The Telocytes in the Subepicardial Niche
Abstract
1. Introduction
2. Materials and Methods
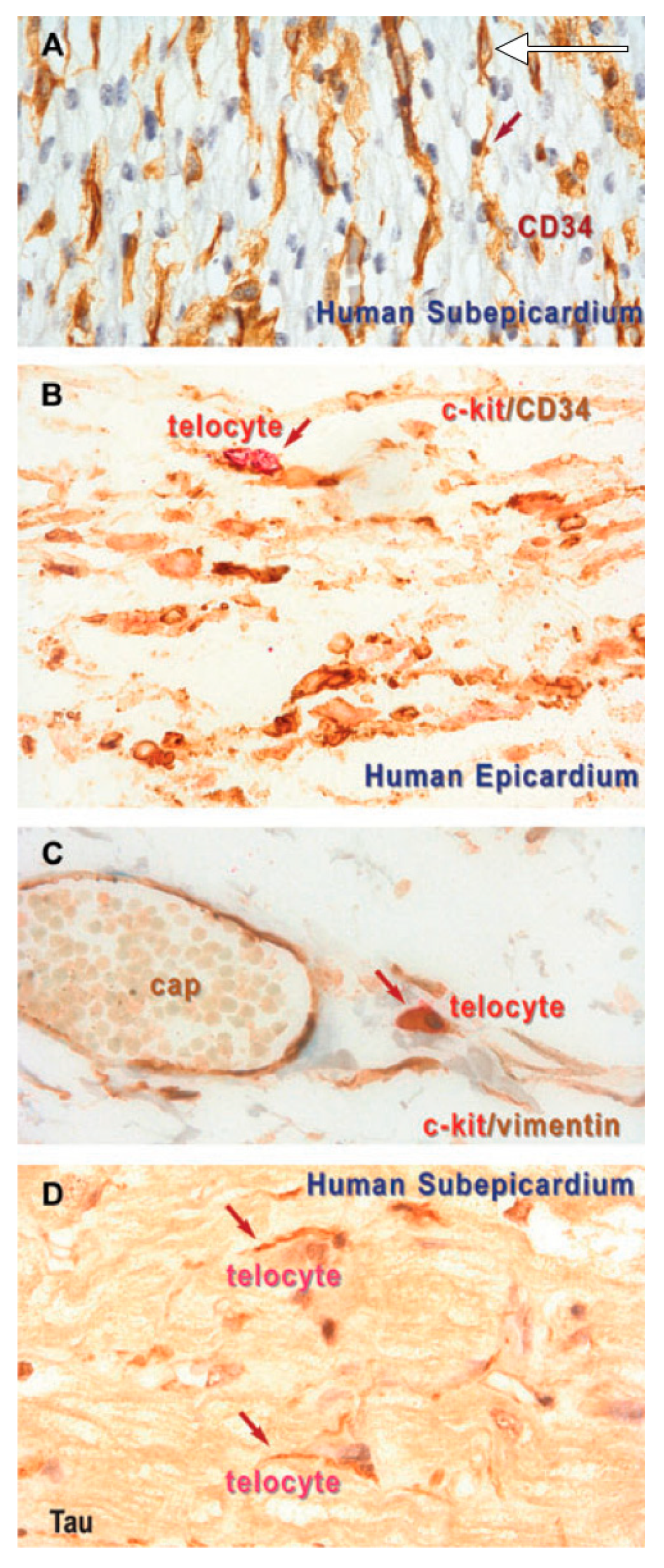
3. Results
4. Discussion
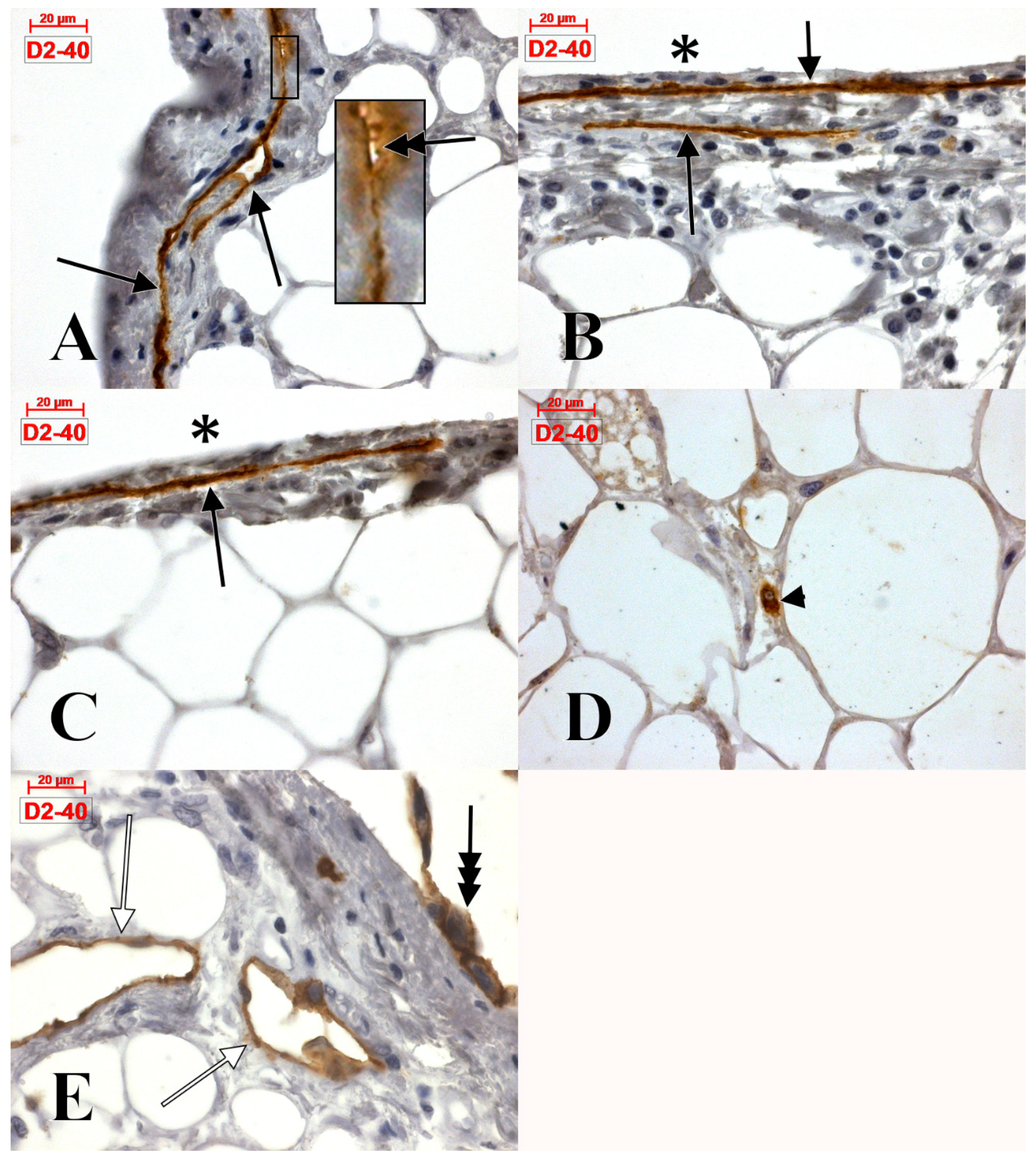
4.1. The Epicardial Lymphatics
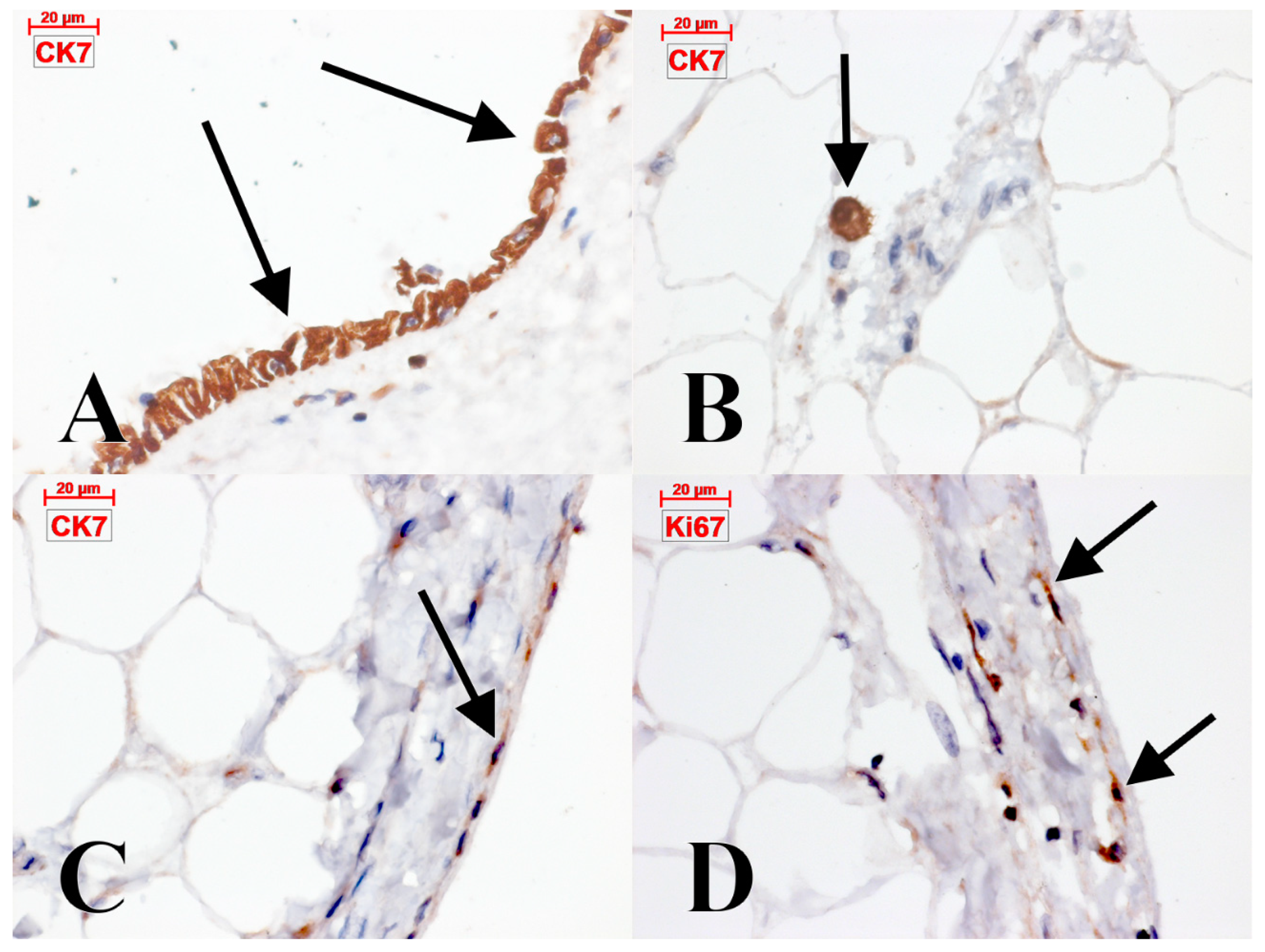
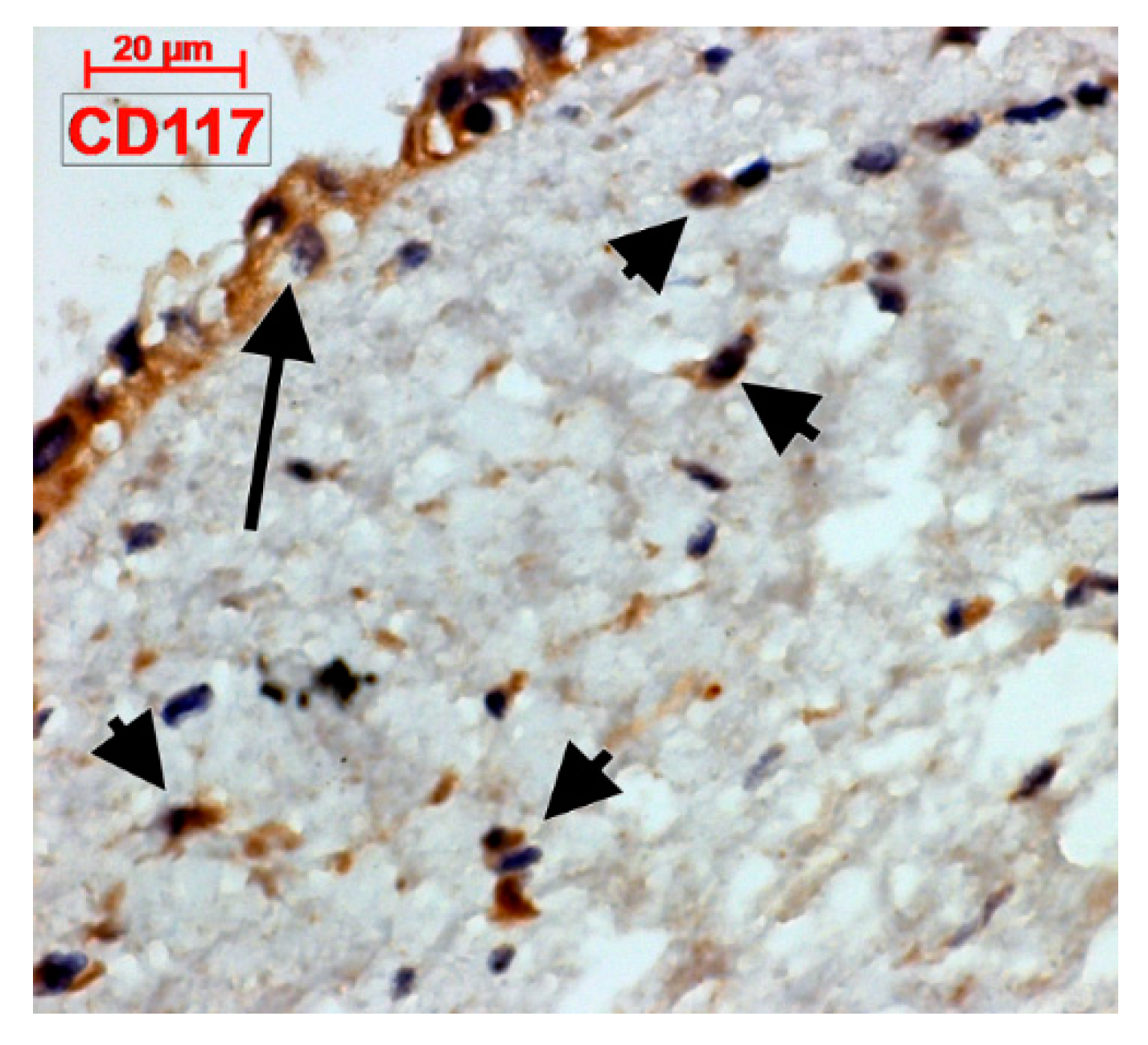
4.2. C-Kit-Positive Cardiac Cells
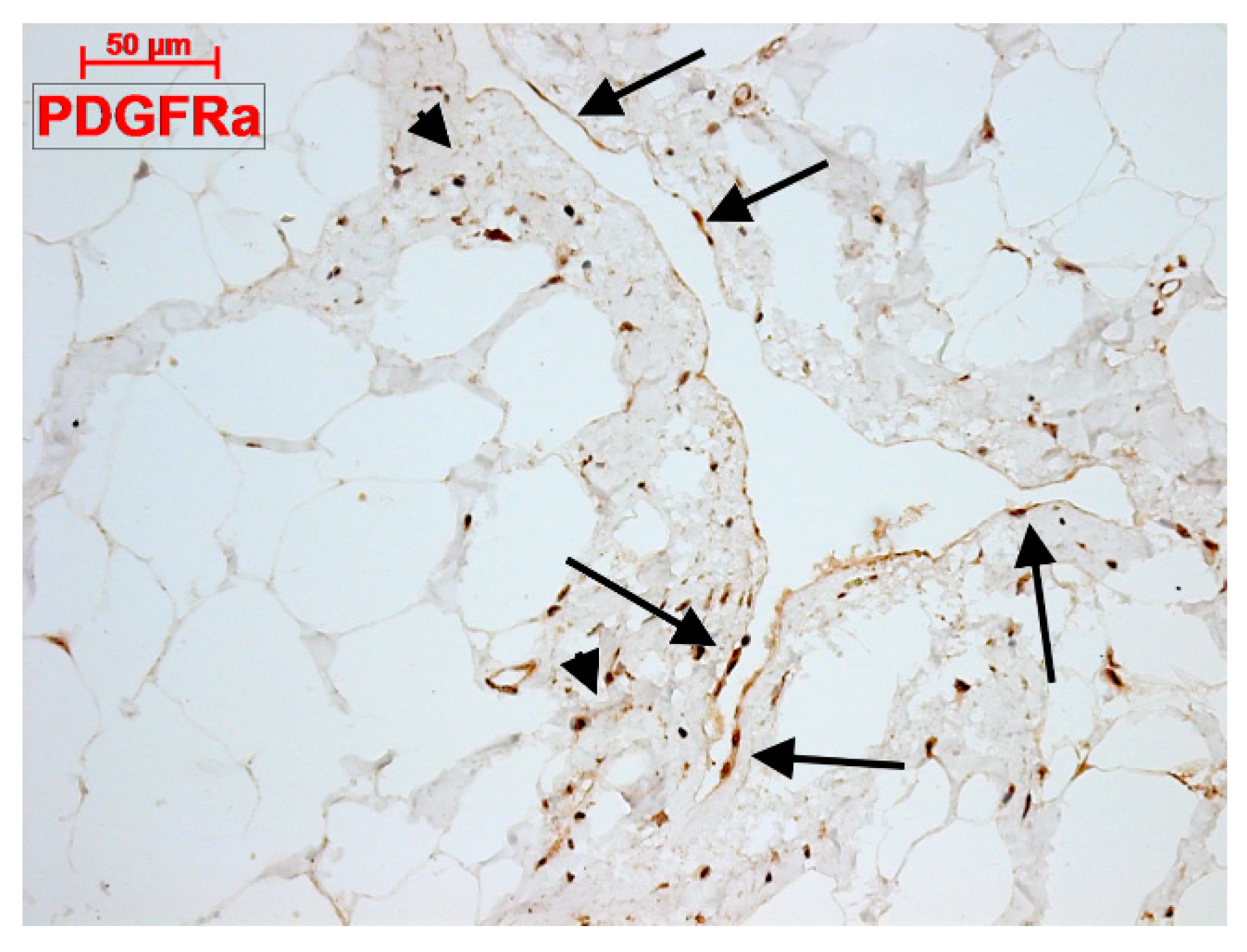
4.3. What Are (Sub)epicardial TCs?
4.4. The Epicardial and Subepicardial Expression of Podoplanin
4.5. Processes of Epicardial Transdifferentiation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Popescu, L.M.; Faussone-Pellegrini, M.S. TELOCYTES—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell. Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Ciontea, S.M.; Radu, E.; Regalia, T.; Ceafalan, L.; Cretoiu, D.; Gherghiceanu, M.; Braga, R.I.; Malincenco, M.; Zagrean, L.; Hinescu, M.E.; et al. C-kit immunopositive interstitial cells (Cajal-type) in human myometrium. J. Cell. Mol. Med. 2005, 9, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Ciontea, S.M.; Popescu, L.M.; Ceafalan, L.; Ardeleanu, C. Interstitial Cajal-like cells (ICLC) as steroid hormone sensors in human myometrium: Immunocytochemical approach. J. Cell. Mol. Med. 2006, 10, 789–795. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Hinescu, M.E.; Andrei, F.; Mandache, E.; Macarie, C.E.; Faussone-Pellegrini, M.S.; Popescu, L.M. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J. Cell. Mol. Med. 2008, 12, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Gherghiceanu, M.; Hinescu, M.E.; Popescu, L.M. Myocardial interstitial Cajal-like cells (ICLC) in caveolin-1 KO mice. J. Cell. Mol. Med. 2009, 13, 202–206. [Google Scholar] [CrossRef]
- Hinescu, M.E.; Gherghiceanu, M.; Mandache, E.; Ciontea, S.M.; Popescu, L.M. Interstitial Cajal-like cells (ICLC) in atrial myocardium: Ultrastructural and immunohistochemical characterization. J. Cell. Mol. Med. 2006, 10, 243–257. [Google Scholar] [CrossRef]
- Hinescu, M.E.; Popescu, L.M. Interstitial Cajal-like cells (ICLC) in human atrial myocardium. J. Cell. Mol. Med. 2005, 9, 972–975. [Google Scholar] [CrossRef]
- Kostin, S.; Popescu, L.M. A distinct type of cell in myocardium: Interstitial Cajal-like cells (ICLCs). J. Cell. Mol. Med. 2009, 13, 295–308. [Google Scholar] [CrossRef]
- Pieri, L.; Vannucchi, M.G.; Faussone-Pellegrini, M.S. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J. Cell. Mol. Med. 2008, 12, 1944–1955. [Google Scholar] [CrossRef]
- Suciu, L.; Popescu, L.M.; Regalia, T.; Ardelean, A.; Manole, C.G. Epicardium: Interstitial Cajal-like cells (ICLC) highlighted by immunofluorescence. J. Cell. Mol. Med. 2009, 13, 771–777. [Google Scholar] [CrossRef]
- Iancu, C.B.; Rusu, M.C.; Mogoanta, L.; Hostiuc, S.; Grigoriu, M. Myocardial Telocyte-Like Cells: A Review Including New Evidence. Cells Tissues Organs 2019. [Google Scholar] [CrossRef]
- Rusu, M.C.; Folescu, R.; Manoiu, V.S.; Didilescu, A.C. Suburothelial interstitial cells. Cells Tissues Organs 2014, 199, 59–72. [Google Scholar] [CrossRef]
- Rusu, M.C.; Hostiuc, S.; Vrapciu, A.D.; Mogoanta, L.; Manoiu, V.S.; Grigoriu, F. Subsets of telocytes: Myocardial telocytes. Ann. Anat. 2017, 209, 37–44. [Google Scholar] [CrossRef]
- Grigoriu, F.; Hostiuc, S.; Vrapciu, A.D.; Rusu, M.C. Subsets of telocytes: The progenitor cells in the human endocardial niche. Rom. J. Morphol. Embryol. 2016, 57, 767–774. [Google Scholar]
- Varga, I.; Polak, S.; Kyselovic, J.; Kachlik, D.; Danisovic, L.; Klein, M. Recently Discovered Interstitial Cell Population of Telocytes: Distinguishing Facts from Fiction Regarding Their Role in the Pathogenesis of Diverse Diseases Called “Telocytopathies”. Medicina 2019, 55, 56. [Google Scholar] [CrossRef]
- Dobra, M.A.; Vrapciu, A.D.; Pop, F.; Petre, N.; Rusu, M.C. The molecular phenotypes of ureteral telocytes are layer-specific. Acta Histochem. 2017. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Traini, C. The telocytes/myofibroblasts 3-D network forms a stretch receptor in the human bladder mucosa. Is this structure involved in the detrusor overactive diseases? Ann. Anat. 2018, 218, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, L.B.; Gorshkov, A.N.; Konovalov, P.V.; Krylova, J.S. Telocytes in the human sinoatrial node. J. Cell. Mol. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Hostiuc, S. Telocytes and telocytes-like cells. Past, present, and above all future. Ann. Anat. 2019. [Google Scholar] [CrossRef]
- Petrea, C.E.; Craitoiu, S.; Vrapciu, A.D.; Manoiu, V.S.; Rusu, M.C. The telopode- and filopode-projecting heterogeneous stromal cells of the human sclera niche. Ann. Anat. 2018, 218, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Petrea, C.E.; Rusu, M.C.; Manoiu, V.S.; Vrapciu, A.D. Telocyte-Like Cells Containing Weibel-Palade Bodies in Rat Lamina Fusca. Ann. Anat. 2018, 218, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.; Schroppel, B.; Dass, M.; Zimmermann, H.; Wolburg, H.; Fallier-Becker, P.; Gevaert, T.; Burkhardt, C.J.; Do, H.M.; Stolzenburg, J.U. 3D-electron microscopic characterization of interstitial cells in the human bladder upper lamina propria. Neurourol. Urodyn. 2018, 37, 89–98. [Google Scholar] [CrossRef]
- Diaz-Flores, L.; Gutierrez, R.; Garcia, M.P.; Saez, F.J.; Diaz-Flores, L., Jr.; Valladares, F.; Madrid, J.F. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol. Histopathol. 2014, 29, 831–870. [Google Scholar] [CrossRef]
- Varga, I.; Kyselovič, J.; Danišovič, Ľ.; Gálfiová, P.; Kachlík, D.; Polák, Š.; Klein, M. Recently discovered interstitial cells termed telocytes: Distinguishing cell-biological and histological facts from fictions. Biologia 2018. [Google Scholar] [CrossRef]
- Varga, I.; Danisovic, L.; Kyselovic, J.; Gazova, A.; Musil, P.; Miko, M.; Polak, S. The functional morphology and role of cardiac telocytes in myocardium regeneration. Can. J. Physiol. Pharmacol. 2016. [Google Scholar] [CrossRef]
- Rusu, M.C.; Hostiuc, S. Critical review: Cardiac telocytes vs cardiac lymphatic endothelial cells. Ann. Anat. 2019, 222, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Manole, C.G.; Gherghiceanu, M.; Ardelean, A.; Nicolescu, M.I.; Hinescu, M.E.; Kostin, S. Telocytes in human epicardium. J. Cell. Mol. Med. 2010, 14, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Gherghiceanu, M.; Manole, C.G.; Faussone-Pellegrini, M.S. Cardiac renewing: Interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J. Cell. Mol. Med. 2009, 13, 866–886. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Popescu, L.M. Human epicardium: Ultrastructural ancestry of mesothelium and mesenchymal cells. J. Cell. Mol. Med. 2009, 13, 2949–2951. [Google Scholar] [CrossRef]
- Rusu, M.C.; Pop, F.; Hostiuc, S.; Dermengiu, D.; Lala, A.I.; Ion, D.A.; Manoiu, V.S.; Mirancea, N. The human trigeminal ganglion: C-kit positive neurons and interstitial cells. Ann. Anat. 2011, 193, 403–411. [Google Scholar] [CrossRef]
- Suciu, L.; Popescu, L.M.; Gherghiceanu, M.; Regalia, T.; Nicolescu, M.I.; Hinescu, M.E.; Faussone-Pellegrini, M.S. Telocytes in human term placenta: Morphology and phenotype. Cells Tissues Organs 2010, 192, 325–339. [Google Scholar] [CrossRef]
- Chang, Y.; Li, C.; Lu, Z.; Li, H.; Guo, Z. Multiple immunophenotypes of cardiac telocytes. Exp. Cell Res. 2015, 338, 239–244. [Google Scholar] [CrossRef]
- Petre, N.; Rusu, M.C.; Pop, F.; Jianu, A.M. Telocytes of the mammary gland stroma. Folia Morphol. 2016, 75, 224–231. [Google Scholar] [CrossRef]
- Iancu, C.B.; Iancu, D.; Rentea, I.; Hostiuc, S.; Dermengiu, D.; Rusu, M.C. Molecular signatures of cardiac stem cells. Rom. J. Morphol. Embryol. 2015, 56, 1255–1262. [Google Scholar]
- Keith, M.C.; Bolli, R. “String Theory” of c-kitpos Cardiac Cells: A New Paradigm Regarding the Nature of These Cells That May Reconcile Apparently Discrepant Results. Circ. Res. 2015, 116, 1216–1230. [Google Scholar] [CrossRef]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; Lecapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef]
- Choi, S.H.; Jung, S.Y.; Asahara, T.; Suh, W.; Kwon, S.-M.; Baek, S.H. Direct comparison of distinct cardiomyogenic induction methodologies in human cardiac-derived c-kit positive progenitor cells. Tissue Eng. Regen. Med. 2012, 9, 311–319. [Google Scholar] [CrossRef]
- Bearzi, C.; Cascapera, S.; Nascimbene, A.; Casarsa, C.; Rastaldo, R.; Hosoda, T.; De Angelis, A.; Rota, M.; Quaini, F.; Urbanek, K.; et al. Late-Breaking Basic Science Abstracts. Late Breaking Developments in Stem Cell Biology and Cardiac Growth Regulation. Circulation 2005, 111, 1720–1724. [Google Scholar]
- Ye, J.; Boyle, A.; Shih, H.; Sievers, R.E.; Zhang, Y.; Prasad, M.; Su, H.; Zhou, Y.; Grossman, W.; Bernstein, H.S.; et al. Sca-1+ cardiosphere-derived cells are enriched for Isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS ONE 2012, 7, e30329. [Google Scholar] [CrossRef]
- Barile, L.; Messina, E.; Giacomello, A.; Marban, E. Endogenous cardiac stem cells. Prog. Cardiovasc. Dis. 2007, 50, 31–48. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Choi, S.H.; Jung, S.Y.; Suh, W.; Baek, S.H.; Kwon, S.M. Establishment of Isolation and Expansion Protocols for Human Cardiac C-kit-Positive Progenitor Cells for Stem Cell Therapy. Transplant. Proc. 2013, 45, 420–426. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, P.; Yao, L.; Su, M.; He, P.; Niu, N.; McNutt, M.A.; Gu, J. CD117-positive cells of the heart: Progenitor cells or mast cells? J. Histochem. Cytochem. 2010, 58, 309–316. [Google Scholar] [CrossRef]
- Veinot, J.P.; Prichett-Pejic, W.; Song, J.; Waghray, G.; Parks, W.; Mesana, T.G.; Ruel, M. CD117-positive cells and mast cells in adult human cardiac valves-observations and implications for the creation of bioengineered grafts. Cardiovasc. Pathol. 2006, 15, 36–40. [Google Scholar] [CrossRef]
- Sandstedt, J.; Jonsson, M.; Lindahl, A.; Jeppsson, A.; Asp, J. C-kit+ CD45- cells found in the adult human heart represent a population of endothelial progenitor cells. Basic Res. Cardiol. 2010, 105, 545–556. [Google Scholar] [CrossRef]
- Di Meglio, F.; Castaldo, C.; Nurzynska, D.; Romano, V.; Miraglia, R.; Bancone, C.; Langella, G.; Vosa, C.; Montagnani, S. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J. Mol. Cell. Cardiol. 2010, 49, 719–727. [Google Scholar] [CrossRef]
- Bronnum, H.; Andersen, D.C.; Schneider, M.; Nossent, A.Y.; Nielsen, S.B.; Sheikh, S.P. Islet-1 is a dual regulator of fibrogenic epithelial-to-mesenchymal transition in epicardial mesothelial cells. Exp. Cell Res. 2013, 319, 424–435. [Google Scholar] [CrossRef]
- Min, K.W. Interstitial cells of Cajal (pICC) in the pancreas. J. Cell. Mol. Med. 2005, 9, 737–739. [Google Scholar] [CrossRef]
- Manetti, M.; Guiducci, S.; Ruffo, M.; Rosa, I.; Faussone-Pellegrini, M.S.; Matucci-Cerinic, M.; Ibba-Manneschi, L. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J. Cell. Mol. Med. 2013, 17, 482–496. [Google Scholar] [CrossRef]
- Metzger, R.; Rolle, U.; Fiegel, H.C.; Franke, F.E.; Muenstedt, K.; Till, H. C-kit receptor in the human vas deferens: Distinction of mast cells, interstitial cells and interepithelial cells. Reproduction 2008, 135, 377–384. [Google Scholar] [CrossRef]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Smith, C.L.; Baek, S.T.; Sung, C.Y.; Tallquist, M.D. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res. 2011, 108, e15–e26. [Google Scholar] [CrossRef]
- Rudat, C.; Norden, J.; Taketo, M.M.; Kispert, A. Epicardial function of canonical Wnt-, Hedgehog-, Fgfr1/2-, and Pdgfra-signalling. Cardiovasc. Res. 2013, 100, 411–421. [Google Scholar] [CrossRef][Green Version]
- Chong, J.J.; Reinecke, H.; Iwata, M.; Torok-Storb, B.; Stempien-Otero, A.; Murry, C.E. Progenitor cells identified by PDGFR-alpha expression in the developing and diseased human heart. Stem Cells Dev. 2013, 22, 1932–1943. [Google Scholar] [CrossRef]
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts 2014, 5, 353–369. [Google Scholar] [CrossRef]
- Manole, C.G.; Gherghiceanu, M.; Simionescu, O. Telocyte dynamics in psoriasis. J. Cell. Mol. Med. 2015, 19, 1504–1519. [Google Scholar] [CrossRef]
- Wang, F.; Bei, Y.; Zhao, Y.; Song, Y.; Xiao, J.; Yang, C. Telocytes in pregnancy-induced physiological liver growth. Cell. Physiol. Biochem. 2015, 36, 250–258. [Google Scholar] [CrossRef]
- Marini, M.; Mencucci, R.; Rosa, I.; Favuzza, E.; Guasti, D.; Ibba-Manneschi, L.; Manetti, M. Telocytes in normal and keratoconic human cornea: An immunohistochemical and transmission electron microscopy study. J. Cell. Mol. Med. 2017, 21, 3602–3611. [Google Scholar] [CrossRef]
- Milia, A.F.; Ruffo, M.; Manetti, M.; Rosa, I.; Conte, D.; Fazi, M.; Messerini, L.; Ibba-Manneschi, L. Telocytes in Crohn’s disease. J. Cell. Mol. Med. 2013, 17, 1525–1536. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Traini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini, M.S. Telocytes express PDGFRalpha in the human gastrointestinal tract. J. Cell. Mol. Med. 2013, 17, 1099–1108. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, L.; Zhong, C.; Fu, S.; Bei, Y.; Huica, R.I.; Wang, F.; Xiao, J. Cardiac telocytes are double positive for CD34/PDGFR-alpha. J. Cell. Mol. Med. 2015, 19, 2036–2042. [Google Scholar] [CrossRef]
- Breiteneder-Geleff, S.; Matsui, K.; Soleiman, A.; Meraner, P.; Poczewski, H.; Kalt, R.; Schaffner, G.; Kerjaschki, D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 1997, 151, 1141–1152. [Google Scholar]
- Al-Rawi, M.A.; Mansel, R.E.; Jiang, W.G. Molecular and cellular mechanisms of lymphangiogenesis. Eur. J. Surg. Oncol. 2005, 31, 117–121. [Google Scholar] [CrossRef]
- Adamczyk, L.A.; Gordon, K.; Kholova, I.; Meijer-Jorna, L.B.; Telinius, N.; Gallagher, P.J.; van der Wal, A.C.; Baandrup, U. Lymph vessels: The forgotten second circulation in health and disease. Virchows Arch. 2016, 469, 3–17. [Google Scholar] [CrossRef]
- Wetterwald, A.; Hoffstetter, W.; Cecchini, M.G.; Lanske, B.; Wagner, C.; Fleisch, H.; Atkinson, M. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone 1996, 18, 125–132. [Google Scholar] [CrossRef]
- Mahtab, E.A.; Wijffels, M.C.; Van Den Akker, N.M.; Hahurij, N.D.; Lie-Venema, H.; Wisse, L.J.; Deruiter, M.C.; Uhrin, P.; Zaujec, J.; Binder, B.R.; et al. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: Correlation with abnormal epicardial development. Dev. Dyn. 2008, 237, 847–857. [Google Scholar] [CrossRef]
- Sawa, Y. New trends in the study of podoplanin as a cell morphological regulator. Jpn. Dent. Sci. Rev. 2010, 46, 165–172. [Google Scholar] [CrossRef][Green Version]
- Astarita, J.L.; Acton, S.E.; Turley, S.J. Podoplanin: Emerging functions in development, the immune system, and cancer. Front. Immunol. 2012, 3, 283. [Google Scholar] [CrossRef]
- Karunamuni, G.; Yang, K.; Doughman, Y.Q.; Wikenheiser, J.; Bader, D.; Barnett, J.; Austin, A.; Parsons-Wingerter, P.; Watanabe, M. Expression of lymphatic markers during avian and mouse cardiogenesis. Anat. Rec. 2010, 293, 259–270. [Google Scholar] [CrossRef]
- Manetti, M.; Rosa, I.; Messerini, L.; Guiducci, S.; Matucci-Cerinic, M.; Ibba-Manneschi, L. A loss of telocytes accompanies fibrosis of multiple organs in systemic sclerosis. J. Cell. Mol. Med. 2014, 18, 253–262. [Google Scholar] [CrossRef]
- Benias, P.C.; Wells, R.G.; Sackey-Aboagye, B.; Klavan, H.; Reidy, J.; Buonocore, D.; Miranda, M.; Kornacki, S.; Wayne, M.; Carr-Locke, D.L.; et al. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci. Rep. 2018, 8, 4947. [Google Scholar] [CrossRef] [PubMed]
- Kholova, I.; Dragneva, G.; Cermakova, P.; Laidinen, S.; Kaskenpaa, N.; Hazes, T.; Cermakova, E.; Steiner, I.; Yla-Herttuala, S. Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions. Eur. J. Clin. Investig. 2011, 41, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Trerotola, M.; Franck, J.; Cardon, T.; Marchisio, M.; Fournier, I.; Salzet, M.; Maffia, M.; Vergara, D. The multiverse nature of epithelial to mesenchymal transition. Semin. Cancer Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.M.; Dronkers, E.; Goumans, M.J. The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate. Pharmacol. Res. 2018, 127, 129–140. [Google Scholar] [CrossRef]
- Moerkamp, A.T.; Lodder, K.; van Herwaarden, T.; Dronkers, E.; Dingenouts, C.K.; Tengstrom, F.C.; van Brakel, T.J.; Goumans, M.J.; Smits, A.M. Human fetal and adult epicardial-derived cells: A novel model to study their activation. Stem Cell Res. Ther. 2016, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Van Tuyn, J.; Atsma, D.E.; Winter, E.M.; van der Velde-van Dijke, I.; Pijnappels, D.A.; Bax, N.A.; Knaan-Shanzer, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; van der Laarse, A.; et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells 2007, 25, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Winter, E.M.; Poelmann, R.E. Epicardium-derived cells (EPDCs) in development, cardiac disease and repair of ischemia. J. Cell. Mol. Med. 2010, 14, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Bax, N.A.; van Oorschot, A.A.; Maas, S.; Braun, J.; van Tuyn, J.; de Vries, A.A.; Groot, A.C.; Goumans, M.J. In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFbeta-signaling and WT1. Basic Res. Cardiol. 2011, 106, 829–847. [Google Scholar] [CrossRef]
- Risebro, C.A.; Vieira, J.M.; Klotz, L.; Riley, P.R. Characterisation of the human embryonic and foetal epicardium during heart development. Development 2015, 142, 3630–3636. [Google Scholar] [CrossRef]
- Viragh, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; Kalman, F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat. Embryol. 1993, 188, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Vrancken Peeters, M.P.; Mentink, M.M.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Cytokeratins as a marker for epicardial formation in the quail embryo. Anat. Embryol. 1995, 191, 503–508. [Google Scholar] [PubMed]
- Manta, L.; Rusu, M.C.; Pop, F. What podoplanin tells us about cells with telopodes. Ann. Anat. 2018, 218, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.N.; Wang, S.; Yang, Q.; Wang, Y.J.; Chen, D.X.; Zhu, X.X. ESC reverses epithelial mesenchymal transition induced by transforming growth factor-beta via inhibition of Smad signal pathway in HepG2 liver cancer cells. Cancer Cell Int. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, J.Y.; Tian, Y.; Cao, Y.; Lian, C.; Ou, Q.; Wu, B.; Jin, C.; Gao, F.; Wang, J.; et al. A cell culture condition that induces the mesenchymal-epithelial transition of dedifferentiated porcine retinal pigment epithelial cells. Exp. Eye Res. 2018, 177, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Vainio, S.; Vassileva, G.; McMahon, A.P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 1994, 372, 679–683. [Google Scholar] [CrossRef]
- Kispert, A.; Vainio, S.; McMahon, A.P. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 1998, 125, 4225–4234. [Google Scholar]
- Gessert, S.; Kuhl, M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ. Res. 2010, 107, 186–199. [Google Scholar] [CrossRef]
- Merki, E.; Zamora, M.; Raya, A.; Kawakami, Y.; Wang, J.; Zhang, X.; Burch, J.; Kubalak, S.W.; Kaliman, P.; Izpisua Belmonte, J.C.; et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc. Natl. Acad. Sci. USA 2005, 102, 18455–18460. [Google Scholar] [CrossRef]
- Compton, L.A.; Potash, D.A.; Mundell, N.A.; Barnett, J.V. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev. Dyn. 2006, 235, 82–93. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iancu, C.B.; Rusu, M.C.; Mogoantă, L.; Hostiuc, S.; Toader, O.D. The Telocytes in the Subepicardial Niche. Appl. Sci. 2019, 9, 1615. https://doi.org/10.3390/app9081615
Iancu CB, Rusu MC, Mogoantă L, Hostiuc S, Toader OD. The Telocytes in the Subepicardial Niche. Applied Sciences. 2019; 9(8):1615. https://doi.org/10.3390/app9081615
Chicago/Turabian StyleIancu, Cristian Bogdan, Mugurel Constantin Rusu, Laurenţiu Mogoantă, Sorin Hostiuc, and Oana Daniela Toader. 2019. "The Telocytes in the Subepicardial Niche" Applied Sciences 9, no. 8: 1615. https://doi.org/10.3390/app9081615
APA StyleIancu, C. B., Rusu, M. C., Mogoantă, L., Hostiuc, S., & Toader, O. D. (2019). The Telocytes in the Subepicardial Niche. Applied Sciences, 9(8), 1615. https://doi.org/10.3390/app9081615






