Cellular Spheroids of Mesenchymal Stem Cells and Their Perspectives in Future Healthcare
Abstract
1. Three-Dimensional (3D) Cell Culture Systems
2. 3D Culture of Cancer Cells or Stem Cells
3. Cell–Material Interface and Interaction in Scaffold-Free Stem Cell 3D Culture Systems
4. Spheroid Culture of MSCs
5. Physical and Chemical Factors Affecting Cell Behaviors in Scaffold-Free 3D Culture Systems
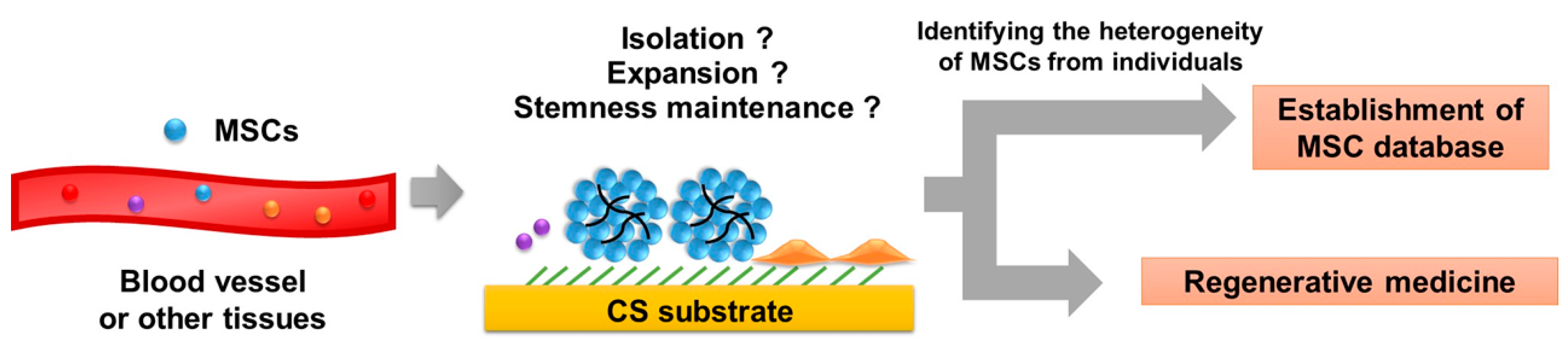
6. Perspectives of MSCs Spheroids in Healthcare
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Eglen, R.M. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [PubMed]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, B.; Zhou, C.; Li, Y.; Li, B.; Yu, M.; Luo, Y.; Gao, L.; Zhang, D.; Xue, Q.; et al. The comparison genomics analysis with glioblastoma multiforme (GBM) cells under 3D and 2D cell culture conditions. Colloids Surf. B Biointerfaces 2018, 172, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Zschenker, O.; Streichert, T.; Hehlgans, S.; Cordes, N. Genome-wide gene expression analysis in cancer cells reveals 3D growth to affect ECM and processes associated with cell adhesion but not DNA repair. PLoS ONE 2012, 7, e34279. [Google Scholar] [CrossRef] [PubMed]
- Polonio-Alcala, E.; Rabionet, M.; Guerra, A.J.; Yeste, M.; Ciurana, J.; Puig, T. Screening of additive manufactured scaffolds designs for triple negative breast cancer 3D cell culture and stem-like expansion. Int. J. Mol. Sci. 2018, 19, 3148. [Google Scholar] [CrossRef]
- Huang, Y.J.; Hsu, S.H. Acquisition of epithelial-mesenchymal transition and cancer stem-like phenotypes within chitosan-hyaluronan membrane-derived 3D tumor spheroids. Biomaterials 2014, 35, 10070–10079. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhan, S.; Rui, C.; Sho, E.; Shi, X.; Ding, Y. Microporous cellulosic scaffold as a spheroid culture system modulates chemotherapeutic responses and stemness in hepatocellular carcinoma. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, R.; Gu, Q.; Liang, L.; Wang, L.; Zhang, Y.; Wang, X.; Liu, X.; Li, Z.; Fang, J.; et al. A fully defined static suspension culture system for large-scale human embryonic stem cell production. Cell Death Dis. 2018, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.R.; Hashino, E. 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat. Protoc. 2014, 9, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Cesarz, Z.; Tamama, K. Spheroid culture of mesenchymal stem cells. Stem Cells Int 2016, 2016, 9176357. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.; Sykova, E.; Kubinova, S. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, Y.; Chen, L.; Liang, W. Safety of neural stem cell transplantation in patients with severe traumatic brain injury. Exp. Ther. Med. 2017, 13, 3613–3618. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Villa, O.; Silva, D.; Gelain, F. 3D culture of adult mouse neural stem cells within functionalized self-assembling peptide scaffolds. Int. J. Nanomed. 2011, 6, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Han, H.W.; Hsu, S.H. Chitosan derived co-spheroids of neural stem cells and mesenchymal stem cells for neural regeneration. Colloids Surf. B Biointerfaces 2017, 158, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Avci, H.X.; Ochalek, A.; Rosingh, L.N.; Molnar, K.; Laszlo, L.; Bellak, T.; Teglasi, A.; Pesti, K.; Mike, A.; et al. Comparison of 2D and 3D neural induction methods for the generation of neural progenitor cells from human induced pluripotent stem cells. Stem Cell Res. 2017, 25, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Shoval, H.; Karsch-Bluman, A.; Brill-Karniely, Y.; Stern, T.; Zamir, G.; Hubert, A.; Benny, O. Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Sci. Rep. 2017, 7, 10428. [Google Scholar] [CrossRef]
- Han, H.W.; Hsu, S.H. Chitosan-hyaluronan based 3D co-culture platform for studying the crosstalk of lung cancer cells and mesenchymal stem cells. Acta Biomater. 2016, 42, 157–167. [Google Scholar] [CrossRef]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef] [PubMed]
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 2011. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, T.; Xu, A.; Zhang, L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell. Mol. Med. 2016, 20, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Camoes, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simoes, S.; Gaspar, M.; Mosqueira, D.; et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res. Ther. 2015, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Castro, E.; Cunningham, C.J.; Miller, J.; Cain, S.A.; Allan, S.M.; Pinteaux, E. Generation of Human Mesenchymal Stem Cell 3D Spheroids Using Low-binding Plates. Bio Protoc. 2018, 8, e2968. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids formation on non-adhesive surfaces by liquid overlay technique: Considerations and practical approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylostalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef]
- Lou, Y.L.; Guo, D.W.; Zhang, H.; Song, L.J. Effectiveness of mesenchymal stems cells cultured by hanging drop vs. conventional culturing on the repair of hypoxic-ischemic-damaged mouse brains, measured by stemness gene expression. Open Life Sci. 2016, 11, 519–523. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, Y.S.; Lee, S.H. Long-duration three-dimensional spheroid culture promotes angiogenic activities of adipose-derived mesenchymal stem cells. Biomol. Ther. 2016, 24, 260–267. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Liu, B.H.; Sieber, M.; Hsu, S.H. Substrate-dependent gene regulation of self-assembled human MSC spheroids on chitosan membranes. BMC Genom. 2014, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.-R.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, S.; Cho, S.-P.; Hong, B.H.; Choung, P.-H.; Chung, T.D.; et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933–938. [Google Scholar] [CrossRef]
- Law, S.; Chaudhuri, S. Mesenchymal stem cell and regenerative medicine: Regeneration versus immunomodulatory challenges. Am. J. Stem Cells 2013, 2, 22–38. [Google Scholar] [PubMed]
- Hsu, S.H.; Hsieh, P.S. Self-assembled adult adipose-derived stem cell spheroids combined with biomaterials promote wound healing in a rat skin repair model. Wound Repair Regen. 2015, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.C.; Whitehead, J.; Zhou, D.; Ho, S.S.; Leach, J.K. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017, 64, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nguyen, A.L.; Shi, S.; Hill, C.; Wilder-Smith, P.; Krasieva, T.B.; Le, A.D. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012, 21, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Muneta, T.; Tsuji, K.; Ichinose, S.; Makino, H.; Umezawa, A.; Sekiya, I. Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration. Arthritis Res. Ther. 2012, 14, R136. [Google Scholar] [CrossRef]
- Suenaga, H.; Furukawa, K.S.; Suzuki, Y.; Takato, T.; Ushida, T. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J. Mater. Sci. Mater. Med. 2015, 26, 254. [Google Scholar] [CrossRef]
- Yanagihara, K.; Uchida, S.; Ohba, S.; Kataoka, K.; Itaka, K. Treatment of Bone Defects by Transplantation of Genetically Modified Mesenchymal Stem Cell Spheroids. Mol. Ther. Methods Clin. Dev. 2018, 9, 358–366. [Google Scholar] [CrossRef]
- Liu, B.H.; Yeh, H.Y.; Lin, Y.C.; Wang, M.H.; Chen, D.C.; Lee, B.H.; Hsu, S.H. Spheroid formation and enhanced cardiomyogenic potential of adipose-derived stem cells grown on chitosan. BioRes. Open Access 2013, 2, 28–39. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Wolint, P.; Wickboldt, N.; Gemayel, G.; Weber, B.; Brokopp, C.E.; Boni, A.; Falk, V.; Bosman, A.; Jaconi, M.E.; et al. Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies. Biomaterials 2013, 34, 6339–6354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Chang, T.M.S. Transdifferentiation of bioencapsulated bone marrow cells into hepatocyte-like cells in the 90% hepatectomized rat model. Liver Transplant. 2006, 12, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Chang, T.M.S. Preliminary study on intrasplenic implantation of artificial cell bioencapsulated stem cells to increase the survival of 90% hepatectomized rats. Artif. Cell Blood Substit. Biotechnol. 2009, 37, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Chang, T.M.S. Intrasplenic transplantation of bioencapsulated mesenchymal stem cells improves the recovery rates of 90% partial hepatectomized rats. Stem Cells Int. 2012, 2012, 697094. [Google Scholar] [CrossRef] [PubMed]
- Talaei-Khozani, T.; Borhani-Haghighi, M.; Ayatollahi, M.; Vojdani, Z. An in vitro model for hepatocyte-like cell differentiation from Wharton’s jelly derived-mesenchymal stem cells by cell-base aggregates. Gastroenterol. Hepatol. Bed Bench 2015, 8, 188–199. [Google Scholar] [PubMed]
- Sun, Y.; Wang, Y.; Zhou, L.; Zou, Y.; Huang, G.; Gao, G.; Ting, S.; Lei, X.; Ding, X. Spheroid-cultured human umbilical cord-derived mesenchymal stem cells attenuate hepatic ischemia-reperfusion injury in rats. Sci. Rep. 2018, 8, 2518. [Google Scholar] [CrossRef]
- Turker, E.; Arslan-Yildiz, A. Recent advances in magnetic levitation: A biological approach from diagnostics to tissue engineering. ACS Biomater. Sci. Eng. 2018, 4, 787–799. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Saburina, I.N.; Gorkun, A.A.; Fidarov, A.F.; Kolokol’tsova, T.D.; Zurina, I.M.; Kosheleva, N.V.; Ustinova, E.E.; Repin, V.S. Induction of Vasculo- and Osteogenesis in Spheroids Formed by Adipose-Derived Stromal Cells. Bull. Exp. Biol. Med. 2018, 166, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Barnes, A.; Genever, P. Analysis of the intrinsic self-organising properties of mesenchymal stromal cells in three-dimensional co-culture models with endothelial cells. Bioengineering 2018, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Williams, S.J.; Ramachandran, K.; Stehno-Bittel, L. Integration of mesenchymal stem cells into islet cell spheroids improves long-term viability, but not islet function. Islets 2017, 9, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Futrega, K.; Atkinson, K.; Lott, W.B.; Doran, M.R. Spheroid coculture of hematopoietic stem/progenitor cells and monolayer expanded mesenchymal stem/stromal cells in polydimethylsiloxane microwells modestly improves in vitro hematopoietic stem/progenitor cell expansion. Tissue Eng. Part C Methods 2017, 23, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Viejo, M.; Menendez-Menendez, Y.; Otero-Hernandez, J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J. Stem Cells 2015, 7, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Tseng, T.C.; Dai, N.T.; Fu, K.Y.; Dai, L.G.; Hsu, S.H. Fast isolation and expansion of multipotent cells from adipose tissue based on chitosan-selected primary culture. Biomaterials 2015, 65, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.C.; Chang, H.H.; Tu, Y.K.; Young, T.H. Efficient transfer of human adipose-derived stem cells by chitosan/gelatin blend films. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1369–1377. [Google Scholar] [CrossRef]
- Wang, L.; Rao, R.R.; Stegemann, J.P. Delivery of mesenchymal stem cells in chitosan/collagen microbeads for orthopedic tissue repair. Cells Tissues Organs 2013, 197, 333–343. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Z.; Law, J.B.; He, A.Y.; Abbas, A.A.; Denslin, V.; Kamarul, T.; Hui, J.H.; Lee, E.H. The Combined effect of substrate stiffness and surface topography on chondrogenic differentiation of mesenchymal stem cells. Tissue Eng. Part A 2017, 23, 43–54. [Google Scholar] [CrossRef]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef]
- Datta, N.; Pham, Q.P.; Sharma, U.; Sikavitsas, V.I.; Jansen, J.A.; Mikos, A.G. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Han, Y.; Li, X.; Liu, C.; Lv, Z.; Wang, X.; Tang, X.; Wang, Z. Carbenoxolone inhibits mechanical stress-induced osteogenic differentiation of mesenchymal stem cells by regulating p38 MAPK phosphorylation. Exp. Ther. Med. 2018, 15, 2798–2803. [Google Scholar] [CrossRef] [PubMed]
- Steward, A.J.; Kelly, D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015, 227, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Saleh, F.A.; Whyte, M.; Genever, P.G. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur. Cell Mater. 2011, 22, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, P.; Chen, L.; Wang, Y.; Wang, Z.; Zhang, B. The effects of spheroid formation of adipose-derived stem cells in a microgravity bioreactor on stemness properties and therapeutic potential. Biomaterials 2015, 41, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Murphy, K.C.; Hung, B.P.; Browne-Bourne, S.; Zhou, D.; Yeung, J.; Genetos, D.C.; Leach, J.K. Measurement of oxygen tension within mesenchymal stem cell spheroids. J. R. Soc. Interface 2017, 14, 20160851. [Google Scholar] [CrossRef]
- Campbell, J.J.; Bader, D.L.; Lee, D.A. Mechanical loading modulates intracellular calcium signaling in human mesenchymal stem cells. J. Appl. Biomater. Biomech. 2008, 6, 9–15. [Google Scholar]
- Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The role of physical stimuli on calcium channels in chondrogenic differentiation of mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 2998. [Google Scholar] [CrossRef]
- Formigli, L.; Meacci, E.; Sassoli, C.; Squecco, R.; Nosi, D.; Chellini, F.; Naro, F.; Francini, F.; Zecchi-Orlandini, S. Cytoskeleton/stretch-activated ion channel interaction regulates myogenic differentiation of skeletal myoblasts. J. Cell. Physiol. 2007, 211, 296–306. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; Li, H.; Wu, Y. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J. Cell. Mol. Med. 2017, 21, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Mege, R.M.; Ishiyama, N. Integration of cadherin adhesion and cytoskeleton at adherens junctions. Cold Spring Harb. Perspect. Biol. 2017, 9, a028738. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Hsieh, P.S.; Tseng, C.S.; Hsu, S.H. The substrate-dependent regeneration capacity of mesenchymal stem cell spheroids derived on various biomaterial surfaces. Biomater. Sci. 2014, 2, 1652–1660. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal stromal/stem Cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J. Cell Biochem. 2012, 113, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.M.; Mauck, R.L. On the origin and impact of mesenchymal stem cell heterogeneity: New insights and emerging tools for single cell analysis. Eur. Cell Mater. 2017, 34, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, G. Circulating mesenchymal stem cells and their clinical implications. J. Orthop. Transl. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Marketou, M.E.; Parthenakis, F.I.; Kalyva, A.; Pontikoglou, C.; Maragkoudakis, S.; Kontaraki, J.E.; Zacharis, E.A.; Patrianakos, A.; Chlouverakis, G.; Papadaki, H.A.; et al. Circulating mesenchymal stem cells in patients with hypertrophic cardiomyopathy. Cardiovasc. Pathol. 2015, 24, 149–153. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.-W.; Asano, S.; Hsu, S.-h. Cellular Spheroids of Mesenchymal Stem Cells and Their Perspectives in Future Healthcare. Appl. Sci. 2019, 9, 627. https://doi.org/10.3390/app9040627
Han H-W, Asano S, Hsu S-h. Cellular Spheroids of Mesenchymal Stem Cells and Their Perspectives in Future Healthcare. Applied Sciences. 2019; 9(4):627. https://doi.org/10.3390/app9040627
Chicago/Turabian StyleHan, Hao-Wei, Shigetaka Asano, and Shan-hui Hsu. 2019. "Cellular Spheroids of Mesenchymal Stem Cells and Their Perspectives in Future Healthcare" Applied Sciences 9, no. 4: 627. https://doi.org/10.3390/app9040627
APA StyleHan, H.-W., Asano, S., & Hsu, S.-h. (2019). Cellular Spheroids of Mesenchymal Stem Cells and Their Perspectives in Future Healthcare. Applied Sciences, 9(4), 627. https://doi.org/10.3390/app9040627






