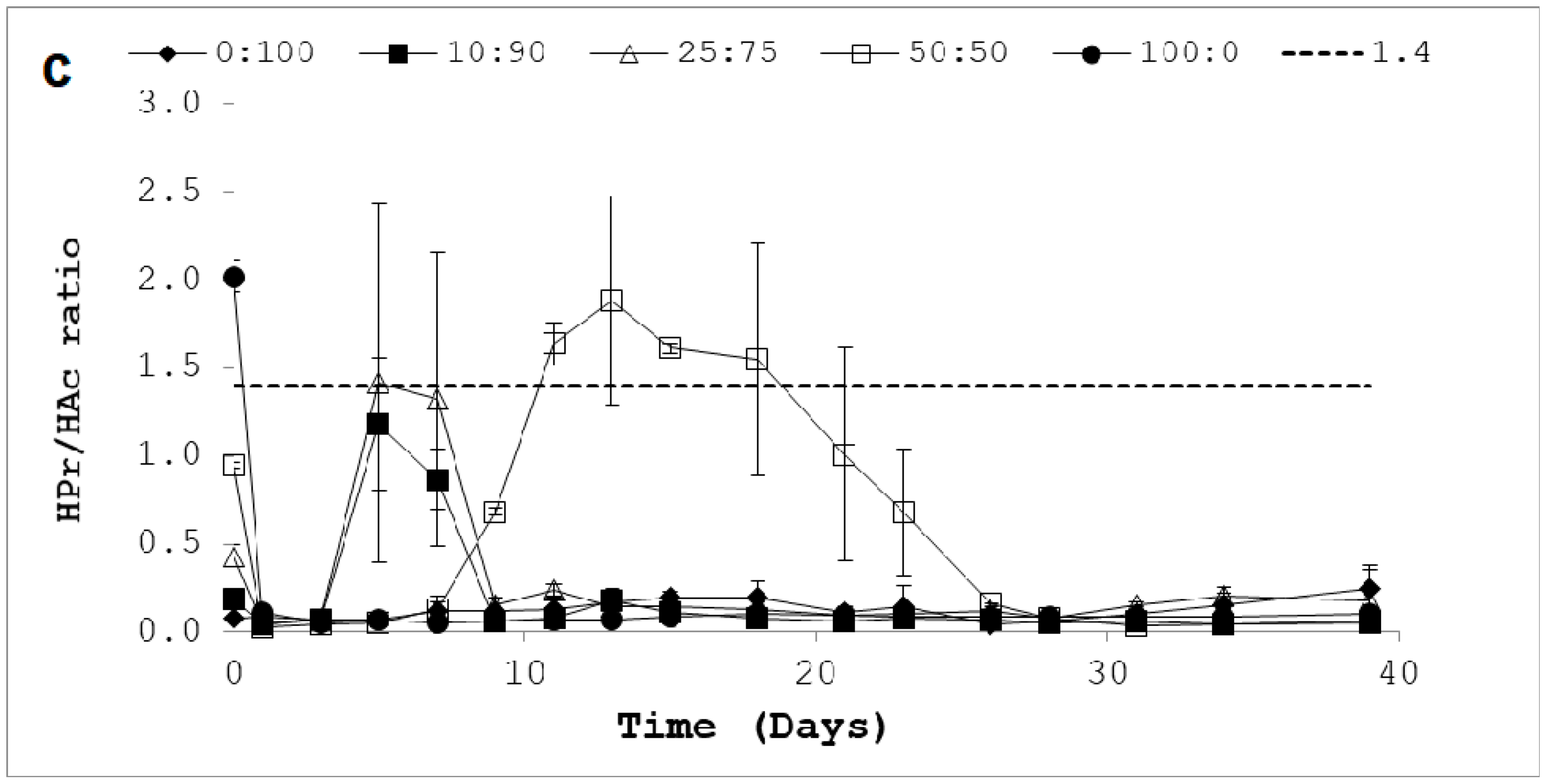
3.2. Process Stability
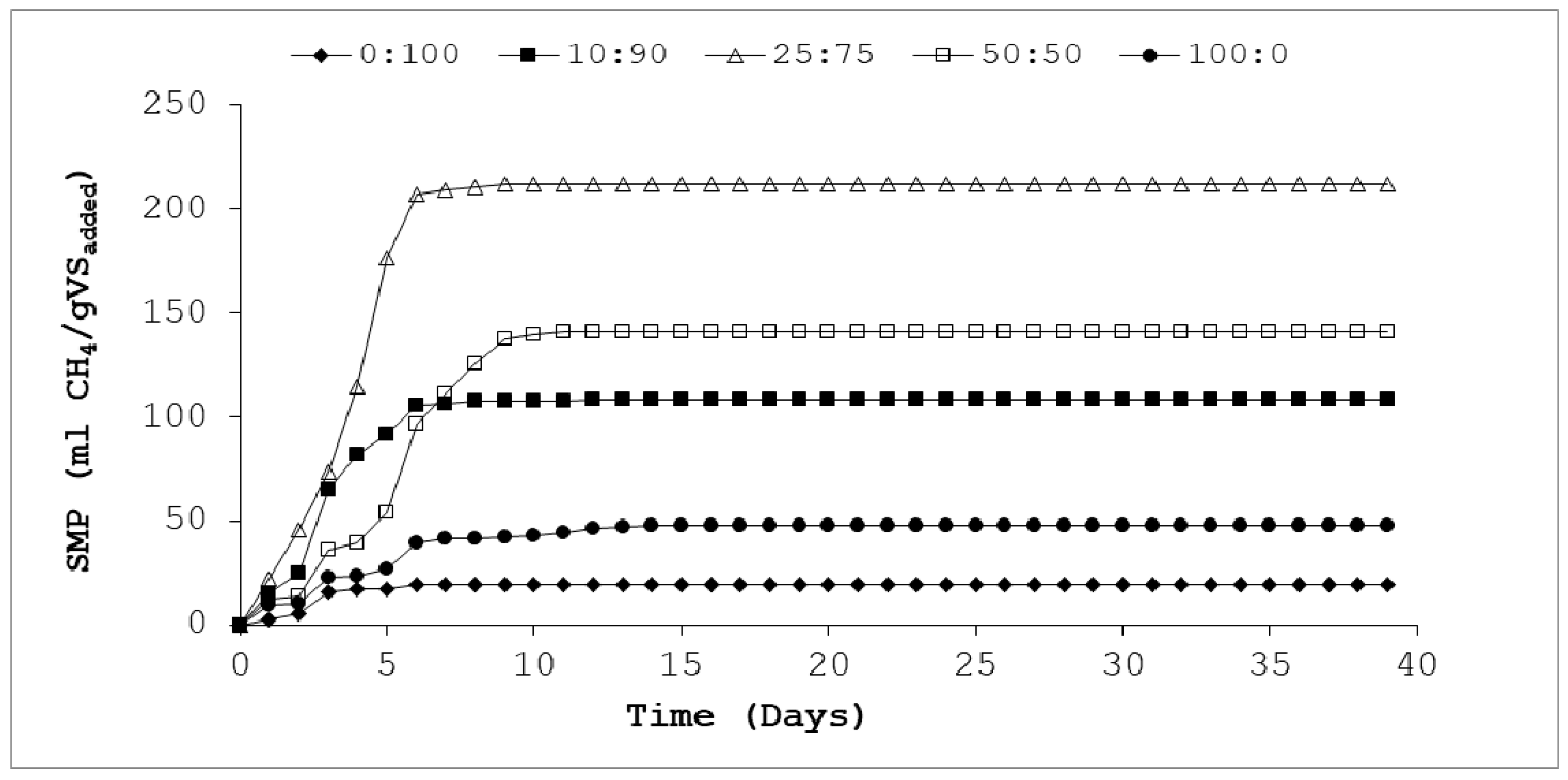
3.3. Methane Production
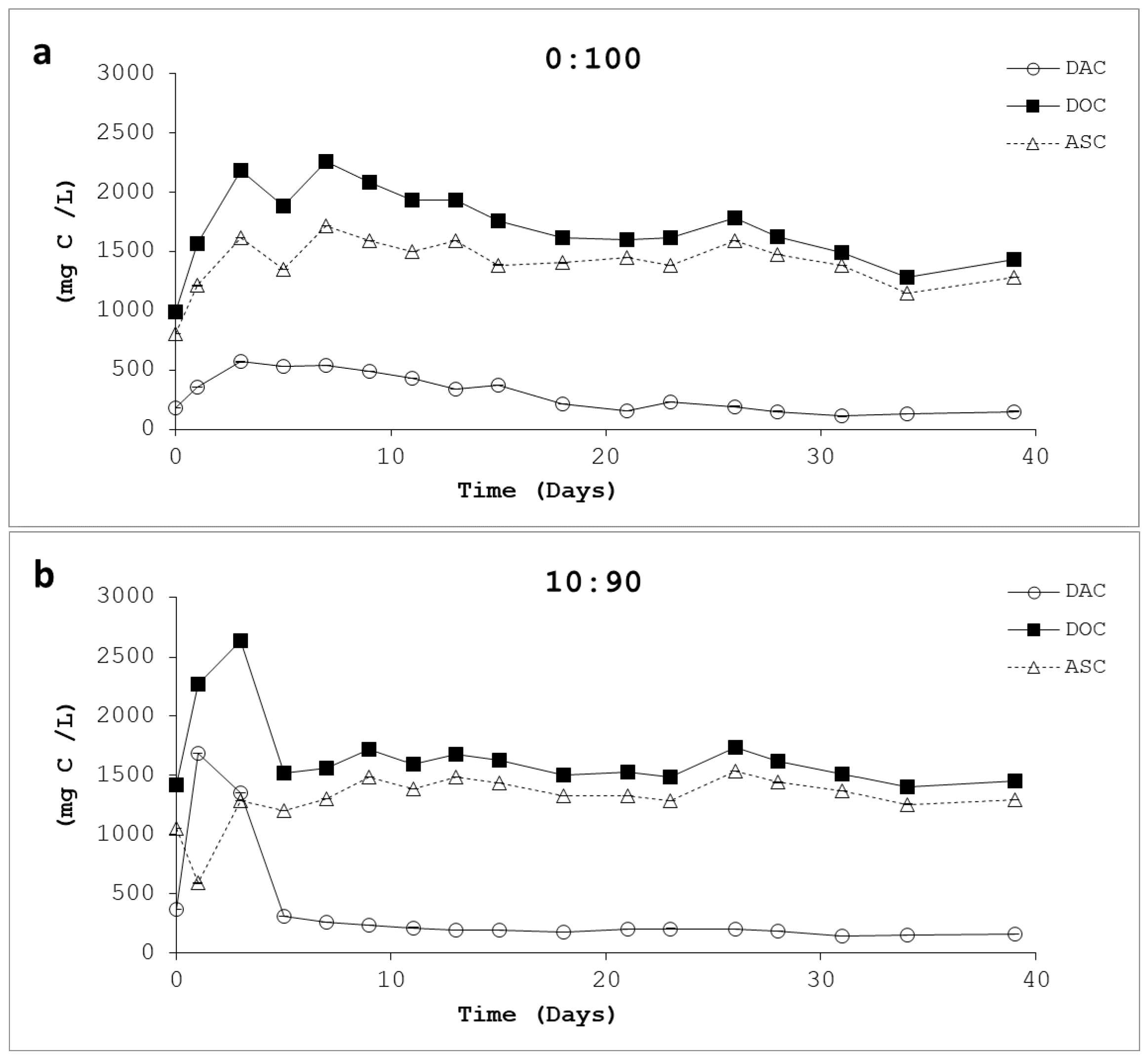
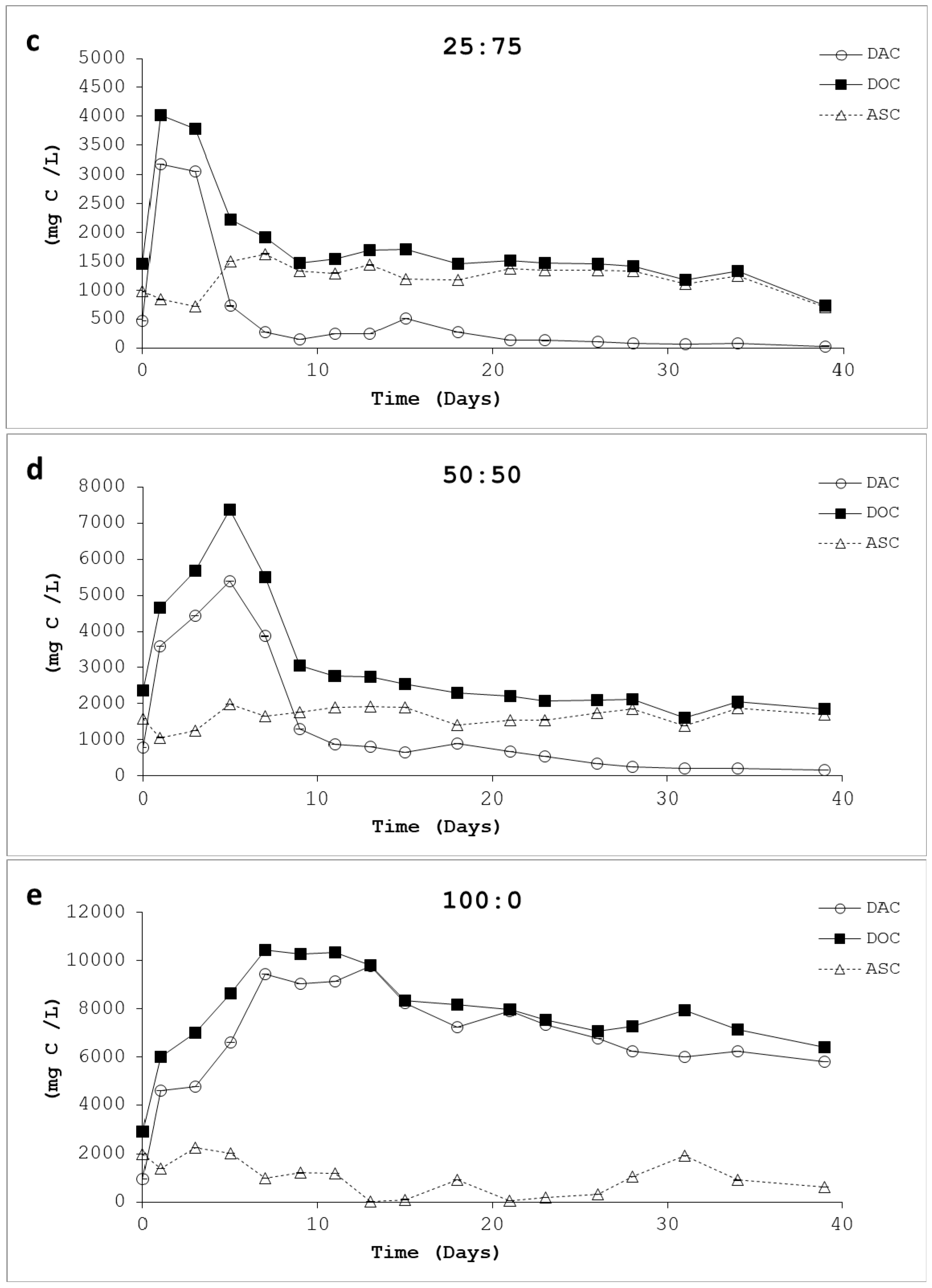
3.4. Acidogenic Substrate as Carbon (ASC)
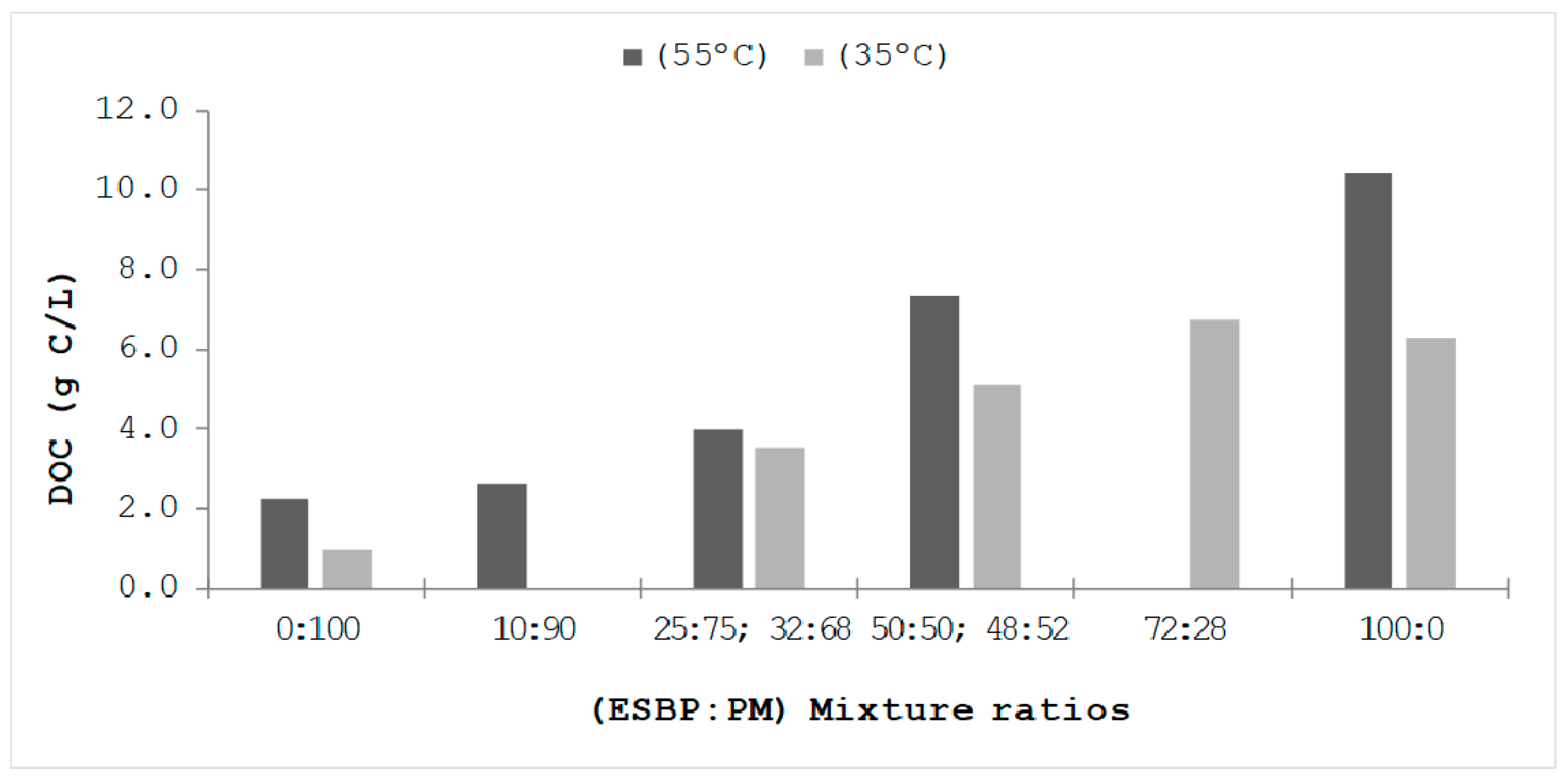
3.5. Influence of the Temperature Range
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Singh, R.; Krishna, B.B.; Mishra, G.; Kumar, J.; Bhaskar, T. Strategies for selection of thermo-chemical processes for the valorisation of biomass. Renew. Energy 2016, 98, 226–237. [Google Scholar] [CrossRef]
- Hutnan, M.; Drtil, M.; Mrafkova, L. Anaerobic biodegradation of sugar beet pulp. Biodegradation 2000, 11, 203–211. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Biomethanization of sugar beet byproduct by semi-continuous single digestion and co-digestion with cow manure. Bioresour. Technol. 2016, 200, 311–319. [Google Scholar] [CrossRef]
- Montañés, R.; Solera, R.; Pérez, M. Anaerobic co-digestion of sewage sludge and sugar beet pulp lixiviation in batch reactors: effect of temperature. Bioresour. Technol. 2015, 180, 177–184. [Google Scholar] [CrossRef]
- Ohuchi, Y.; Ying, C.; Lateef, S.A.; Ihara, I.; Iwasaki, M.; Inoue, R.; Umetsu, K. Anaerobic co-digestion of sugar beet tops silage and dairy cow manure under thermophilic condition. J. Mater. Cycles Waste Manag. 2015, 17, 540–546. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Fdez-Güelfo, L.A.; Álvarez-Gallego, C.; Sales, D.; Romero, L.I. New indirect parameters for interpreting a destabilization episode in an anaerobic reactor. Chem. Eng. J. 2012, 180, 32–38. [Google Scholar] [CrossRef]
- Velásquez Piñas, J.A.; Venturini, O.J.; Silva Lora, E.E.; Calle Roalcaba, O.D. Technical assessment of mono-digestion and co-digestion systems for the production of biogas from anaerobic digestion in Brazil. Renew. Energy 2018, 117, 447–458. [Google Scholar] [CrossRef]
- Salsabil, M.R.; Laurent, J.; Casellas, M.; Dagot, C. Techno-economic evaluation of thermal treatment, ozonation and sonication for the reduction of wastewater biomass volume before aerobic or anaerobic digestion. J. Hazard. Mater. 2010, 174, 323–333. [Google Scholar] [CrossRef]
- Aboudi, K.; Gómez-Quiroga, X.; Álvarez-Gallego, C.J.; Quiroga-Alonso, J.M.; Romero-García, L.I. Effect of Temperature on Biohydrogen and Biomethane Productions by Anaerobic Digestion of Sugar Beet by-Products. Int. J. Environ. Sci. Dev. 2018, 8, 762–766. [Google Scholar] [CrossRef]
- Zahedi, S. Energy efficiency: Importance of indigenous microorganisms contained in the municipal solid wastes. Waste Manag. 2018, 78, 763–769. [Google Scholar] [CrossRef]
- Zahedi, S.; Dahunsi, S.O.; Perez, M.; Solera, R. Assessment of chemical inhibitor addition to improve the gas production from biowaste. Waste Biomass Valoriz. 2017, 10, 1091–1099. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Comparison of mesophilic and thermophilic dry anaerobic digestion of OFMSW: Kinetic analysis. Chem. Eng. J. 2013, 232, 59–64. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Preze, M.; Romero, L.I. Bio-methanization of organic fraction from municipal solid waste: Temperature effects. Polish J. Chem. Technol. 2013, 15, 99–106. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Palatsi, J.; Illa, J.; Prenafeta-Boldú, F.X.; Laureni, M.; Fernandez, B.; Angelidaki, I.; Flotats, X. Long-chain fatty acids inhibition and adaptation process in anaerobic thermophilic digestion: Batch tests, microbial community structure and mathematical modelling. Bioresour. Technol. 2010, 101, 2243–2251. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Sanaei-Moghadam, A.; Abbaspour-Fard, M.H.; Aghel, H.; Aghkhani, M.H.; Abedini-Torghabeh, J. Enhancement of Biogas Production by Co-digestion of Potato Pulp with Cow Manure in a CSTR System. Appl. Biochem. Biotechnol. 2014, 173, 1858–1869. [Google Scholar] [CrossRef]
- Rodriguez-Verde, I.; Regueiro, L.; Carballa, M.; Hospido, A.; Lema, J.M. Assessing anaerobic co-digestion of pig manure with agroindustrial wastes: the link between environmental impacts and operational parameters. Sci. Total Environ. 2014, 497–498, 475–483. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Semi-continuous anaerobic co-digestion of sugar beet byproduct and pig manure: Effect of the organic loading rate (OLR) on process performance. Bioresour. Technol. 2015, 194, 283–290. [Google Scholar] [CrossRef]
- Fang, C.; Boe, K.; Angelidaki, I. Anaerobic co-digestion of by-products from sugar production with cow manure. Water Res. 2011, 45, 3473–3480. [Google Scholar] [CrossRef]
- Umetsu, K.; Yamazaki, S.; Kishimoto, T.; Takahashi, J.; Shibata, Y.; Zhang, C.; Misaki, T.; Hamamoto, O.; Ihara, I.; Komiyama, M. Anaerobic co-digestion of dairy manure and sugar beets. Int. Congr. Ser. 2006, 1293, 307–310. [Google Scholar] [CrossRef]
- Wu, X.; Yao, W.; Zhu, J.; Miller, C. Biogas and CH(4) productivity by co-digesting swine manure with three crop residues as an external carbon source. Bioresour. Technol. 2010, 101, 4042–4047. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Influence of total solids concentration on the anaerobic co-digestion of sugar beet by-products and livestock manures. Sci. Total Environ. 2017, 586, 438–445. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Li, Y. Solid-state anaerobic digestion of lignocellulosic biomass: Recent progress and perspectives. Bioresour. Technol. 2016, 205, 239–249. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Liotta, F.; d’Antonio, G.; Esposito, G.; Fabbricino, M.; Frunzo, L.; van Hullebusch, E.D.; Lens, P.N.L.; Pirozzi, F. Effect of moisture on disintegration kinetics during anaerobic digestion of complex organic substrates. Waste Manag. Res. 2014, 32, 40–48. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- APHA-AWWA-WPCF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Álvarez-Gallego, C.J. Testing Different Procedures for the Start-Up of a Dry Anaerobic Co-Digestion Process of OFMSW and Sewage Sludge at Thermophilic Range, University of Cadiz. 2005. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=52224 (accessed on 30 September 2018).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Angeriz-Campoy, R.; Fdez-Güelfo, L.A.; Tyagi, V.K.; Álvarez-Gallego, C.J.; Romero-García, L.I. New criteria to determine the destabilization of the acidogenic anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW) with mixed sludge (MS). Bioresour. Technol. 2018, 248, 174–179. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Evaluation of methane generation and process stability from anaerobic co-digestion of sugar beet by-product and cow manure. J. Biosci. Bioeng. 2016, 121, 566–572. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Yuan, Y.-X.; Dai, Y.-M.; Li, D.; Li, Z.-D.; Liu, X.-F.; Zhang, X.-Y.; Yan, Z.-Y. Methane production characteristics and microbial community dynamics of mono-digestion and co-digestion using corn stalk and pig manure. Int. J. Hydrogen Energy 2017, 42, 4893–4901. [Google Scholar] [CrossRef]
- Guwy, A.J.; Hawkes, F.R.; Wilcox, S.J.; Hawkes, D.L. Neural network and on-off control of bicarbonate alkalinity in a fluidised-bed anaerobic digester. Water Res. 1997, 31, 2019–2025. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Thermophilic anaerobic digestion of livestock waste: the effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Callaghan, F.J.; Wase, D.A.J.; Thayanithy, K.; Forster, C.F. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass Bioenergy 2002, 22, 71–77. [Google Scholar] [CrossRef]
- Switzenbaum, M.S.; Giraldo-Gomez, E.; Hickeyt, R.F. Monitoring of the anaerobic methane fermentation process. Enzyme Microb. Technol. 1990, 12, 722–730. [Google Scholar] [CrossRef]
- Zickefoose, C.; Hayes, R.B.J.; Bryant, J.O. Anaerobic Sludge Digestion: Operations Manual. EPA 430=9-76-001; National Technical Information Service, Ed.; United States Environmental Protection Agency: Washington, DC, USA, 1976. [Google Scholar]
- Hill, D.T.; Cobb, S.A.; Bolte, J.P. Using Volatile Fatty Acid Relationships to Predict Anaerobic Digester Failure. Trans. ASAE 1987, 30, 496. [Google Scholar] [CrossRef]
- Pullammanappallil, P.C.; Chynoweth, D.P.; Lyberatos, G.; Svoronos, S.A. Stable performance of anaerobic digestion in the presence of a high concentration of propionic acid. Bioresour. Technol. 2001, 78, 165–169. [Google Scholar] [CrossRef]
- Barredo, M.S.; Evison, L.M. Effect of propionate toxicity on methanogen-enriched sludge, Methanobrevibacter smithii, and Methanospirillum hungatii at different pH values. Appl. Environ. Microbiol. 1991, 57, 1764–1769. [Google Scholar] [PubMed]
- Wang, Y.; Li, G.; Chi, M.; Sun, Y.; Zhang, J.; Jiang, S.; Cui, Z. Effects of co-digestion of cucumber residues to corn stover and pig manure ratio on methane production in solid state anaerobic digestion. Bioresour. Technol. 2018, 250, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Improvement of Exhausted Sugar Beet Cossettes Anaerobic Digestion Process by Co-Digestion with Pig Manure. Energy Fuels 2015, 29, 754–762. [Google Scholar]
- Yang, Z.; Wang, W.; He, Y.; Zhang, R.; Liu, G. Effect of ammonia on methane production, methanogenesis pathway, microbial community and reactor performance under mesophilic and thermophilic conditions. Renew. Energy 2018, 125, 915–925. [Google Scholar] [CrossRef]
- Selvam, A.; Xu, D.; Zhao, Z. Fate of tetracycline, sulfonamide and fluoroquinolone resistance genes and the changes in bacterial diversity during composting of swine manure. Bioresour. Technol. 2012, 126, 383–390. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Johnson, T.A.; Su, J.-Q.; Qiao, M.; Guo, G.-X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef]
- Zhang, J.; Sui, Q.; Zhong, H.; Meng, X.; Wang, Z.; Wang, Y.; Wei, Y. Impacts of zero valent iron, natural zeolite and Dnase on the fate of antibiotic resistance genes during thermophilic and mesophilic anaerobic digestion of swine manure. Bioresour. Technol. 2018, 258, 135–141. [Google Scholar] [CrossRef]
- Montañés Alonso, R.; Solera del Río, R.; Pérez García, M. Thermophilic and mesophilic temperature phase anaerobic co-digestion (TPAcD) compared with single-stage co-digestion of sewage sludge and sugar beet pulp lixiviation. Biomass Bioenergy 2016, 93, 107–115. [Google Scholar] [CrossRef]
- Smith, P.H.; Mah, R.A. Kinetics of acetate metabolism during sludge digestion. Appl. Microbiol. 1966, 14, 368–371. [Google Scholar]
- Chan, P.C.; de Toledo, R.A.; Shim, H. Anaerobic co-digestion of food waste and domestic wastewater. Effect of intermittent feeding on short and long chain fatty acids accumulation. Renew. Energy 2018, 124, 129–135. [Google Scholar] [CrossRef]
- Rasit, N.; Idris, A.; Harun, R.; Wan Ab Karim Ghani, W.A. Effects of lipid inhibition on biogas production of anaerobic digestion from oily effluents and sludges: An overview. Renew. Sustain. Energy Rev. 2015, 45, 351–358. [Google Scholar] [CrossRef]

| Component | Units | ESBP | PM | Inoculum |
|---|---|---|---|---|
| pH | - | 6.22 ± 0.03 | 8.26 ± 0.10 | 7.96 ± 0.08 |
| TS | g/kg | 857.7 ± 0.04 | 276.3 ± 0.27 | 55.67 ± 0.15 |
| VS | g/kg | 758.6 ± 0.06 | 156.8 ± 0.30 | 28.43 ± 0.11 |
| sCOD | g/kg | 21.0 ± 0.42 | 7.0 ± 0.28 | 3.4 ± 0.39 |
| TVFA | g/kg | 1.99 ± 0.31 | 1.2 ± 0.02 | 0.72 ± 0.10 |
| Alkalinity | g/kg | 2.19 ± 0.10 | 33.1 ± 0.50 | 11.8 ± 0.77 |
| C/N ratio | - | 37.4 ± 0.22 | 3.03 ± 0.27 | - |
| Pectins | % | 55.54 | - | 69.31 |
| Hemicellulose | % | 22.52 | - | 11.29 |
| Cellulose | % | 21.14 | - | 11.90 |
| Lignin | % | 3.50 | - | 5.61 |
| Assay | Reference | CH4 (HAc) (L/Lreactor) | CH4 (HPr) (L/Lreactor) | CH4 (HBu) (L/Lreactor) | CH4 (ASC) (L/Lreactor) | CH4 (VFAs+ASC) (L) | CH4 (Produced) (L) | CH4 (Non-Produced) (%) |
|---|---|---|---|---|---|---|---|---|
| 0:100T | This study | 0.052 | 0.010 | 0.077 | 0.106 | 0.42 | 0.86 | 32.7 |
| 10:90T | 0.100 | 0.006 | 0.042 | 0.100 | 0.42 | 4.03 | 9.40 | |
| 25:75T | 0.039 | 0.011 | 0.012 | 0.100 | 0.27 | 9.16 | 2.90 | |
| 50:50T | 0.064 | 0.009 | 0.051 | 0.103 | 0.39 | 8.43 | 4.40 | |
| 100:0T | 4.056 | 0.499 | 1.121 | 0.098 | 9.82 | 2.92 | 77.1 | |
| 0:100M | Aboudi et al. [44] | 0.004 | 0.002 | 0.002 | 0.102 | 0.22 | 7.63 | 2.8 |
| 32:68M | 0.003 | 0.000 | 0.002 | 0.100 | 0.21 | 8.48 | 2.4 | |
| 48:52M | 0.011 | 0.001 | 0.004 | 0.099 | 0.23 | 9.34 | 2.4 | |
| 72:28M | 0.041 | 0.025 | 0.003 | 0.099 | 0.34 | 10.19 | 3.2 | |
| 100:0M | 0.266 | 0.200 | 0.022 | 0.107 | 5.19 | 11.04 | 32.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Quiroga, X.; Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Enhancement of Methane Production in Thermophilic Anaerobic Co-Digestion of Exhausted Sugar Beet Pulp and Pig Manure. Appl. Sci. 2019, 9, 1791. https://doi.org/10.3390/app9091791
Gómez-Quiroga X, Aboudi K, Álvarez-Gallego CJ, Romero-García LI. Enhancement of Methane Production in Thermophilic Anaerobic Co-Digestion of Exhausted Sugar Beet Pulp and Pig Manure. Applied Sciences. 2019; 9(9):1791. https://doi.org/10.3390/app9091791
Chicago/Turabian StyleGómez-Quiroga, Xiomara, Kaoutar Aboudi, Carlos José Álvarez-Gallego, and Luis Isidoro Romero-García. 2019. "Enhancement of Methane Production in Thermophilic Anaerobic Co-Digestion of Exhausted Sugar Beet Pulp and Pig Manure" Applied Sciences 9, no. 9: 1791. https://doi.org/10.3390/app9091791
APA StyleGómez-Quiroga, X., Aboudi, K., Álvarez-Gallego, C. J., & Romero-García, L. I. (2019). Enhancement of Methane Production in Thermophilic Anaerobic Co-Digestion of Exhausted Sugar Beet Pulp and Pig Manure. Applied Sciences, 9(9), 1791. https://doi.org/10.3390/app9091791








