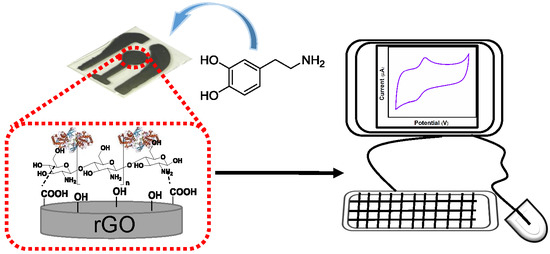
Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Apparatus
2.3. Fabrication of the SPCE Electrode
2.4. Fabrication of rGO/SPCE Electrode
2.5. Fabrication of Chitosan/rGO/SPCE Electrode
2.6. Fabrication of Tyrosinase/Chitosan/rGO/SPCE
2.7. Preparation of Human Urine Samples
3. Results and Discussion
3.1. Characterizations of Tyrosinase/Chitosan/rGO Biocomposites
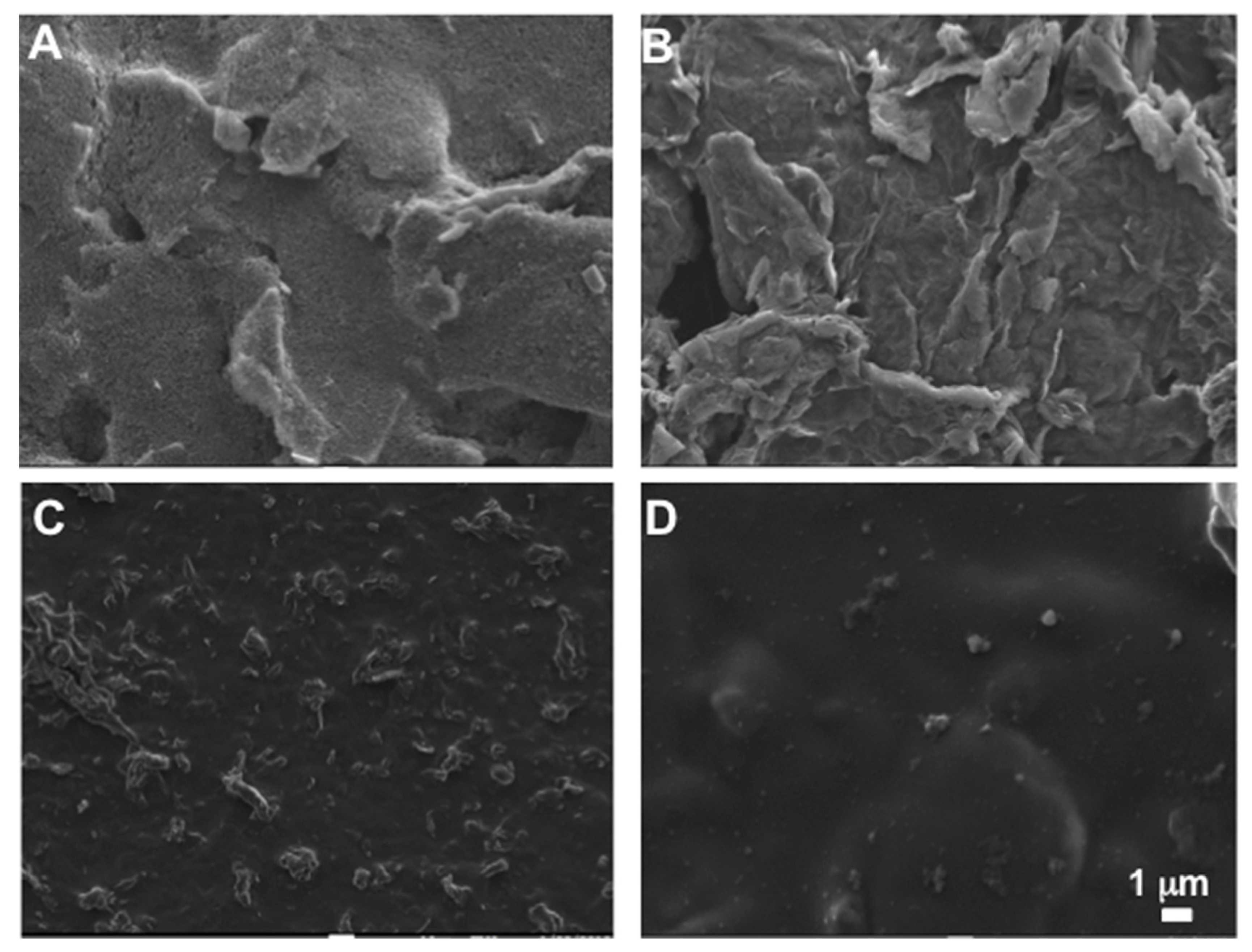
3.1.1. Morphology Analysis
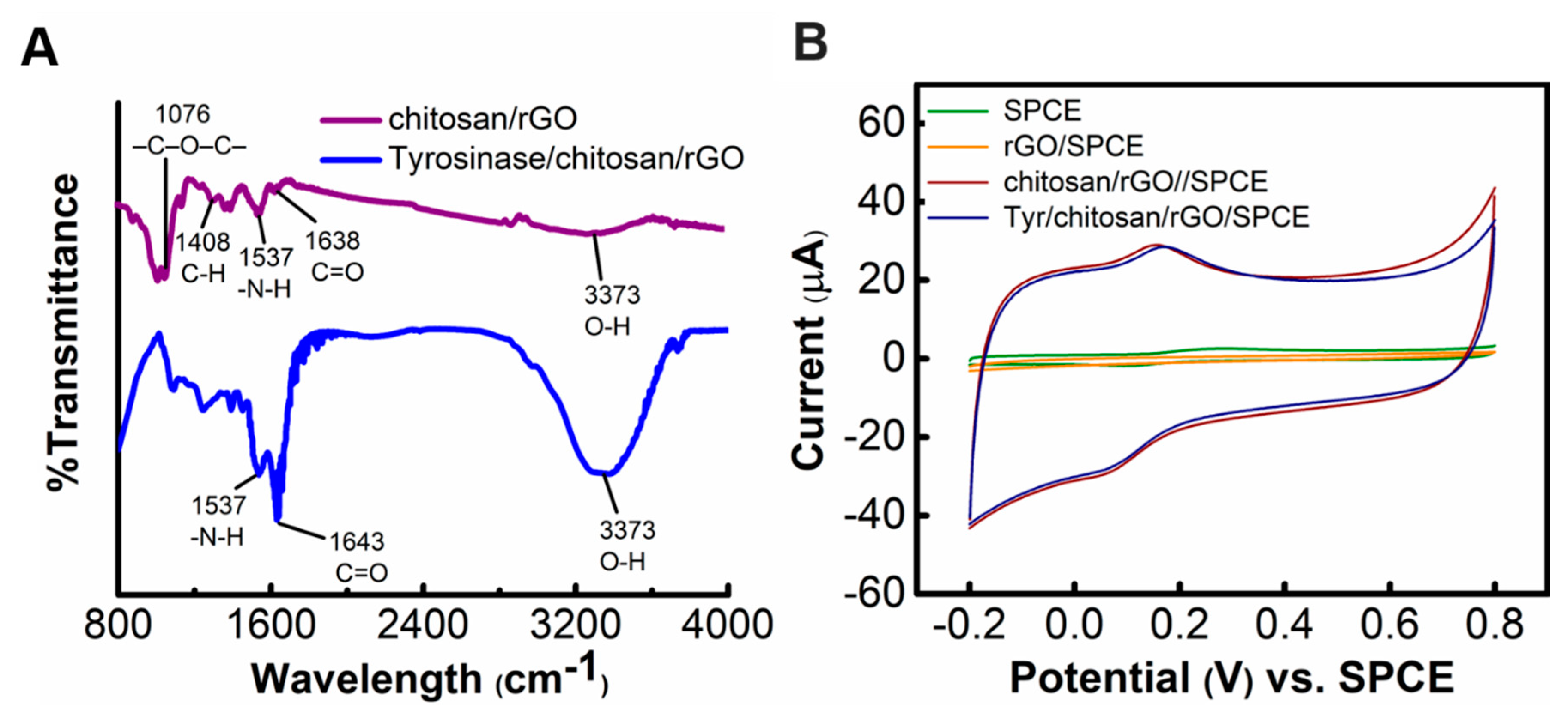
3.1.2. Cyclic Voltammetry
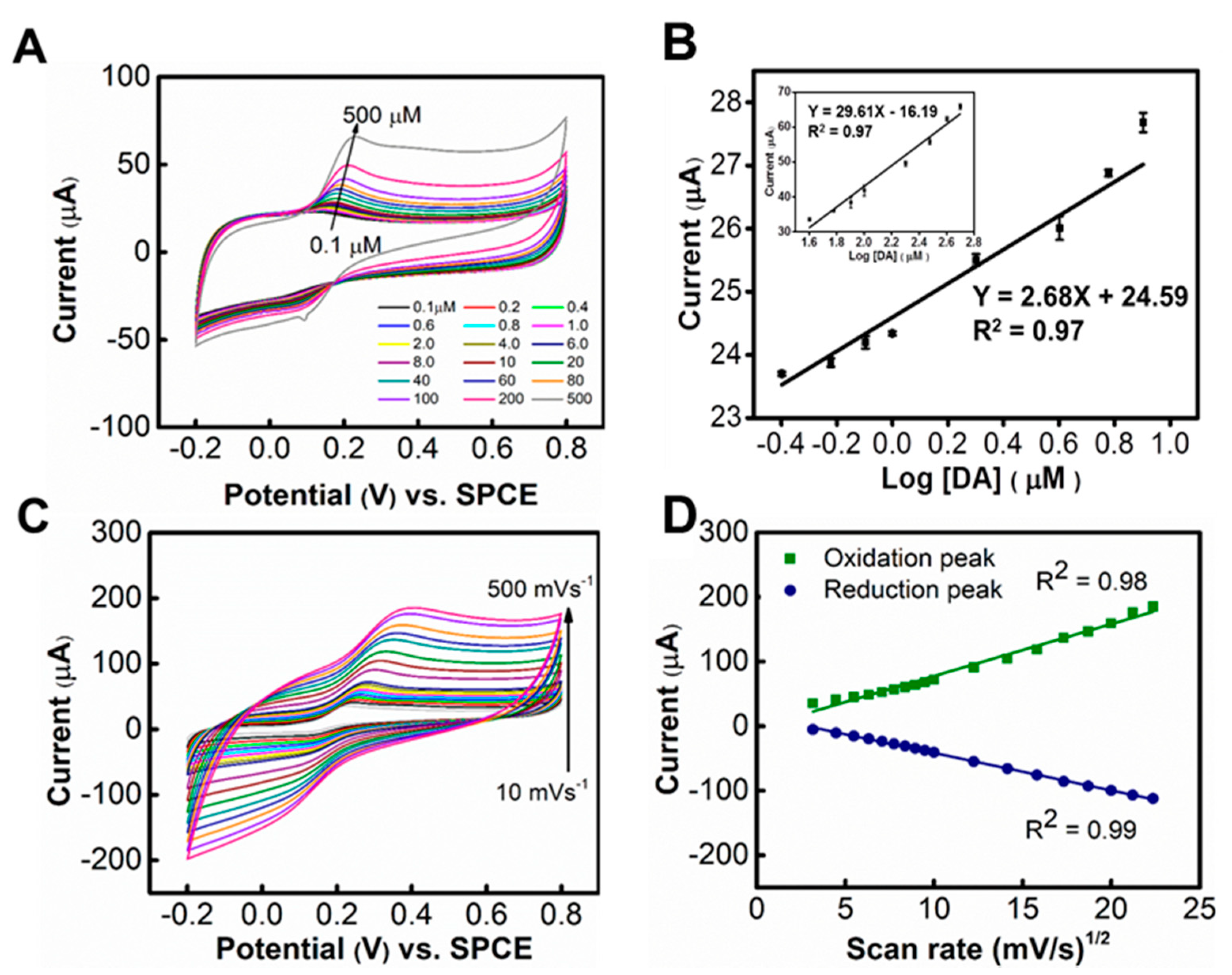
3.2. Detection of DA
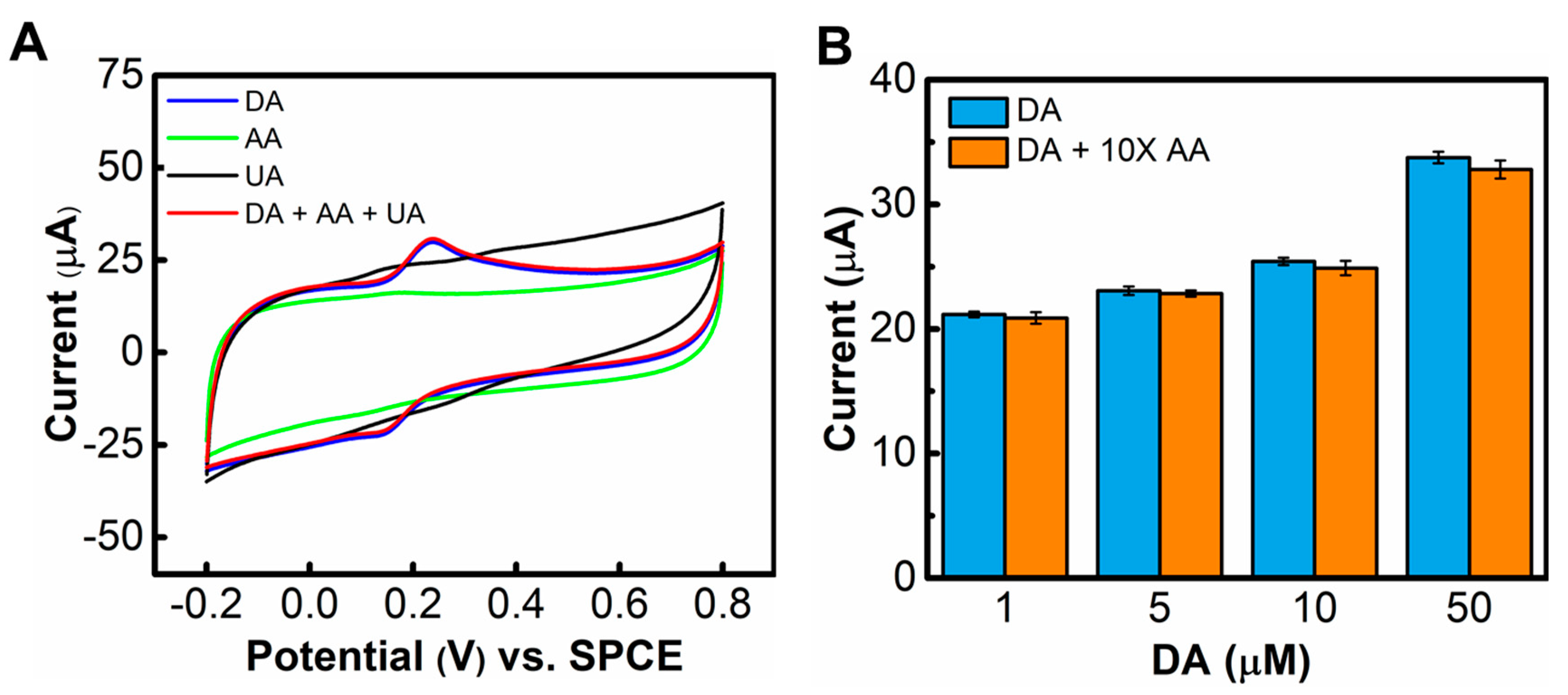
3.3. Interference
3.4. Detection of DA in Human Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Clark, K.L.; Noudoost, B. The role of prefrontal catecholamines in attention and working memory. Front. Neural Circuits 2014, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, S.; Sidhureddy, B.; Cirone, J.; Chen, A.C. Nanomaterials-based electrochemical sensors for in vitro and in vivo analyses of neurotransmitters. Appl. Sci. 2018, 8, 1504. [Google Scholar] [CrossRef]
- O’Neill, R.D. Microvoltammetric techniques and sensors for monitoring neurochemical dynamics in vivo. A review. Analyst 1994, 119, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ensafi, M.T.; Khayamian, T. Simultaneous determination of ascorbic acid, dopamine, and uric acid by differential pulse voltammetry using tiron modified glassy carbon electrode. Int. J. Electrochem. Sci. 2010, 4, 26895–26901. [Google Scholar]
- Lin, L.; Chen, J.; Yao, H.; Chen, Y.; Zheng, Y.; Lin, X. Simultaneous determination of dopamine, ascorbic acid and uric acid at poly (evans blue) modified glassy carbon electrode. Bioelectrochemistry 2008, 73, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Covalent modification of glassy carbon electrode with cysteine for the determination of dopamine in the presence of ascorbic acid. Microchim. Acta 2007, 161, 191–200. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, M.; Li, C.; Sun, D.; Yang, B. Porous boron-doped diamond electrode for detection of dopamine and pyridoxine in human serum. Electrochim. Acta 2017, 258, 744–753. [Google Scholar] [CrossRef]
- Dinesh, B.; Saraswathi, R.; Senthil Kumar, A. Water based homogenous carbon ink modified electrode as an efficient sensor system for simultaneous detection of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2017, 233, 92–104. [Google Scholar] [CrossRef]
- Hu, G.Z.; Zhang, D.P.; Wu, W.L.; Yang, Z.S. Selective determination of dopamine in the presence of high concentration of ascorbic acid using nano-au self-assembly glassy carbon electrode. Colloids Surf. B 2008, 62, 199–205. [Google Scholar] [CrossRef]
- Liu, T.; Li, M.; Li, Q. Electroanalysis of dopamine at a gold electrode modified with n-acetylcysteine self-assembled monolayer. Talanta 2004, 63, 1053–1059. [Google Scholar] [CrossRef]
- Oko, D.N.; Garbarino, S.; Zhang, J.; Xu, Z.; Chaker, M.; Ma, D.; Guay, D.; Tavares, A.C. Dopamine and ascorbic acid electro-oxidation on au, aupt and pt nanoparticles prepared by pulse laser ablation in water. Electrochim. Acta 2015, 159, 174–183. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, X.; Niu, Y.; Wu, F.; Hu, Y.; Yang, Y. Voltammetric dopamine sensor based on a gold electrode modified with reduced graphene oxide and Mn3O4 on gold nanoparticles. Microchim. Acta 2017, 184, 2081–2088. [Google Scholar] [CrossRef]
- Zhou, C.; Li, S.; Zhu, W.; Pang, H.; Ma, H. A sensor of a polyoxometalate and Au–Pd alloy for simultaneously detection of dopamine and ascorbic acid. Electrochim. Acta 2013, 113, 454–463. [Google Scholar] [CrossRef]
- Pruneanu, S.; Biris, A.R.; Pogacean, F.; Socaci, C.; Coros, M.; Rosu, M.C.; Watanabe, F.; Biris, A.S. The influence of uric and ascorbic acid on the electrochemical detection of dopamine using graphene-modified electrodes. Electrochim. Acta 2015, 154, 197–204. [Google Scholar] [CrossRef]
- Karuppiah, C.; Palanisamy, S.; Chen, S.-M.; Ramaraj, S.K.; Periakaruppan, P. A novel and sensitive amperometric hydrazine sensor based on gold nanoparticles decorated graphite nanosheets modified screen printed carbon electrode. Electrochim. Acta 2014, 139, 157–164. [Google Scholar] [CrossRef]
- Yu, H.N.; Pang, Y.C.; Tang, J.Y. Polyaniline nanofiber modified platinum electrode used to determination of dopamine by square wave voltammetry technique. Int. J. Electrochem. Sci. 2015, 10, 8353–8360. [Google Scholar]
- Caetano, F.R.; Gevaerd, A.; Castro, E.G.; Bergamini, M.F.; Zarbin, A.J.G.; Marcolino-Junior, L.H. Electroanalytical application of a screen-printed electrode modified by dodecanethiol-stabilized platinum nanoparticles for dapsone determination. Electrochim. Acta 2012, 66, 265–270. [Google Scholar] [CrossRef]
- Wu, L.; Feng, L.; Ren, J.; Qu, X. Electrochemical detection of dopamine using porphyrin-functionalized graphene. Biosens. Bioelectron. 2012, 34, 57–62. [Google Scholar] [CrossRef]
- Zhang, L.; Ning, L.; Li, S.; Pang, H.; Zhang, Z.; Ma, H.; Yan, H. Selective electrochemical detection of dopamine in the presence of uric acid and ascorbic acid based on a composite film modified electrode. RSC Adv. 2016, 6, 66468–66476. [Google Scholar] [CrossRef]
- Gorle, D.B.; Kulandainathan, M.A. Electrochemical sensing of dopamine at the surface of a dopamine grafted graphene oxide/poly(methylene blue) composite modified electrode. RSC Adv. 2016, 6, 19982–19991. [Google Scholar] [CrossRef]
- Martin, C.; Grgicak, C. The effect of repeated activation on screen-printed carbon electrode cards. J. Electrochem. Soc. 2014, 61, 1–8. [Google Scholar] [CrossRef]
- Nagles, E.; García-Beltrán, O.; Calderón, J.A. Evaluation of the usefulness of a novel electrochemical sensor in detecting uric acid and dopamine in the presence of ascorbic acid using a screen-printed carbon electrode modified with single walled carbon nanotubes and ionic liquids. Electrochim. Acta 2017, 258, 512–523. [Google Scholar] [CrossRef]
- Yang, C.H.; Chen, C.W.; Lin, Y.K.; Yeh, Y.C.; Hsu, C.C.; Fan, Y.J.; Yu, I.S.; Chen, J.Z. Atmospheric-pressure plasma jet processed carbon-based electrochemical sensor integrated with a 3D-printed microfluidic channel. J. Electrochem. Soc. 2017, 164, B534–B541. [Google Scholar] [CrossRef]
- Du, C.X.; Han, L.; Dong, S.L.; Li, L.H.; Wei, Y. A novel procedure for fabricating flexible screen-printed electrodes with improved electrochemical performance. Iop Conf. Ser. Mater. Sci. Eng. 2016, 137, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wang, T.; Li, D.; Xi, F.; Wang, J.; Wang, E. Functionalization of monolithic and porous three-dimensional graphene by one-step chitosan electrodeposition for enzymatic biosensor. ACS Appl. Mater. Interfaces 2014, 6, 19997–20002. [Google Scholar] [CrossRef] [PubMed]
- Reza, K.K.; Ali, M.A.; Srivastava, S.; Agrawal, V.V.; Biradar, A.M. Tyrosinase conjugated reduced graphene oxide based biointerface for bisphenol a sensor. Biosens. Bioelectron. 2015, 74, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Deng, D.; Jin, J.; Lu, X.; Chen, J. Nanographene-based tyrosinase biosensor for rapid detection of bisphenol a. Biosens. Bioelectron. 2012, 35, 193–199. [Google Scholar] [CrossRef]
- Dutta, G.; Tan, C.; Siddiqui, S.; Arumugam, P.U. Enabling long term monitoring of dopamine using dimensionally stable ultrananocrystalline diamond microelectrodes. Mater. Res. Express 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Rani, V.S.V.; Arun, K.; Dhanasekaran, T.; Narayanan, V.; Jesudurai, D. Amperometric nanomolar detection of dopamine using metal free carbon nanotubes synthesized by a simple chemical approach. Mater. Res. Express 2018, 5, 9. [Google Scholar]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Quinson, J.; Hidalgo, R.; Ash, P.A.; Dillon, F.; Grobert, N.; Vincent, K.A. Comparison of carbon materials as electrodes for enzyme electrocatalysis: Hydrogenase as a case study. Faraday Discuss. 2014, 172, 473–496. [Google Scholar] [CrossRef]
- Alarcon-Angeles, G.; Guix, M.; Silva, W.C.; Ramirez-Silva, M.T.; Palomar-Pardave, M.; Romero-Romo, M.; Merkoci, A. Enzyme entrapment by beta-cyclodextrin electropolymerization onto a carbon nanotubes-modified screen-printed electrode. Biosens. Bioelectron. 2010, 26, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Zejli, H.; Hidalgo-Hidalgo de Cisneros, J.L.; Naranjo-Rodriguez, I.; Liu, B.; Temsamani, K.R.; Marty, J.L. Phenol biosensor based on sonogel-carbon transducer with tyrosinase alumina sol-gel immobilization. Anal. Chim. Acta 2008, 612, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Dincer, A.; Becerik, S.; Aydemir, T. Immobilization of tyrosinase on chitosan-clay composite beads. Int. J. Biol. Macromol. 2012, 50, 815–820. [Google Scholar] [CrossRef]
- Ragusa, A.; Priore, P.; Giudetti, A.M.; Ciccarella, G.; Gaballo, A. Neuroprotective investigation of chitosan nanoparticles for dopamine delivery. Appl. Sci. 2018, 8, 474. [Google Scholar] [CrossRef]
- Justin, R.; Chen, B. Strong and conductive chitosan–reduced graphene oxide nanocomposites for transdermal drug delivery. J. Mater. Chem. B 2014, 2, 3759–3770. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.-M. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef]
- Zhou, T.; Qi, X.; Bai, H.; Fu, Q. The different effect of reduced graphene oxide and graphene oxide on the performance of chitosan by using homogenous fillers. RSC Adv. 2016, 6, 34153–34158. [Google Scholar] [CrossRef]
- Liu, B.; Lian, H.T.; Yin, J.F.; Sun, X.Y. Dopamine molecularly imprinted electrochemical sensor based on graphene–chitosan composite. Electrochim. Acta 2012, 75, 108–114. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. New approach for porous chitosan-graphene matrix preparation through enhanced amidation for synergic detection of dopamine and uric acid. ACS Omega 2017, 2, 3043–3054. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Appliction of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Parlak, O.; Turner, A.P.; Tiwari, A. On/off-switchable zipper-like bioelectronics on a graphene interface. Adv. Mater. 2014, 26, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Ferapontova, E.E. Electrochemical analysis of dopamine: Perspectives of specific in vivo detection. Electrochim. Acta 2017, 245, 664–671. [Google Scholar] [CrossRef]
- Reza, K.K.; Ali, M.A.; Singh, M.K.; Agrawal, V.V.; Biradar, A.M. Amperometric enzymatic determination of bisphenol a using an ito electrode modified with reduced graphene oxide and mn3o4 nanoparticles in a chitosan matrix. Microchim. Acta 2017, 184, 1809–1816. [Google Scholar] [CrossRef]
| Sample Used | Added (μM) | Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Urine sample | 8 | 7.81 | 0.831 | 97.6 |
| 2 | 1.91 | 0.897 | 95.7 | |
| 0.4 | 0.37 | 0.424 | 93.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-Y.; Chou, Y.-C.; Tsai, J.-H.; Huang, T.-M.; Chen, J.-Z.; Yeh, Y.-C. Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine. Appl. Sci. 2019, 9, 622. https://doi.org/10.3390/app9040622
Liu C-Y, Chou Y-C, Tsai J-H, Huang T-M, Chen J-Z, Yeh Y-C. Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine. Applied Sciences. 2019; 9(4):622. https://doi.org/10.3390/app9040622
Chicago/Turabian StyleLiu, Cheng-You, Yi-Chieh Chou, Jui-Hsuan Tsai, Tzu-Ming Huang, Jian-Zhang Chen, and Yi-Chun Yeh. 2019. "Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine" Applied Sciences 9, no. 4: 622. https://doi.org/10.3390/app9040622
APA StyleLiu, C.-Y., Chou, Y.-C., Tsai, J.-H., Huang, T.-M., Chen, J.-Z., & Yeh, Y.-C. (2019). Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine. Applied Sciences, 9(4), 622. https://doi.org/10.3390/app9040622