Hydrochemical Processes and Isotopic Study of Geothermal Springs within Soutpansberg, Limpopo Province, South Africa
Abstract
1. Introduction
2. Study Area
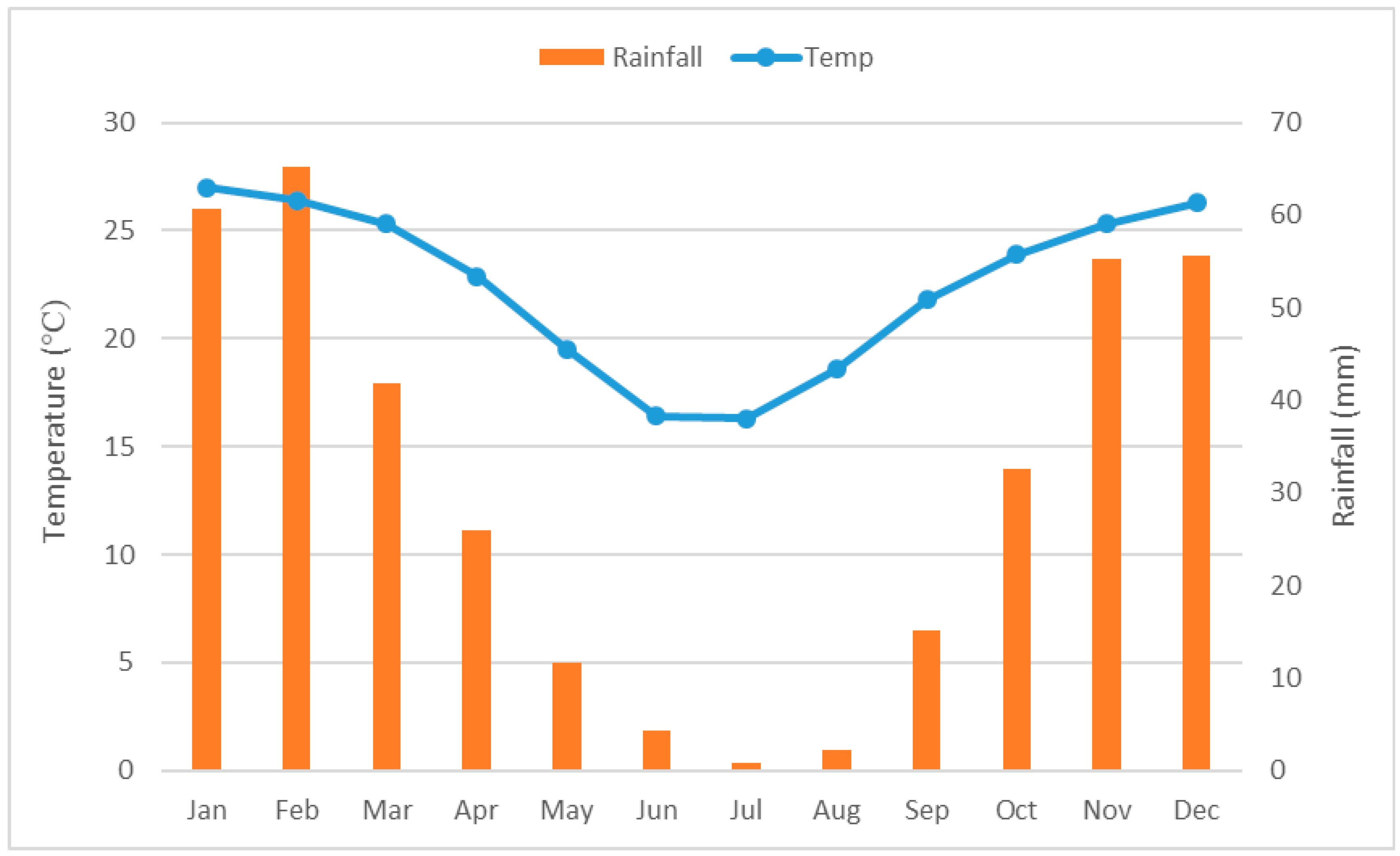
2.1. Climate of the Study Area
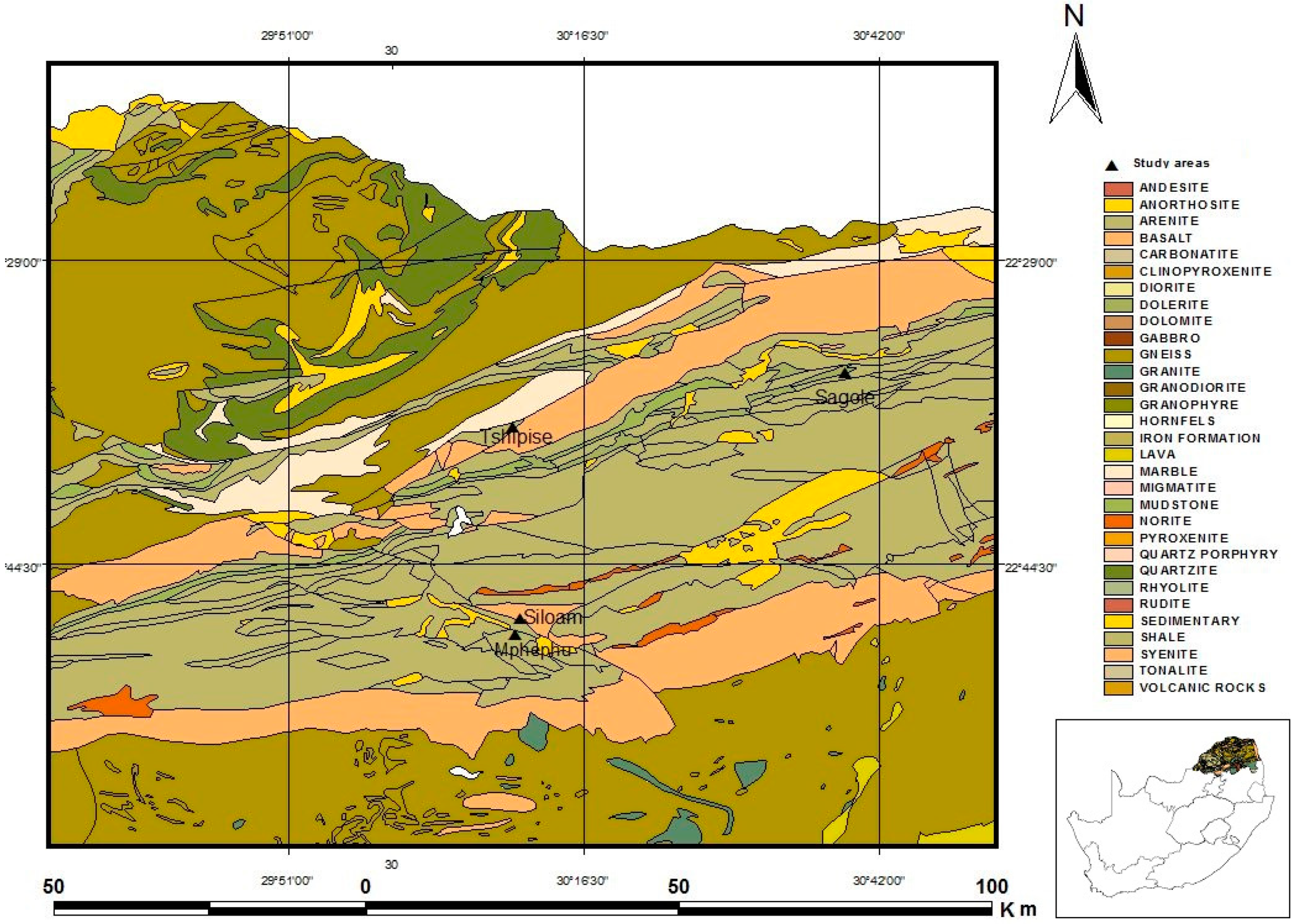
2.2. Geology of Study Area
3. Materials and Methods
3.1. Sampling and Sample Pretreatment
3.2. Analytical Methods
4. Results and Discussion
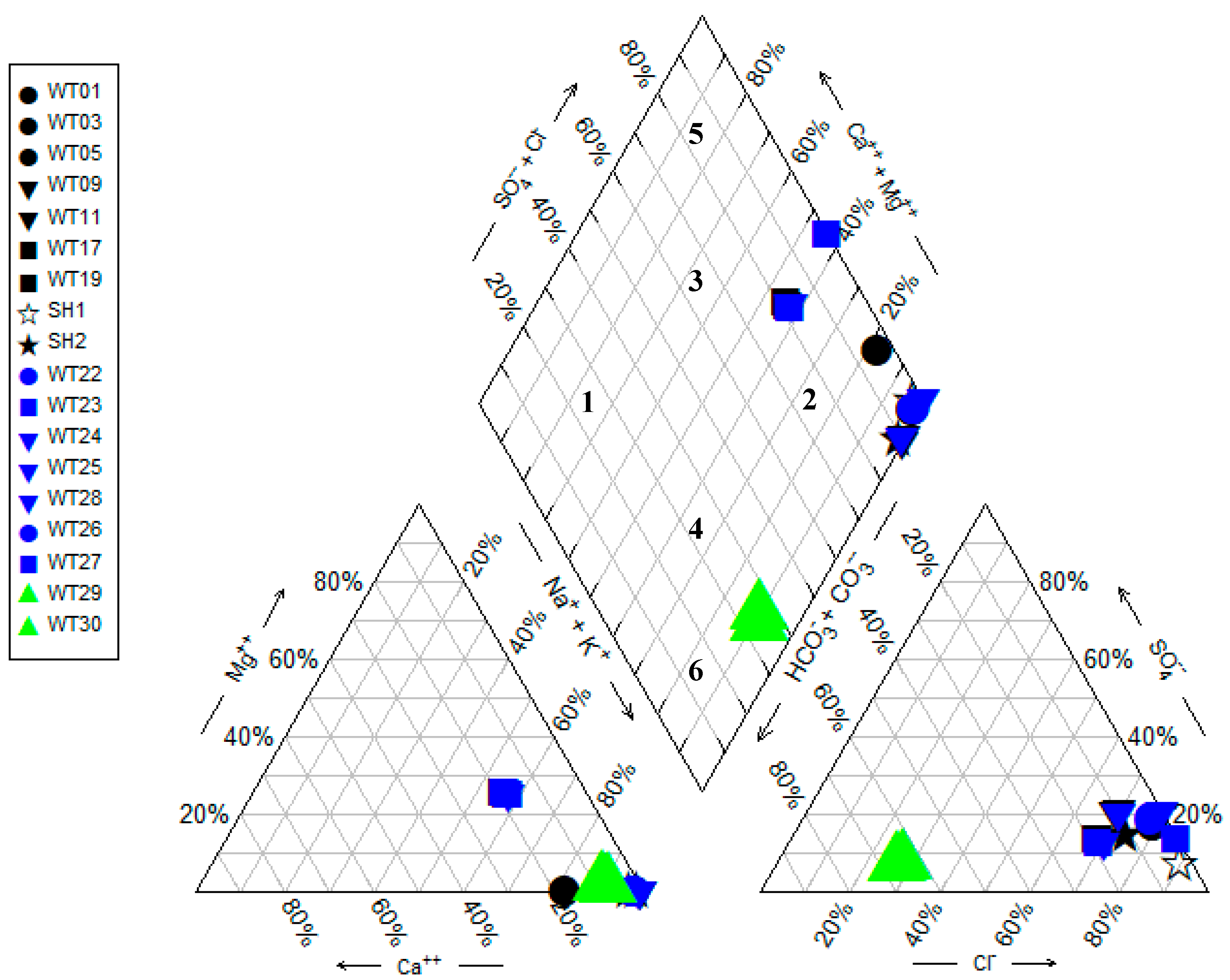
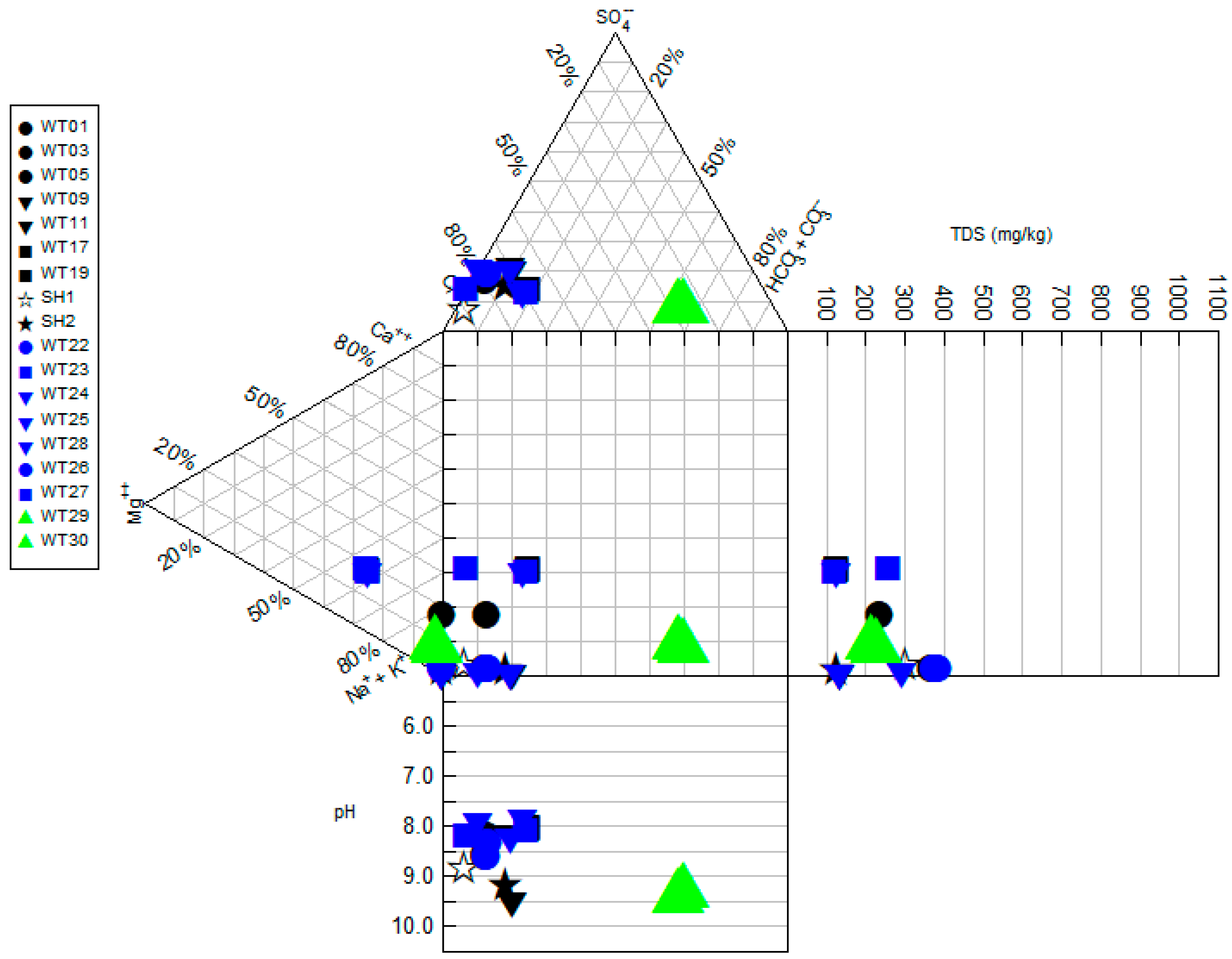
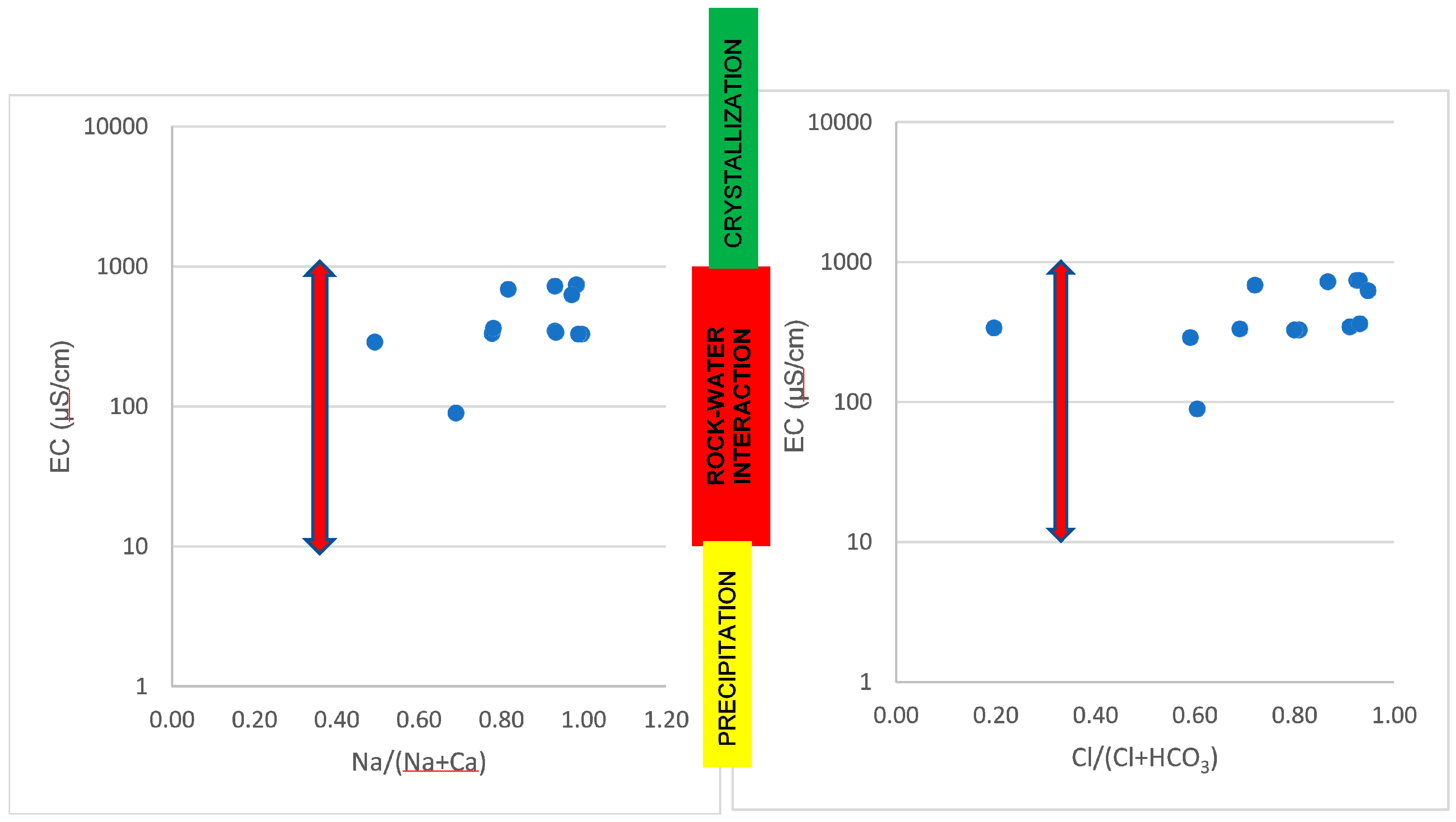
4.1. Physiochemical and Hydrochemical Parameters of the Geothermal Springs
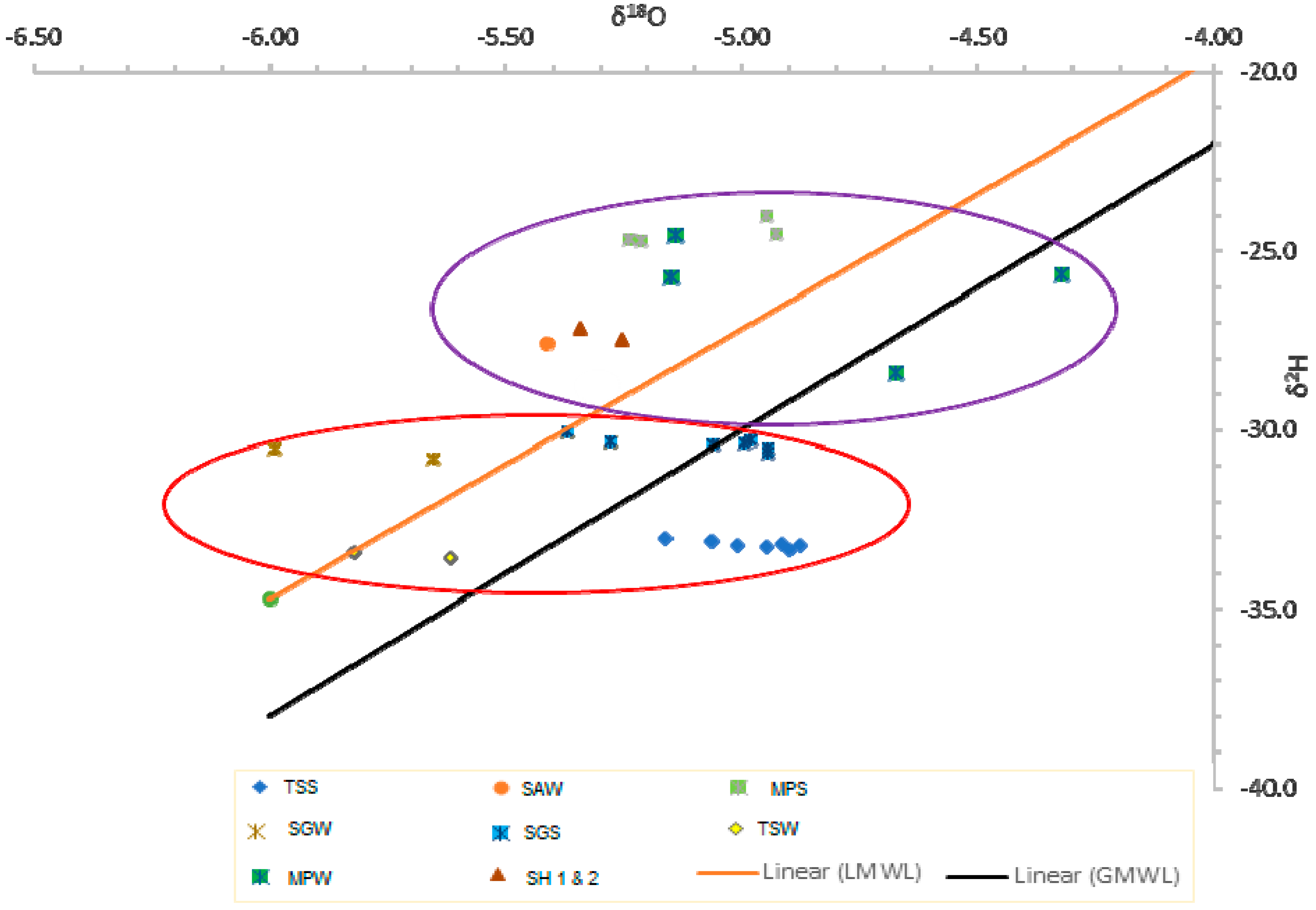
4.2. Isotopic Composition
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kent, L.E. Thermal waters of the Union of South Africa and South West Africa. S. Afr. J. Geol. 1949, 52, 231–264. [Google Scholar]
- Durowoju, O.S.; Odiyo, J.O.; Ekosse, G.E. Hydrogeochemical setting of geothermal springs in Limpopo Province—Review, South Africa. Res. J. Chem. Env. 2015, 19, 77–88. [Google Scholar] [CrossRef]
- Olivier, J.; Van Niekerk, H.J.; Van Der Walt, I.J. Physical and Chemical characteristics of thermal springs in the Waterberg area of Limpopo Province, South Africa. Water SA 2008, 34, 163–174. [Google Scholar]
- Van Vuuren, K. Die Warmwaterbronne van Suidwes-Kaapland: HulleVerbreiding, Eienskappe en Benutting. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 1990. [Google Scholar]
- Lund, J.W. Balneological Use of Thermal Water in the USA; GHC Bulletin: Klamath Falls, OR, USA, 2000; Volume 21, pp. 31–34. [Google Scholar]
- Gonfiantini, R. On the Isotopic Composition of Precipitation. Hydrologie et Geochimie Isotopique. In Proceedings of the International Symposium in Memory of J.-Ch. Fontes, Paris, France, 1–2 June 1995. [Google Scholar]
- Al-Ruwaih, F.M.; Mohamed, S. Hydrochemical Processes and Environmental Isotopic Study of Groundwater in Kuwait. Water Int. 2004, 29, 158–166. [Google Scholar] [CrossRef]
- Rajmohan, N.; Elango, L. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ. Geol. 2004, 46, 47–61. [Google Scholar] [CrossRef]
- Ako, A.A.; Shimada, J.; Ichiyanagi, K.; Koike, K.; Hosono, T.; Eyong, G.E.T.; Iskandar, I. Isotope hydrology and hydrochemistry of water resources in the banana plain (mungo-division) of the Cameroon Volcanic line. J. Environ. Hydrol. 2010, 18, 1–20. [Google Scholar]
- Rindl, M.R. The medicinal springs of South Africa (Supplement). S. Afr. J. Sci. 1916, 13, 528–552. [Google Scholar]
- Rindl, M.R. The medicinal springs of South Africa (Supplement). S. Afr. J. Sci. 1930, 27, 213–226. [Google Scholar]
- Kent, L.E. The warm springs of Loubad, near Nylstroom, Transvaal. Trans. R. Soc. S. Afr. 1948, 31, 151–168. [Google Scholar] [CrossRef]
- Kent, L.E.; Russell, H.D. The warm spring of Buffelshoek, near Thabazimbi, Transvaal. Trans. R. Soc. S. Afr. 1950, 32, 161–175. [Google Scholar] [CrossRef]
- Mazor, E.; Verhagen, B.T. Dissolved ions, stable and radioactive isotopes and noble gases in thermal waters of South Africa. J. Hydrol. 1983, 63, 315–329. [Google Scholar] [CrossRef]
- Olivier, J.; Venter, J.S.; Jonker, C.Z. Thermal and Chemical Characteristics of Thermal Springs in the Northern Part of the Limpopo Province, South Africa. Water SA 2011, 34, 163–174. [Google Scholar] [CrossRef]
- Durowoju, O.S.; Odiyo, J.O.; Ekosse, G.E. Variations of Heavy Metals from Geothermal Spring to Surrounding Soil and Mangifera Indica–Siloam Village, Limpopo Province. Sustainability 2016, 8, 60. [Google Scholar] [CrossRef]
- Durowoju, O.S.; Odiyo, J.O.; Ekosse, G.E. Horizontal variation in trace elements and soil characteristics at Siloam and Tshipise geothermal springs, Limpopo Province, South Africa. Water SA 2016, 42, 4. [Google Scholar] [CrossRef]
- Durowoju, O.S.; Odiyo, J.O.; Ekosse, G.E. Geochemistry of Siloam and Tshipise Geothermal Springs, Limpopo Province, South Africa. Am. J. Environ. Sci. 2018. [Google Scholar] [CrossRef]
- Makungo, R.; Odiyo, J.O.; Ndiritu, J.G.; Mwaka, B. Rainfall-runoff modelling approach for ungauged catchments: A case study of Nzhelele River sub-quaternary catchment. Phys. Chem. Earth 2010, 35, 596–607. [Google Scholar] [CrossRef]
- Kent, L.E. The thermal waters in the Republic of South Africa. In Proceedings of the Symposium II on Mineral and Thermal Waters of the World, B—Overseas Countries, Academia, Prague, Czech Republic, 1969; Volume 19, pp. 143–164. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, R. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Makungo, T.E. The Adequacy of Water Supply to Meet the Demand in Siloam Village of Limpopo Province of South Africa. Bachelor’s Thesis, University of Venda, Thohoyandou, South Africa, 2008. [Google Scholar]
- Department of Water Affairs and Forestry (DWAF). Luvuvhu/Letaba Water Management Area: Water Resource Situation Assessment; Report NO P0200/00/030/; WSM (pty) Ltd.: Pretoria, South Africa, 2001.
- Brandl, G.; Soutpansberg Group. Catalogue of South Africa Lithostratigraphic Units; Council for Geoscience: Pretoria, South Africa, 1999; pp. 6–41. [Google Scholar]
- Brandl, G.; Council for Geoscience. The Geology of the Alldays Area: Explanation: Sheet 2228; Council for Geoscience: Pretoria, South Africa, 2002; p. 71. [Google Scholar]
- Johnson, M.R.; Anhaeusser, C.R.; Thomas, R.J. The geology of South Africa; Geological Society of South Africa: Johannesburg, South Africa; Council for Geosciences: Pretoria, South Africa, 2006; pp. 301–318. [Google Scholar]
- Brandl, G.; Geological Survey. The Geology of the Pietersburg Area: Explanation of Sheet 2328; Government Printer: Pietersburg, South Africa, 1986; p. 43.
- Mundalamo, H.R. Investigation of Water Quality in Nzhelele Valley, Limpopo Province, South Africa. Bachelor’s Thesis, University of Venda, Thohoyandou, South Africa, 2003. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Guideline for Water Reuse; EPA/625/R-04/108; EPA: Washington, DC, USA, 2004.
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington DC, USA, 1998. [Google Scholar]
- United State Environmental Protection Agency (USEPA). Method 200.8. Determination of Trace Elements in Waters and Wastes by ICP, Revision 5.4. 1994. Available online: http://www.epa.gov/sam/pdfs/EPA-200.8.pdf (accessed on 11 November 2017).
- United State Environmental Protection Agency (USEPA). Determination of Inorganic Anion in Water by Ion Chromatography; Method 300.0. Rev 2.1; US Environmental Agency: Cincinnati, OH, USA, 1993.
- Dolnikowski, G.G. A New Sample Preparation Method for Isotope Ratio Mass Spectrometry of 2H-Enriched Samples Generated by the Doubly Labeled Water Method. Obesity 1955. [Google Scholar] [CrossRef]
- Durowoju, O.S.; Odiyo, J.O.; Ekosse, G.E. Determination of isotopic composition of rainwater to generate local meteoric water line in Thohoyandou, Limpopo Province, South Africa. Water SA 2019, accepted. [Google Scholar]
- Todd, D.K. Groundwater Hydrology, 2nd ed.; John Wiley: New York, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, ON, Canada, 1980; ISBN 0 471 87616X. [Google Scholar]
- Department of Water Affairs and Forestry (DWAF). South African Water Quality Guidelines, Volume 1: Domestic Water Use, 2nd ed.; DWAF: Pretoria, South Africa, 1996.
- Piper, A.M. A graphic procedure in geochemical interpretation of water analyses. Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar] [CrossRef]
- Durov, S.A. Classification of natural waters and graphical representation of their composition. Dokl. Akad. Nauk. USSR 1948, 59, 87–90. [Google Scholar]
- Yahaya, M.I.; Mohammad, S.; Abdullahi, B.K. Seasonal variations of heavy metals concentrations in Abattoir dumping site soil in Nigeria. J. Appl. Sci. Environ. Manag. 2009, 13, 9–13. [Google Scholar] [CrossRef]
- Lipfert, G.; Reeve, A.S.; Sidle, W.; Marvinney, R. Geochemical patterns in an arsenic-tainted, fracture—Bedrock groundwater system in Northport, Maine USA. Appl. Geochem. 2006, 26, 747–762. [Google Scholar]
- Bond, G.W. A geochemical survey of the underground water supplies of the Union of South Africa. Memoirs Geol. Surv. S. Afr. 1946, 41, 208. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Aghazadeh, N.; Mogaddam, A.A. Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh area, Northwest of Iran. J. Environ. Prot. 2010, 1, 30–40. [Google Scholar] [CrossRef]
- Schoeller, H. Geochemistry of groundwater. In Groundwater Studies—An international Guide for Research and Practice; UNESCO: Paris, France, 1977; pp. 1–18. [Google Scholar]
- Glover, E.T.; Akiti, T.T.; Osae, S. Major ion chemistry and identification of hydrogeochemical processes of groundwater in Accra Plains. Geoscience 2012, 50, 10279–10288. [Google Scholar]
- Gurdak, J.J.; Hanson, R.T.; McMahon, P.B.; Bruce, B.W.; McCray, J.E.; Thyne, G.D.; Reedy, R.C. Climate variability controls on unsaturated water and chemical movement, High Plans Aquifer, USA. Vadose Zone J. 2007, 6, 533–547. [Google Scholar] [CrossRef]
- Kebede, S. Groundwater in Ethiopia; Springer: Berlin/Heidelberg, Germany, 2013; pp. 187–203. [Google Scholar]
- Gat, J.R.; Gonfiantini, R. Stable Isotope Hydrology: Dueterium and Oxygen-18 in the Water Cycle; International Atomic Energy Agency: Vienna, Austria, 1981. [Google Scholar]
- Kebede, S.; Travi, Y. Origin of the δ18O and δ2H composition of meteoric waters in Ethiopia. Quart. Int. 2012, 257, 4–12. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Coplen, T.B.; Herczeg, A.L.; Barnes, C. Isotope engineering-using stable isotopes of water molecule to solve practical problems. In Environmental Tracers in Catchment Hydrology; Cook, P., Herczeg, A.L., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 2000; pp. 79–110. [Google Scholar]
- Gat, J.R. Isotope Hydrology: A Study of the Water Cycle; Series on Environmental Science and Management; Imperial College Press: London, UK, 2010. [Google Scholar]
- Edmunds, W.M.; Fellman, E.; Goni, I.B.; Prudhomme, C. Spatial temporal distribution of groundwater recharge in northern Nigeria. Hydrogeol. J. 2002, 10, 205–215. [Google Scholar] [CrossRef]
- Fantong, W.Y.; Satake, H.; Ayonghe, S.N.; Aka, F.T.; Kasuyoshi, A. Hydrogeochemical controls and usability of groundwater in the semi-arid Mayo Tsanaga River Basin: Far north province, Cameroon. J. Environ. Earth Sci. 2008. [Google Scholar] [CrossRef]
- Odiyo, J.O.; Makungo, R. Fluoride concentrations in groundwater and impact on human health in Siloam Village, Limpopo Province, South Africa. Water SA 2012, 38, 731–736. [Google Scholar] [CrossRef]
- Mahamat, H.B.; Coz, M.L.; Abderamane, H.; Sardini, P.; Razack, M. Hydrochemical and Isotopic Characteristics of the Basement Aquifer in the Wadi Fira Area, Eastern Chad. J. Water Resour. Prot. 2017, 9, 1688–1708. [Google Scholar] [CrossRef][Green Version]



| Sampling Site | Sites Coordinates | Altitude (m) | Surface Geology and Geological Structures |
|---|---|---|---|
| Mphephu | 30°10′35.582″ E 22°54′26.280″ S | 890 | Quartzite and Sandstone Reverse fault between Waterberg Group quartzite and Dominion Reef lava |
| Sagole | 30°39′07.128″ E 22°31′49.440″ S | 450 | Mudstone, shale, subordinate micaceous sandstone, carbonaceous shale, siltstone, micaceous sandstone. Mikambeni Formation and Madzaringwe Formation, Karoo Supergroup |
| Siloam | 30°10′59.988″ E 22°52′58.800″ S | 835 | Basalt, minor tuff Sibasa Formation, Soutpansberg Group |
| Tshipise | 30°10′20.712″ E 22°36′31.320″ S | 520 | Basalt, minor andesite, cream-coloured sandstone, dolerite sills, and dykes Intersection of 2 post-Permian faults in upper Karoo |
| SAGOLE | TSHIPISE | MPHEPHU | SILOAM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SGW | SGS | TSW | TSS | MPW | MPS | SAW | SH1 | SH2 | |
| Temp (°C) | 42.4 ± 1.45 | 44.8 ± 2.12 | 54.6 ± 2.26 | 55.4 ± 2.21 | 41.3 ± 1.23 | 42.7 ± 1.01 | 67.7 ± 1.68 | 45.2 ± 0.00 | 48.4 ± 0.01 |
| pH | 8.82 ± 0.95 | 7.98 ± 0.22 | 8.46 ± 0.22 | 8.47 ± 0.21 | 8.05 ± 0.02 | 8.15 ± 0.07 | 9.39 ± 0.06 | 8.86 ± 0.00 | 9.19 ± 0.10 |
| SAR | 33.88 ± 546 | 19.20 ± 15.48 | 25.75 ± 0.98 | 25.45 ± 1.29 | 2.07 ± 0.06 | 2.18 ± 0.06 | 7.39 ± 0.04 | 17.25 ± 0.00 | 19.04 ± 0.02 |
| EC (μS/cm) | 330 ± 0.00 | 347.33 ± 16.17 | 746.67 ± 5.77 | 745 ± 7.07 | 335 ± 7.07 | 365 ± 21.21 | 340 ± 2.07 | 630 ± 0.00 | 330 ± 0.00 |
| TDS (mg/L) | 133.13 ± 1.85 | 196.70 ± 122.43 | 377.48 ± 5.36 | 390.61 ± 7.63 | 124.38 ± 1.41 | 120.84 ± 1.19 | 215.18 ± 9.25 | 305 ± 0.00 | 130.12 ± 0.00 |
| Alkalinity (mg/L) | 10.50 ± 4.24 | 6.50 ± 5.89 | 11.12 ± 0.54 | 10.75 ± 0.35 | 12.50 ± 0.00 | 6.00 ± 8.49 | 107.52 ± 1.36 | 10 ± 0.00 | 12 ± 0.00 |
| Na (mg/L) | 64.20 ± 1.84 | 57.13 ± 11.98 | 157.67 ± 4.51 | 154.50 ± 4.95 | 42.50 ± 1.27 | 42.35 ± 1.06 | 78.77 ± 7.54 | 118 ± 0.00 | 62.7 ± 0.00 |
| K (mg/L) | 1.98 ± 0.01 | 2.04 ± 0.05 | 4.55 ± 0.06 | 4.84 ± 0.05 | 2.06 ± 0.04 | 2.11 ± 0.01 | 2.61 ± 0.06 | 2.73 ± 0.00 | 2.21 ± 0.01 |
| Ca (mg/L) | 0.29 ± 0.11 | 4.27 ± 6.61 | 2.84 ± 0.07 | 2.79 ± 0.10 | 12.20 ± 0.00 | 11.90 ± 0.28 | 5.69 ± 0.05 | 3.53 ± 0.00 | 0.81 ± 0.00 |
| Mg (mg/L) | 0.00 ± 0.00 | 3.47 ± 6.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 10.50 ± 0.00 | 10.35 ± 0.07 | 1.04 ± 0.08 | 0 ± 0.00 | 0 ± 0.00 |
| F (mg/L) | 0.77 ± 0.15 | 2.60 ± 1.71 | 5.01 ± 0.63 | 5.98 ± 0.08 | 2.69 ± 0.01 | 4.16 ± 2.48 | 6.51 ± 0.08 | 4.55 ± 0.00 | 4.95 ± 0.00 |
| NO3 (mg/L) | 0.99 ± 0.36 | 1.71 ± 0.85 | 2.13 ± 1.80 | 5.85 ± 1.48 | 3.02 ± 0.40 | 6.25 ± 3.23 | 0.60 ± 0.03 | 0.17 ± 0.00 | 1.31 ± 0.00 |
| Cl (mg/L) | 41.34 ± 0.30 | 81.15 ± 75.10 | 151.86 ± 0.28 | 156.67 ± 0.02 | 33.90 ± 0.06 | 98.82 ± 86.34 | 24.11 ± 0.77 | 153.3 ± 0.00 | 38.9 ± 0.00 |
| SO4 (mg/L) | 16.95 ± 0.54 | 27.89 ± 27.23 | 45.81 ± 2.15 | 51.78 ± 0.42 | 9.21 ± 0.03 | 21.14 ± 18.31 | 8.99 ± 0.06 | 16.45 ± 0.00 | 10.55 ± 0.00 |
| PO4 (mg/L) | 0.92 ± 0.82 | 1.30 ± 2.14 | 1.38 ± 1.22 | 2.14 ± 1.46 | 1.28 ± 0.74 | 2.26 ± 3.09 | 0.42 ± 0.06 | 1.15 ± 0.00 | 1.52 ± 0.00 |
| CO3 (mg/L) | 1.50 ± 2.12 | 0.00 ± 0.00 | 0.58 ± 0.50 | 0.60 ± 0.42 | 0.00 ± 0.00 | 0.00 ± 0.00 | 16.13 ± 0.41 | 1.8 ± 0.00 | 2.4 ± 0.00 |
| HCO3 (mg/L) | 9.76 ± 0.86 | 7.93 ± 7.19 | 12.38 ± 0.37 | 11.90 ± 0.43 | 15.25 ± 0.00 | 7.32 ± 10.35 | 98.75 ± 2.08 | 8.54 ± 0.00 | 9.76 ± 0.00 |
| δ18O (‰) | −5.82 | −5.08 | −5.73 | −4.98 | −4.92 | −4.82 | −5.41 | −5.34 | −5.25 |
| δ2H (‰) | −30.7 | −30.4 | −33.5 | −33.2 | 26.1 | −24.6 | −27.2 | −27.14 | −27.46 |
| CAI-1 | −0.6 | 0.27 | −0.07 | −0.02 | −0.31 | 0.55 | −2.38 | 0.21 | −0.67 |
| CAI-2 | −0.85 | 0.59 | −0.17 | −0.04 | −0.39 | 1.57 | −0.46 | 1.21 | −1.08 |
| Temp | pH | SAR | EC | TDS | Alkalinity | Na | K | Ca | Mg | F | NO3 | Cl | SO4 | PO4 | CO3 | HCO3 | δ18O | δ2H | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | 1.00 | ||||||||||||||||||
| pH | 0.58 | 1.00 | |||||||||||||||||
| SAR | 0.01 | 0.19 | 1.00 | ||||||||||||||||
| EC | 0.27 | −0.07 | 0.41 | 1.00 | |||||||||||||||
| TDS | 0.48 | 0.03 | 0.42 | 0.95 | 1.00 | ||||||||||||||
| Alkalinity | 0.81 | 0.62 | −0.31 | −0.23 | −0.01 | 1.00 | |||||||||||||
| Na | 0.47 | 0.15 | 0.52 | 0.96 | 0.97 | −0.04 | 1.00 | ||||||||||||
| K | 0.50 | 0.00 | 0.40 | 0.92 | 0.92 | −0.04 | 0.94 | 1.00 | |||||||||||
| Ca | −0.23 | −0.54 | −0.89 | −0.29 | −0.37 | 0.05 | −0.47 | −0.30 | 1.00 | ||||||||||
| Mg | −0.47 | −0.65 | −0.79 | −0.44 | −0.55 | −0.17 | −0.63 | −0.44 | 0.95 | 1.00 | |||||||||
| F | 0.79 | 0.42 | −0.23 | 0.46 | 0.54 | 0.50 | 0.52 | 0.58 | −0.03 | −0.27 | 1.00 | ||||||||
| NO3 | −0.14 | −0.57 | −0.26 | 0.21 | 0.08 | −0.33 | 0.05 | 0.32 | 0.49 | 0.52 | 0.16 | 1.00 | |||||||
| Cl | 0.02 | −0.28 | 0.31 | 0.91 | 0.83 | −0.43 | 0.79 | 0.74 | −0.16 | −0.26 | 0.33 | 0.33 | 1.00 | ||||||
| SO4 | 0.20 | −0.36 | 0.50 | 0.81 | 0.80 | −0.34 | 0.78 | 0.87 | −0.27 | −0.30 | 0.26 | 0.49 | 0.78 | 1.00 | |||||
| PO4 | −0.35 | −0.54 | −0.07 | 0.32 | 0.13 | −0.65 | 0.13 | 0.31 | 0.25 | 0.34 | 0.12 | 0.87 | 0.51 | 0.52 | 1.00 | ||||
| CO3 | 0.80 | 0.72 | −0.25 | −0.24 | −0.02 | 0.99 | −0.04 | −0.07 | −0.05 | −0.26 | 0.50 | −0.41 | −0.43 | −0.38 | −0.67 | 1.00 | |||
| HCO3 | 0.81 | 0.58 | −0.33 | −0.23 | −0.01 | 1.00 | −0.04 | −0.03 | 0.08 | −0.14 | 0.49 | −0.31 | −0.43 | −0.33 | −0.64 | 0.98 | 1.00 | ||
| δ18O | −0.24 | −0.51 | −0.68 | −0.16 | −0.21 | −0.18 | −0.34 | −0.15 | 0.68 | 0.69 | 0.17 | 0.66 | 0.03 | −0.03 | 0.61 | −0.24 | −0.16 | 1.00 | |
| δ2H | −0.36 | −0.37 | −0.61 | −0.35 | −0.43 | −0.06 | −0.46 | −0.35 | 0.69 | 0.71 | −0.28 | 0.09 | −0.41 | −0.45 | −0.07 | −0.14 | −0.03 | 0.42 | 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durowoju, O.S.; Butler, M.; Ekosse, G.-I.E.; Odiyo, J.O. Hydrochemical Processes and Isotopic Study of Geothermal Springs within Soutpansberg, Limpopo Province, South Africa. Appl. Sci. 2019, 9, 1688. https://doi.org/10.3390/app9081688
Durowoju OS, Butler M, Ekosse G-IE, Odiyo JO. Hydrochemical Processes and Isotopic Study of Geothermal Springs within Soutpansberg, Limpopo Province, South Africa. Applied Sciences. 2019; 9(8):1688. https://doi.org/10.3390/app9081688
Chicago/Turabian StyleDurowoju, Olatunde Samod, Mike Butler, Georges-Ivo Ekosse Ekosse, and John Ogony Odiyo. 2019. "Hydrochemical Processes and Isotopic Study of Geothermal Springs within Soutpansberg, Limpopo Province, South Africa" Applied Sciences 9, no. 8: 1688. https://doi.org/10.3390/app9081688
APA StyleDurowoju, O. S., Butler, M., Ekosse, G.-I. E., & Odiyo, J. O. (2019). Hydrochemical Processes and Isotopic Study of Geothermal Springs within Soutpansberg, Limpopo Province, South Africa. Applied Sciences, 9(8), 1688. https://doi.org/10.3390/app9081688






