Recent Lipid Membrane-Based Biosensing Platforms
Abstract
1. Introduction
- Adequate robustness for long-term stability under storage and operation.
- Simple and non-expertise demanding reproducible fabrication.
- Stable membrane state, preferably liquid crystalline, that allows lateral mobility.
- Stable and reproducible membrane morphology, preferably defect-free for easier control on ion transport.
- Adequate hydration in the hydrophilic region in order to support protein-based interactions.
- Compatible coupling to transducers.
- Fit-for-purpose detection strategy that will ensure optimal detection without catastrophic perturbations of the lipid organization.
- Carefully managed strength of immobilized biology in order to avoid leakage but maintain functionality.
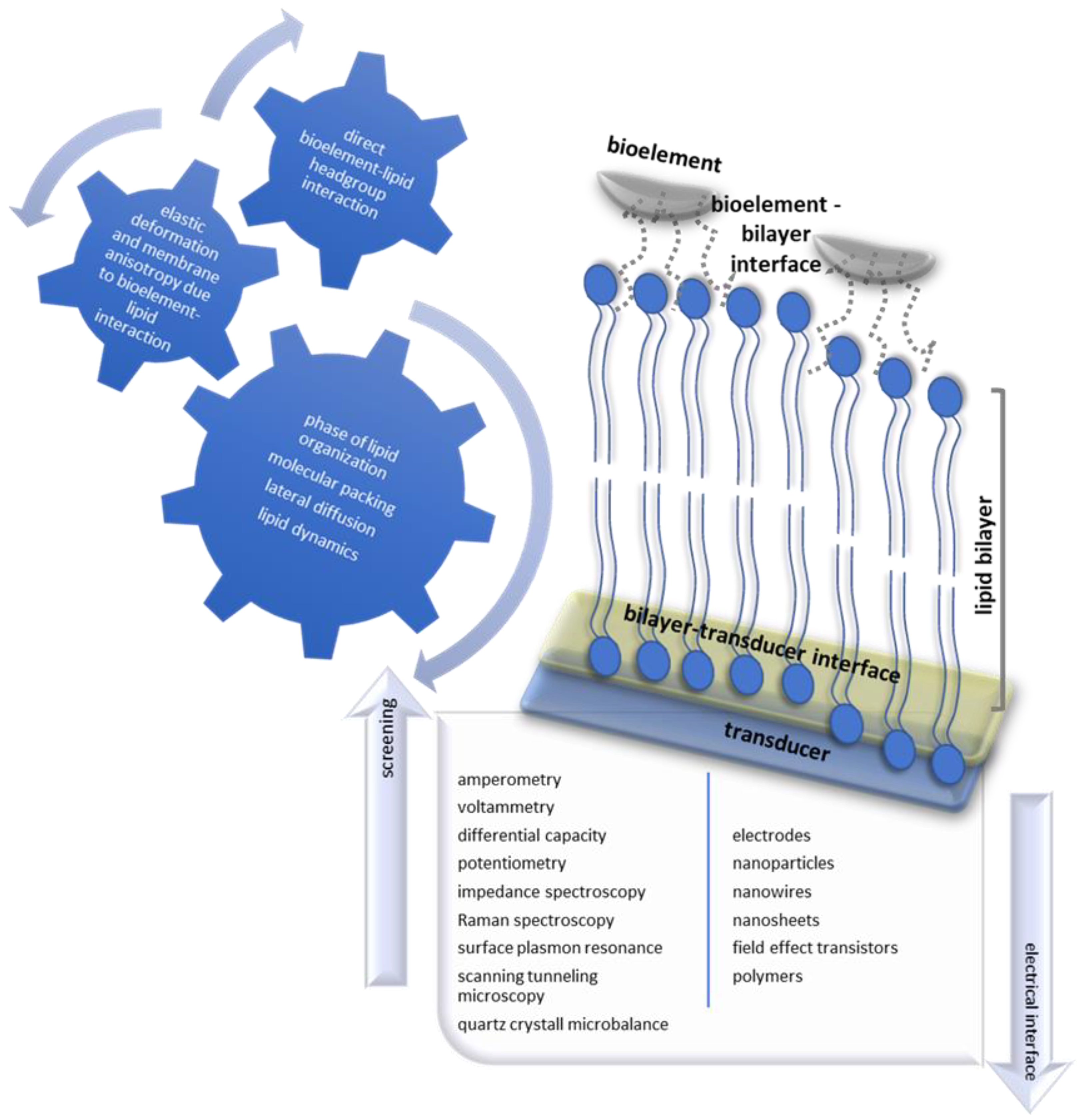
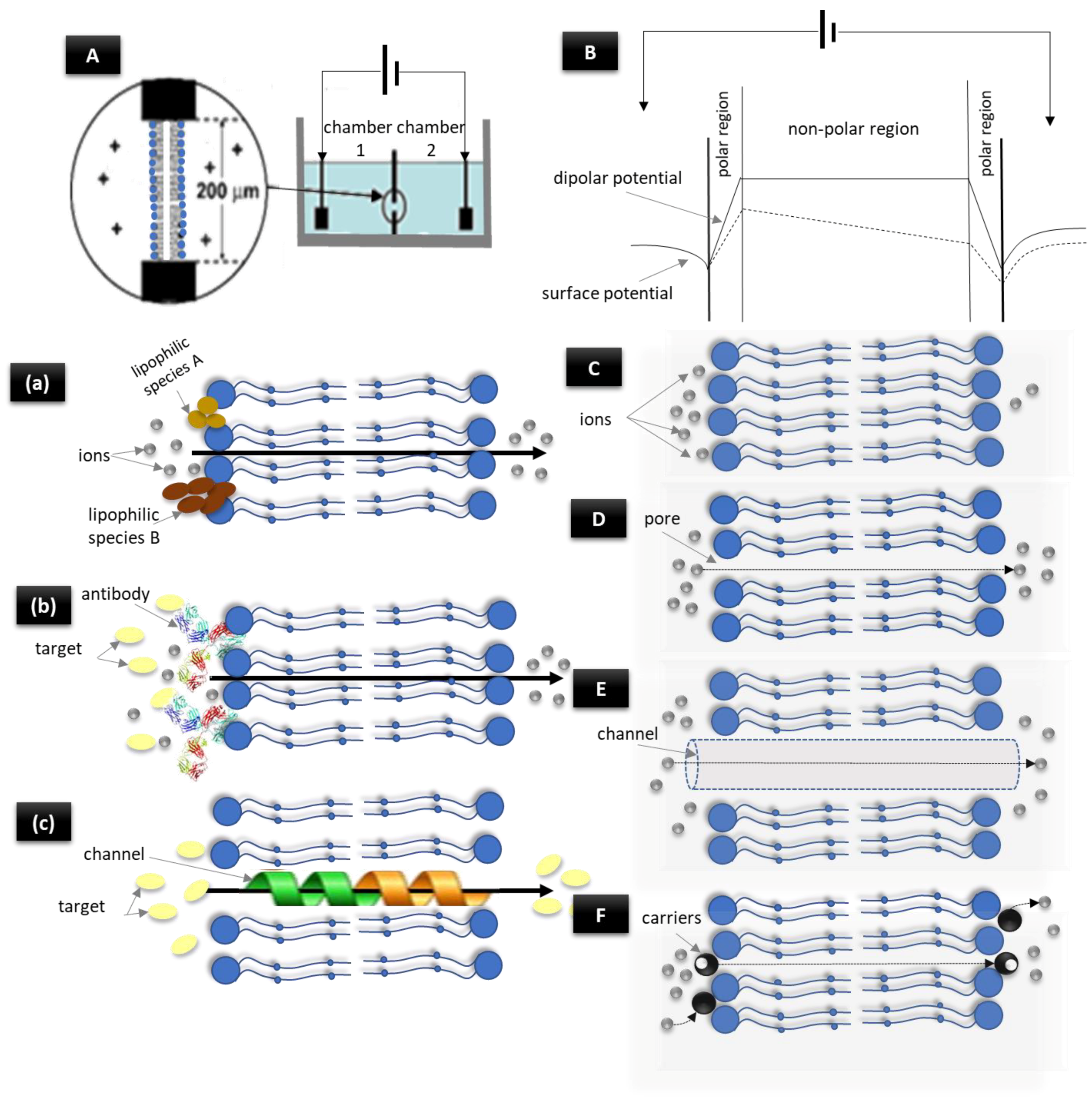
2. The Electroanalytical Aspect of Lipid Membrane Biosensors
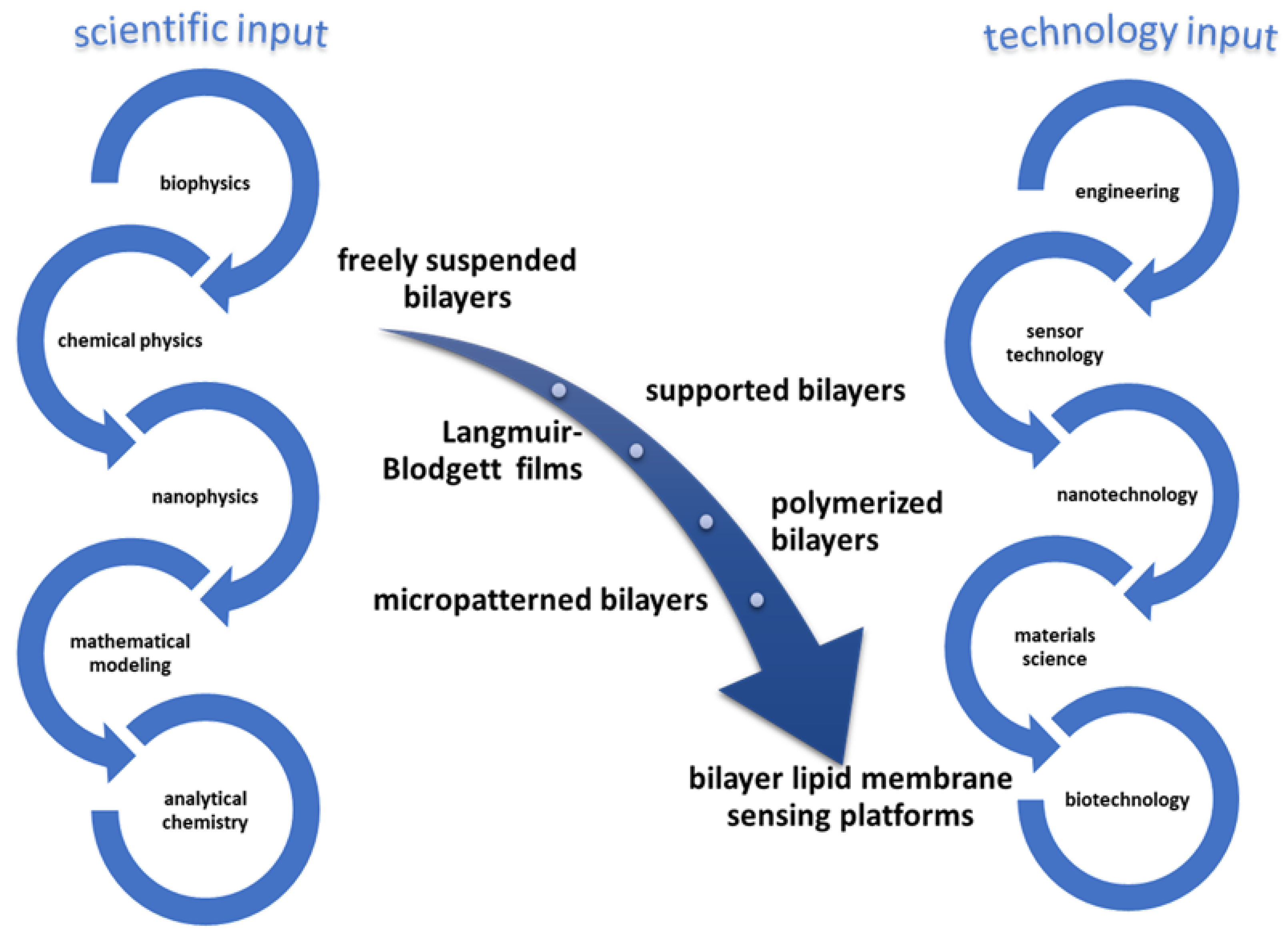
3. Lipid Membrane-Based Biosensing
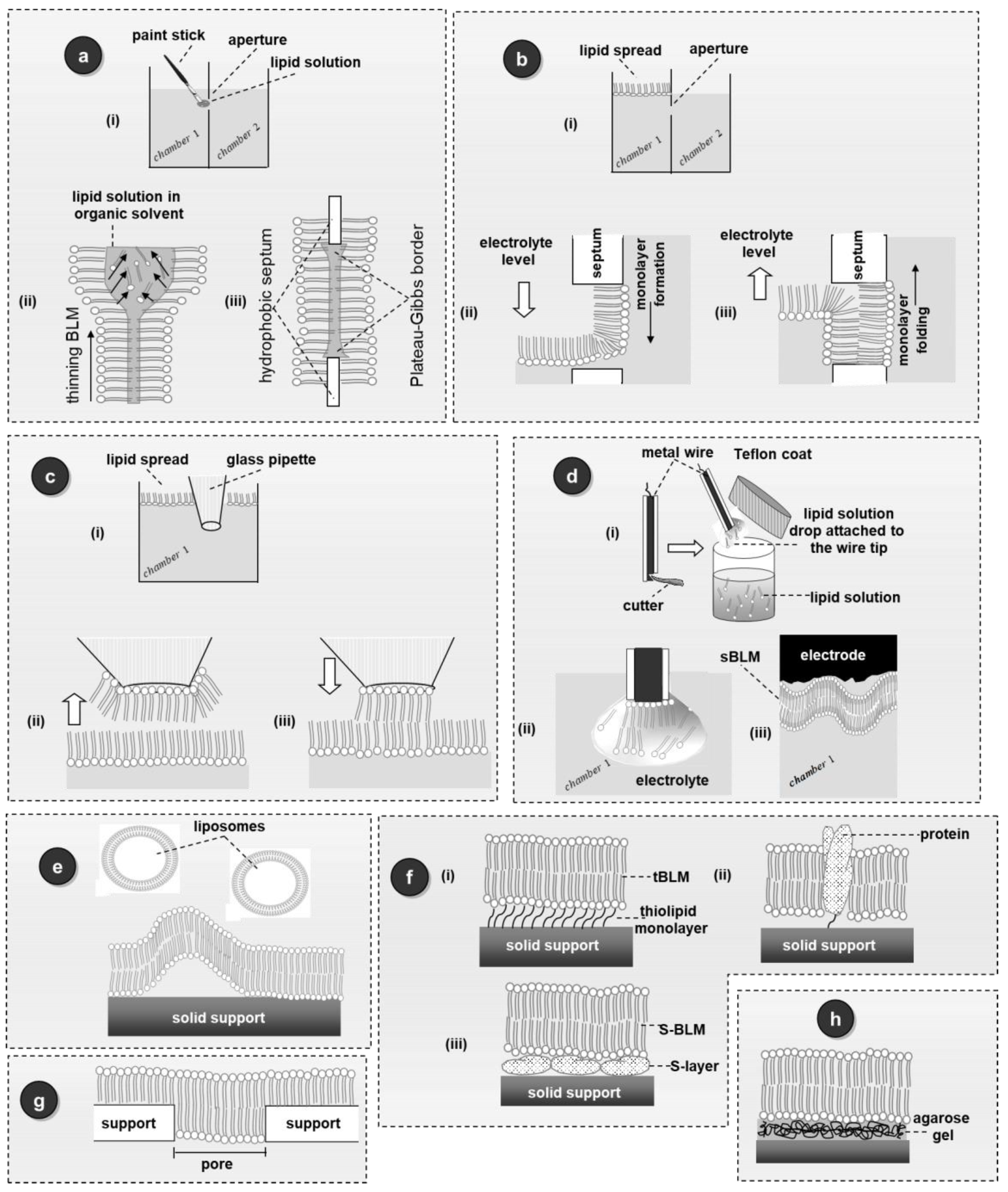
4. Methods for the Preparation of the Most Important Platforms of Stabilized Biosensors Based on Lipid Membranes
4.1. Stabilized Lipid Films Formed on a Glass Fiber Filter
4.2. Polymer-Supported Bilayer Lipid Membranes
4.3. Polymeric Lipid Membranes Supported on Graphene Microelectrodes
4.4. Polymer Lipid Films Supported on ZnO Microelectrodes
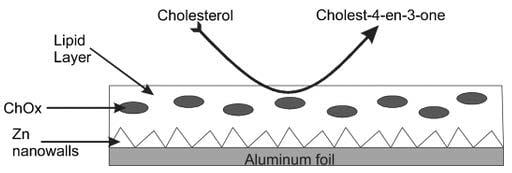
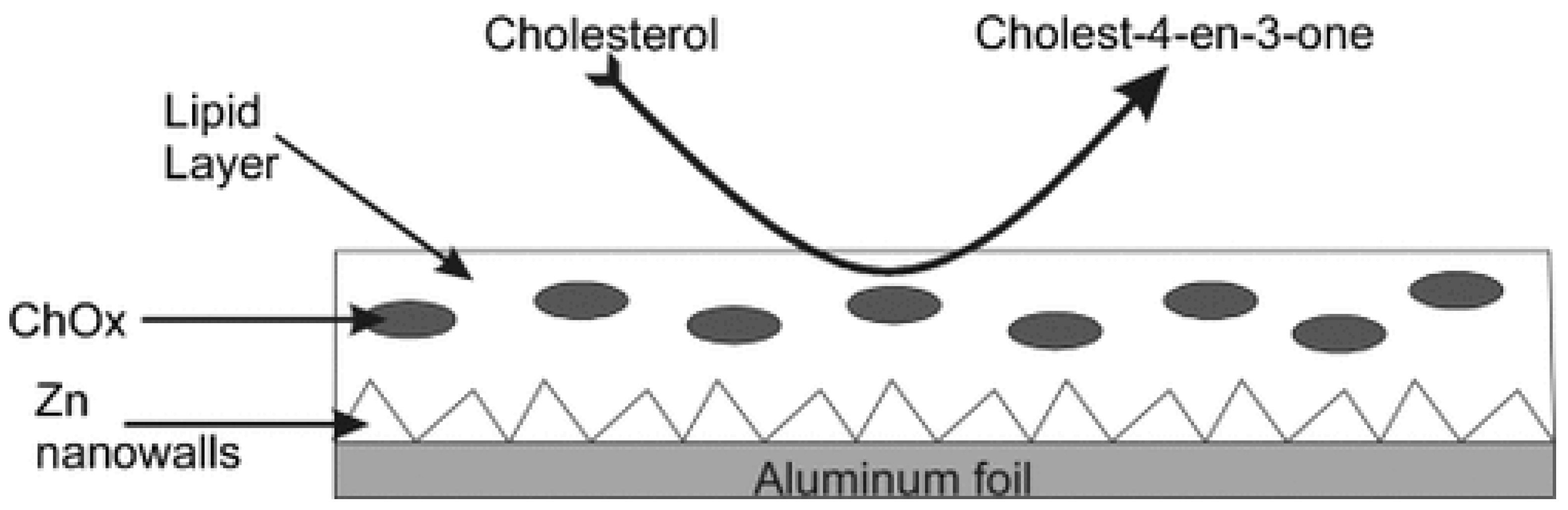
4.4.1. Potentiometric Biosensors Based on ZnO Nanowalls and Stabilized Polymerized Lipid Film
4.4.2. Potentiometric Biosensors Based on Lipid Stabilized Membranes ZnO Nanowires
5. Practical Applications of Lipid Membrane Devices in Food, Environmental, and Clinical Analysis
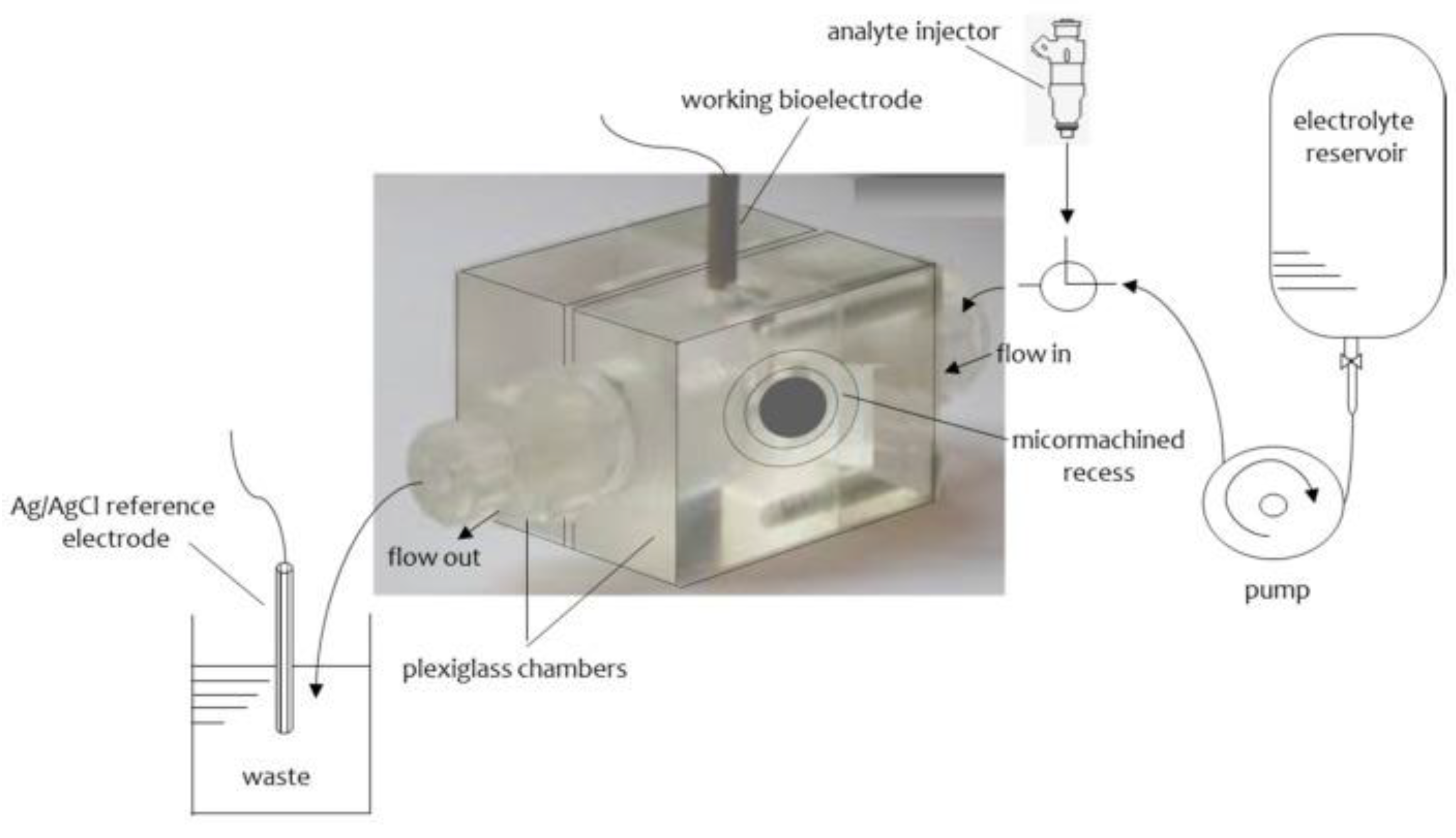
5.1. Applications of Lipid Film Sensors Based on Glass Microfilter
5.2. Applications of Lipid Film Devices Based on Polymeric Lipid Membranes
5.3. Applications of Graphene Based Devices
5.4. Applications of the ZnO Nanoelectrode-Based Devices
6. Challenges and Advances
7. Conclusions and Future Prospects
Author Contributions
Conflicts of Interest
References
- Siontorou, C.G.; Nikoleli, G.-P.; Nikolelis, D.P.; Karapetis, S.K. Artificial lipid membranes: Past, present, and future. Membranes 2017, 7, 38. [Google Scholar] [CrossRef]
- Mazur, F.; Bally, M.; Städler, B.; Chandrawati, R. Liposomes and lipid bilayers in biosensors. Adv. Colloid Interface Sci. 2017, 249, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.F.; Leitch, J.J.; Lipkowski, J. Electrode-supported biomimetic membranes: An electrochemical and surface science approach for characterizing biological cell membranes. Curr. Opin. Electrochem. 2018, 12, 60–72. [Google Scholar] [CrossRef]
- Gould, S.B. Membranes and evolution. Curr. Biol. 2018, 28, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.; Hannesschlaeger, C.; Goss, K.-U.; Pohl, P. Passive permeability of planar lipid bilayers to organic anions. Biophys. J. 2018, 115, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Zatloukalova, M.; Novak, D. Electrochemistry of membrane proteins and protein–lipid assemblies. Curr. Opin. Electrochem. 2018, 12, 73–80. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Nikolelis, D.P.; Evtugyn, G.; Hianik, T. Advances in lipid film based biosensors. TrAC Trends Anal. Chem. 2016, 79, 210–221. [Google Scholar] [CrossRef]
- Raghunathan, K.; Kenworthy, A.K. Dynamic pattern generation in cell membranes: Current insights into membrane organization. BBA-Biomembranes 2018, 1860, 2018–2031. [Google Scholar] [CrossRef]
- Kalyankar, N.D.; Sharma, M.K.; Vaidya, S.V.; Calhoun, D.; Maldarelli, C.; Couzis, A.; Gilchrist, L. Arraying of intact liposomes into chemically functionalized microwells. Langmuir 2006, 22, 5403–5411. [Google Scholar] [CrossRef] [PubMed]
- Siontorou, C.G. Bilayer lipid membrane constructs: A strategic technology evaluation approach. In Advanced Bioelectronic Materials; Tiwari, A., Patra, H.K., Turner, A.P.F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 311–353. [Google Scholar]
- Cranfield, C.G.; Cornell, B.A.; Grage, S.L.; Duckworth, P.; Carne, S.; Ulrich, A.S.; Martinac, B. Transient potential gradients and impedance measures of tethered bilayer lipid membranes: Pore-forming peptide insertion and the effect of electroporation. Biophys. J. 2014, 106, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Nikolelis, D.P.; Siontorou, C.G. Flow injection monitoring and analysis of mixtures of simazine, atrazine, and propazine using filter-supported bilayer lipid membranes (BLMs). Electroanalysis 1996, 8, 907–912. [Google Scholar] [CrossRef]
- Michaloliakos, A.I.; Nikoleli, G.P.; Siontorou, C.G.; Nikolelis, D.P. Rapid flow injection electrochemical detection of Arochlor 1242 using stabilized lipid membranes with incorporated sheep anti-PCB antibody. Electroanalysis 2012, 24, 495–501. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Simantiraki, M.G.; Siontorou, C.G.; Toth, K. Flow injection analysis of carbofuran in foods using air stable lipid film based acetylcholinesterase biosensor. Anal. Chim. Acta 2005, 537, 169–177. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Siontorou, C.G.; Krull, U.J.; Katrivanos, P.L. Ammonium ion minisensors from self-assembled bilayer lipid membranes using gramicidin as an ionophore. Modulation of ammonium selectivity by platelet-activating factor. Anal. Chem. 1996, 15, 1735–1741. [Google Scholar] [CrossRef]
- Vallejo, A.E.; Gervasi, C.A. EIS studies of valinomycin-mediated K+ transport through supported lipid bilayers. J. Electroanal. Chem. 2007, 603, 51–58. [Google Scholar] [CrossRef]
- Su, Z.F.; Shodiev, M.; Leitch, J.J.; Abbasi, F.; Lipkowski, J. Role of transmembrane potential and defects on the permeabilization of lipid bilayers by alamethicin, an ion-channel-forming peptide. Langmuir 2018, 34, 6249–6260. [Google Scholar] [CrossRef]
- Pantoja, R.; Sigg, D.; Blunck, R.; Benzanilla, F.; Heath, J.R. Bilayer reconstitution of voltage-dependent ion channels using a microfabricated silicon chip. Biophys. J. 2001, 81, 2389–2394. [Google Scholar] [CrossRef]
- Ben Chaim, Y.; Chanda, B.; Dascal, N.; Bezanilla, F.; Parnas, I.; Parnas, H. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature 2006, 444, 106–109. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Mely, Y.; Demchenko, A.P.; Duportail, G. Simultaneous probing of hydration and polarity of lipid bilayers with 3-hydroxyflavone fluorescent dyes. BBA-Biomembranes 2004, 1665, 6–19. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Duportail, G.; Mely, Y.; Demchenko, A.P. Ultrasensitive two-color fluorescence probes for dipole potential in phospholipid membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 11219–11224. [Google Scholar] [CrossRef]
- Starke-Peterkovic, T.; Turner, N.; Else, P.L.; Clarke, R.J. Electric field strength of membrane lipids from vertebrate species: Membrane lipid composition and Na+-K+-ATPase molecular activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 663–670. [Google Scholar] [CrossRef]
- Peterson, U.; Mannock, D.A.; Lewis, R.N.A.H.; Pohl, P.; McElhaney, R.N.; Pohl, E.E. Origin of membrane dipole potential: Contribution of the phospholipid fatty acid chains. Chem. Phys. Lipids 2002, 117, 19–27. [Google Scholar] [CrossRef]
- Warshaviak, D.T.; Muellner, M.J.; Chachisvilis, M. Effect of membrane tension on the electric field and dipole potential of lipid bilayer membrane. Biochim. Biophys. Acta 2011, 1808, 2608–2617. [Google Scholar] [CrossRef]
- Wiegand, G.; Arribas-Layton, N.; Hillebrandt, H.; Sackmann, E.; Wagner, P. Electrical properties of supported lipid bilayer membranes. J. Phys. Chem. B 2002, 106, 4245–4254. [Google Scholar] [CrossRef]
- Legiň, M.; Laputková, G.; Sabo, J.; Vojčíková, L. Impedance Spectroscopy of Bilayer Lipid Membranes Self-assembled on Agar Support—Interaction with HDL. Physiol. Res. 2007, 56 (Suppl. 1), S85–S91. [Google Scholar]
- Liu, X.; Li, L.; Mason, A.J. High-throughput impedance spectroscopy biosensor array chip. Philos. Trans. R. Soc. A 2014, 372, 20130107. [Google Scholar] [CrossRef]
- Wüstner, D.; Sklenar, H. Atomistic Monte Carlo simulation of lipid membranes. Int. J. Mol. Sci. 2014, 15, 1767–1803. [Google Scholar] [CrossRef]
- Xing, C.; Faller, R. What is the difference between a supported and a free bilayer? Insights from Molecular Modeling on Different Scales. In Advances in Planar Lipid Bilayers and Liposomes; Iglič, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 127–157. [Google Scholar]
- Khan, M.S.; Dosoky, N.S.; Williams, J.D. Engineering lipid bilayer membranes for protein studies. Int. J. Mol. Sci. 2013, 14, 21561–21597. [Google Scholar] [CrossRef]
- Ackerman, D.G.; Feigenson, G.W. Lipid bilayers: Clusters, domains and phases. Essays Biochem. 2015, 57, 33–42. [Google Scholar] [CrossRef]
- Siontorou, C.G.; Nikolelis, D.P.; Piunno, P.A.E.; Krull, U.J. Detection of DNA hybridization using self-assembled bilayer lipid membranes (BLMs). Electroanalysis 1997, 57, 1067–1071. [Google Scholar] [CrossRef]
- Komura, S.; Shirotori, H.; Olmsted, P.D. Phase behaviour of three-component lipid mixtures. J. Phys. Condens. Matter 2005, 17, S2951–S2956. [Google Scholar] [CrossRef][Green Version]
- Komura, S.; Andelman, D. Physical aspects of heterogeneities in multi-component lipid membranes. Adv. Colloid Interface Sci. 2014, 208, 34–46. [Google Scholar] [CrossRef]
- Dimitrov, D.S.; Jain, R.K. Membrane stability, Biochim. Biophys. Acta 1984, 779, 437–468. [Google Scholar]
- Siontorou, C.G.; Georgopoulos, K.N. A biosensor platform for soil management: The case of nitrites. J. Clean. Prod. 2016, 111, 133–142. [Google Scholar] [CrossRef]
- Oshima, A.; Sumitomo, K. Vesicle fusion with bilayer lipid membrane controlled by electrostatic interaction. Biochem. Biophys. Rep. 2017, 11, 58–63. [Google Scholar] [CrossRef]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: A combined QCM-D and AFM study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef]
- Morigaki, K.; Tawa, K. Vesicle fusion studied by surface plasmon resonance and surface plasmon fluorescence spectroscopy. Biophys. J. 2006, 91, 1380–1387. [Google Scholar] [CrossRef][Green Version]
- Rebaud, S.; Maniti, O.; Girard-Egrot, A.P. Tethered bilayer lipid membranes (tBLMs): Interest and applications for biological membrane investigations. Biochimie 2014, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kongsuphol, O.; Fang, K.B.; Ding, Z. Lipid bilayer technologies in ion channel recordings and their potential in drug screening assay. Sens. Actuators B Chem. 2013, 185, 530–542. [Google Scholar] [CrossRef]
- Schuster, B.; Pum, D.; Sleytr, U.B. S-layer stabilized lipid membranes. Biointerphases 2008, 3, FA3–FA11. [Google Scholar] [CrossRef]
- Römer, W.; Steinem, C. Impedance analysis and single-channel recordings on nano-black lipid membranes based on porous alumina. Biophys. J. 2004, 86, 955–965. [Google Scholar]
- Siontorou, C.G.; Nikolelis, D.P.; Krull, U.J. Flow injection monitoring and analysis of mixtures of hydrazine compounds using filter-supported bilayer lipid membranes with incorporated DNA. Anal. Chem. 2000, 72, 180–186. [Google Scholar] [CrossRef]
- Andersson, M.; Keizer, H.M.; Zhu, C.; Fine, D.; Dodabalapur, A.; Duran, R.S. Detection of single ion channel activity on a chip using tethered bilayer membranes. Langmuir 2007, 23, 2924–2927. [Google Scholar] [CrossRef]
- Phung, T.; Zhang, Y.; Dunlop, J.; Dalziel, J. Bilayer lipid membranes supported on Teflon filters: A functional environment for ion channels. Biosens. Bioelectron. 2011, 26, 3127–3135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Jin, G. Chitosan cushioned phospholipid membrane and its application in imaging ellipsometry based-biosensor. Appl. Surf. Sci. 2011, 257, 9407–9413. [Google Scholar] [CrossRef][Green Version]
- Kibrom, A.; Roskamp, R.F.; Jonas, U.; Menges, B.; Knoll, W.; Paulsen, H.; Naumann, R.L.C. Hydrogel-supported protein-tethered bilayer lipid membranes: A new approach toward polymer-supported lipid membranes. Soft Matter 2011, 7, 237–246. [Google Scholar] [CrossRef]
- Shoaib, T.; Nalam, P.C.; He, Y.; Chen, Y.; Espinosa-Marzal, R.M. Assembly, morphology, diffusivity, and indentation of hydrogel-supported lipid bilayers. Langmuir 2017, 33, 7105–7117. [Google Scholar] [CrossRef] [PubMed]
- Khomutov, G.B.; Kim, V.P.; Potapenkov, K.V.; Parshintsev, A.A.; Soldatov, E.S.; Usmanov, N.N.; Saletsky, A.M.; Sybachin, A.V.; Yaroslavov, A.A.; Migulin, V.A.; et al. Langmuir monolayers and Langmuir-Blodgett films of pH-sensitive lipid. Colloids Surf. A 2017, 532, 150–154. [Google Scholar] [CrossRef]
- Chen, X.; Lenhert, S.; Hirtz, M.; Lu, N.; Fuchs, H.; Chi, L. Langmuir–Blodgett patterning: A bottom–up way to build mesostructures over large areas. Acc. Chem. Res. 2007, 40, 393–401. [Google Scholar] [CrossRef]
- Siontorou, C.G.; Georgopoulos, K.N.; Nikoleli, G.P.; Nikolelis, D.P.; Karapetis, S.K.; Bratakou, S. Protein-based graphene biosensors: Optimizing artificial chemoreception in bilayer lipid membranes. Membranes 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Wilk, S.J.; Petrossian, L.; Goryll, M.; Thornton, T.J.; Goodnick, S.M.; Tang, J.M.; Eisenberg, R.S. Integrated electrodes on a silicon based ion channel measurement platform. Biosens. Bioelectron. 2008, 23, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tabata, K.V.; Noji, H.; Takeuchi, S. Electrophysiological recordings of single ion channels in planar lipid bilayers using a polymethyl methacrylate microfluidic chip. Biosens. Bioelectron. 2007, 22, 1111–1115. [Google Scholar] [CrossRef]
- Reimhult, E.; Baumann, M.; Kaufmann, S.; Kumar, K.; Spycher, P. Advances in nanopatterned and nanostructured supported lipid membranes and their applications. Biotechnol. Genet. Eng. Rev. 2010, 27, 185–216. [Google Scholar] [CrossRef] [PubMed]
- Lenhert, S.; Sun, P.; Wang, Y.; Fuchs, H.; Mirkin, C. Massively parallel dip-pen nanolithography of heterogeneous supported phospholipid multilayer patterns. Small 2007, 3, 71–75. [Google Scholar] [CrossRef]
- Furukawa, K.; Aiba, T. Supported lipid bilayer composition microarray fabricated by pattern-guided self-spreading. Langmuir 2011, 27, 7341–7344. [Google Scholar] [CrossRef]
- Heath, G.R.; Li, M.; Rong, H.; Radu, V.; Frielingsdorf, S.; Lenz, O.; Butt, J.N.; Jeuken, L.J. Multilayered lipid membrane stacks for biocatalysis using membrane enzymes. Adv. Funct. Mater. 2017, 27, 1606265. [Google Scholar] [CrossRef]
- Penkauskas, T.; Preta, G. Biological applications of tethered bilayer lipid membranes. Biochimie 2019, 157, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Revzin, A.; Maverakis, E.; Chang, H.C. Biosensors for immune cell analysis-A perspective. Biomicrofluidics 2012, 6, 021301. [Google Scholar] [CrossRef]
- Diculescu, V.C.; Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Applications of a DNA-electrochemical biosensor. Trends Anal. Chem. 2016, 79, 23–36. [Google Scholar] [CrossRef]
- Osaki, T.; Takeuchi, S. Artificial cell membrane systems for biosensing applications. Anal. Chem. 2017, 89, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Osaki, T.; Takeuchi, S. Membrane protein-based biosensors. J. R. Soc. Interface 2018, 15, 20170952. [Google Scholar] [CrossRef]
- Nikoleli, G.P.; Nikolelis, D.P.; Siontorou, C.G.; Karapetis, S. Lipid membrane nanosensors for environmental monitoring: The art, the opportunities, and the challenges. Sensors 2018, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Andreou, V.G.; Nikolelis, D.P. Flow injection monitoring of aflatoxin M1 in milk and milk preparations using filter-supported bilayer lipid membranes. Anal. Chem. 1998, 70, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Nikolelis, D.P.; Siontorou, C.G.; Andreou, V.G.; Krull, U.J. Stabilized bilayer-lipid membranes for flow-through experiments. Electroanalysis 1995, 7, 531–536. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Raftopoulou, G.; Nikoleli, G.-P.; Simantiraki, M. Stabilized lipid membrane based biosensors with incorporated enzyme for repetitive uses. Electroanalysis 2006, 18, 2467–2474. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Raftopoulou, G.; Chatzigeorgiou, P.; Nikoleli, G.-P.; Viras, K. Optical portable biosensors based on stabilized lipid membrane for the rapid detection of doping materials in human urine. Sens. Actuators B Chem. 2008, 130, 577–582. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Israr, M.Q.; Tzamtzis, N.; Nikolelis, D.P.; Willander, M.; Psaroudakis, N. Structural characterization of graphene nanosheets for miniaturization of potentiometric urea lipid film based biosensors. Electroanalysis 2012, 24, 1285–1295. [Google Scholar] [CrossRef]
- Bratakou, S.; Nikoleli, G.-P.; Nikolelis, D.P.; Psaroudakis, N. Development of a potentiometric chemical sensor for the rapid detection of carbofuran based on air stable lipid films with incorporated calix[4]arene phosphoryl receptor using graphene electrodes. Electroanalysis 2015, 27, 2608–2613. [Google Scholar] [CrossRef]
- Bratakou, S.; Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Tzamtzis, N. Electrochemical biosensor for naphthalene acetic acid in fruits and vegetables based on lipid films with incorporated auxin-binding protein receptor using graphene electrodes. Electroanalysis 2016, 28, 2171–2177. [Google Scholar] [CrossRef]
- Karapetis, S.; Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Tzamtzis, N.; Psaroudakis, N. Development of an electrochemical biosensor for the rapid detection of cholera toxin based on air stable lipid films with incorporated ganglioside GM1 using graphene electrodes. Electroanalysis 2016, 28, 1584–1590. [Google Scholar] [CrossRef]
- Bratakou, S.; Nikoleli, G.-P.; Siontorou, G.C.; Nikolelis, D.P.; Karapetis, S.; Tzamtzis, N. Development of an electrochemical biosensor for the rapid detection of saxitoxin based on air stable lipid films with incorporated Anti-STX using graphene electrodes. Electroanalysis 2017, 29, 990–997. [Google Scholar] [CrossRef]
- Naval, A.P.; Katzenmeyer, A.M.; Gosho, Y.; Tekin, B.; Islam, M.S. Sonochemical approach for rapid growth of zinc oxide nanowalls. Appl. Phys. A 2012, 107, 661–667. [Google Scholar]
- Psychoyios, V.N.; Nikoleli, G.-P.; Tzamtzis, N.; Nikolelis, D.P.; Psaroudakis, N.; Danielsson, B.; Israr, M.Q.; Willander, M. Potentiometric cholesterol biosensor based on ZnO nanowalls and stabilized polymerized lipid film. Electroanalysis 2013, 25, 367–372. [Google Scholar] [CrossRef]
- Usman Ali, S.M.; Alvi, N.H.; Ibupoto, Z.; Nur, O.; Willander, M.; Danielsson, B. Selective potentiometric determination of uric acid with uricase immobilized on ZnO nanowires. Sens. Actuators B 2011, 152, 241–247. [Google Scholar] [CrossRef]
- Vaface, M.; Youzbashizade, H. Production of zinc oxide nanoparticles by liquid phase processing: An investigation on optical properties. Mater. Sci. Forum 2007, 553, 252–256. [Google Scholar] [CrossRef]
- Tzamtzis, N.; Psychoyios, V.N.; Nikoleli, G.-P.; Willander, M.; Qadir Israr, M. Flow Potentiometric Injection Analysis of Uric Acid Using Lipid Stabilized Films with Incorporated Uricase on ZnO Nanowires. Electroanalysis 2012, 24, 1719–1725. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Andreou, V.G. Electrochemical transduction of interactions of atrazine with bilayer lipid membranes. Electroanalysis 2005, 8, 643–647. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Raftopoulou, G.; Simantiraki, M.; Psaroudakis, N.; Nikoleli, G.-P.; Hianik, T. Preparation of a selective receptor for carbofuran for the development of a simple optical spot test for its rapid detection using stabilized in air lipid films with incorporated receptor. Anal. Chim. Acta 2008, 620, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Nikolelis, D.P.; Ntanos, N.; Nikoleli, G.-P.; Tampouris, K. Development of an electrochemical biosensor for the rapid detection of naphthalene acetic acid in fruits by using air stable lipid films with incorporated auxin-binding protein 1 receptor. Protein Pept. Lett. 2008, 15, 789–794. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Raftopoulou, G.; Psaroudakis, N.; Nikoleli, G.-P. Development of an electrochemical chemosensor for the rapid detection of zinc based on air stable lipid films with incorporated calix4arene phosphoryl receptor. Int. J. Environ. Anal. Chem. 2009, 89, 211–222. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Bratakou, S.; Karapetis, S.; Tzamtzis, N. Biosensors based on lipid modified graphene microelectrodes. Carbon 2017, 3, 9. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Nikolelis, D.P.; Tzamtzis, N. Development of an electrochemical biosensor for the rapid detection of cholera toxin using air stable lipid films with incorporated ganglioside GM1. Electroanalysis 2011, 23, 2182–2189. [Google Scholar] [CrossRef]
- Zang, J.; Li, C.M.; Cui, X.; Wang, J.; Sun, X.; Dong, H.; Sun, C.Q. Tailoring Zinc Oxide Nanowires for High Performance Amperometric Glucose Sensor. Electroanalysis 2007, 19, 1008–1014. [Google Scholar] [CrossRef]
- Ali, S.M.U.; Ibupoto, Z.H.; Salman, S.; Nur, O.; Willander, M.; Danielsson, B. Selective determination of urea using urease immobilized on ZnO nanowires. Sens. Actuators B 2011, 160, 637–643. [Google Scholar] [CrossRef]
- Hsu, C.L.; Lin, J.H.; Hsu, D.X.; Wang, S.H.; Lin, S.Y.; Hsueh, T.J. Enhanced non-enzymatic glucose biosensor of ZnO nanowires via decorated Pt nanoparticles and illuminated with UV/green light emitting diodes. Sens. Actuators B. 2017, 238, 150–159. [Google Scholar] [CrossRef]
- Miao, F.; Lu, X.; Tao, B.; Li, R.; Chu, P.K. Glucose oxidase immobilization platform based on ZnO nanowires supported by silicon nanowires for9glucose biosensing. Microelectron. Eng. 2016, 149, 153–158. [Google Scholar] [CrossRef]
- Fung, C.M.; Lloyd, J.S.; Samavat, S.; Deganello, D.; Teng, K.S. Facile fabrication of electrochemical ZnO nanowire glucose biosensor using roll to roll printing technique. Sens. Actuators B 2017, 247, 807–813. [Google Scholar] [CrossRef]
- Mansor, N.A.; Zain, Z.M.; Hamzah, H.H.; Noorden, M.S.A.; Jaapar, S.S.; Beni, V.; Ibupoto, Z.H. Detection of Breast Cancer 1 (BRCA1) gene using an electrochemical DNA biosensor based on immobilized ZnO nanowires. Open J. Appl. Biosens. 2014, 3, 9–17. [Google Scholar] [CrossRef]
- Cao, X.; Cao, X.; Guo, H.; Li, T.; Jie, Y.; Wang, N.; Wang, Z.L. Piezotronic effect enhanced label-free detection of DNA using a schottky-contacted ZnO nanowire biosensor. ACS Nano 2016, 10, 8038–8044. [Google Scholar] [CrossRef]
- Burns, J.R.; Seifert, A.; Fertig, N.; Howorka, S. A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane. Nat. Nanotechnol. 2016, 11, 152–156. [Google Scholar] [CrossRef]
- Siontorou, C.G.; Batzias, F.A. A methodological combined framework for roadmapping biosensor research: A fault tree analysis approach within a strategic technology evaluation frame. Crit. Rev. Biotechnol. 2013, 34, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Moulick, R.G.; Panaitov, G.; Choi, S.E.; Mayer, D.; Offenhausser, A. Patterning artificial lipid bilayer on nanostructured surfaces. Int. J. Nanomed. 2018, 13, 55–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.; Lee, C. Toward advanced neural interfaces for the peripheral nervous system (PNS) and their future applications. Curr. Opin. Biomed. Eng. 2018, 6, 130–137. [Google Scholar] [CrossRef]
- Bizzotto, D.; Burgess, I.J.; Doneux, T.; Sagara, T.; Yu, H.-Z. Beyond simple cartoons: Challenges in characterizing electrochemical biosensor interfaces. ACS Sens. 2018, 3, 5–12. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, M.-T.; Bratakou, S.; Bendos, D.K. Recent Lipid Membrane-Based Biosensing Platforms. Appl. Sci. 2019, 9, 1745. https://doi.org/10.3390/app9091745
Nikoleli G-P, Siontorou CG, Nikolelis M-T, Bratakou S, Bendos DK. Recent Lipid Membrane-Based Biosensing Platforms. Applied Sciences. 2019; 9(9):1745. https://doi.org/10.3390/app9091745
Chicago/Turabian StyleNikoleli, Georgia-Paraskevi, Christina G. Siontorou, Marianna-Thalia Nikolelis, Spyridoula Bratakou, and Dimitrios K. Bendos. 2019. "Recent Lipid Membrane-Based Biosensing Platforms" Applied Sciences 9, no. 9: 1745. https://doi.org/10.3390/app9091745
APA StyleNikoleli, G.-P., Siontorou, C. G., Nikolelis, M.-T., Bratakou, S., & Bendos, D. K. (2019). Recent Lipid Membrane-Based Biosensing Platforms. Applied Sciences, 9(9), 1745. https://doi.org/10.3390/app9091745