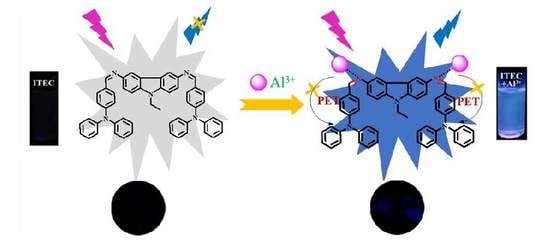
A New “Turn-On” Fluorescence Probe for Al3+ Detection and Application Exploring in Living Cell and Real Samples
Abstract
1. Introduction
2. Experimental
2.1. Materials and Instrumentation
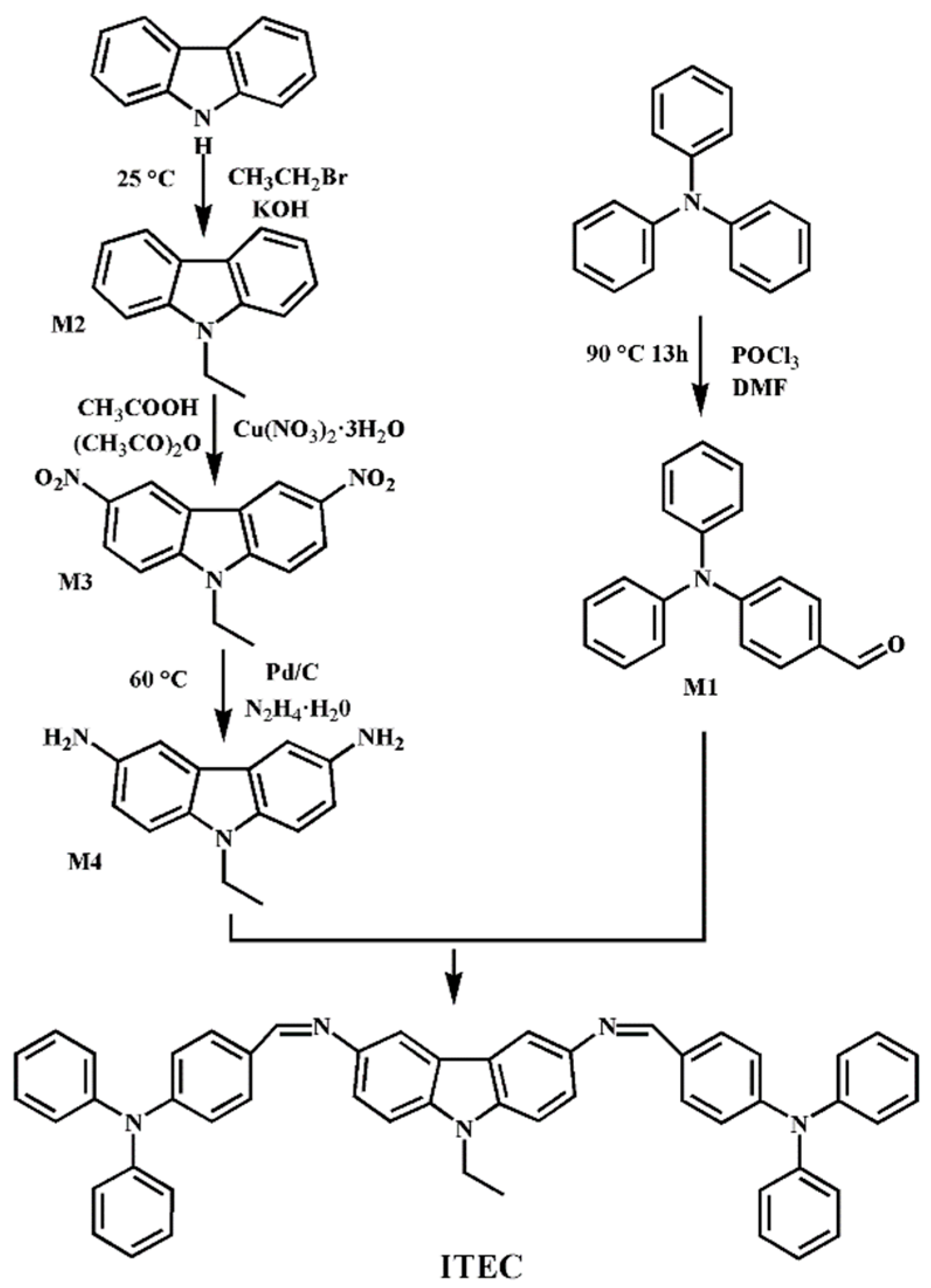
2.2. Synthesis of ITEC
2.3. The Synthesis Procedure of ITEC
2.4. Absorption and Fluorescence Studies
2.5. General Processes for Cell Culture and Fluorescence Imaging
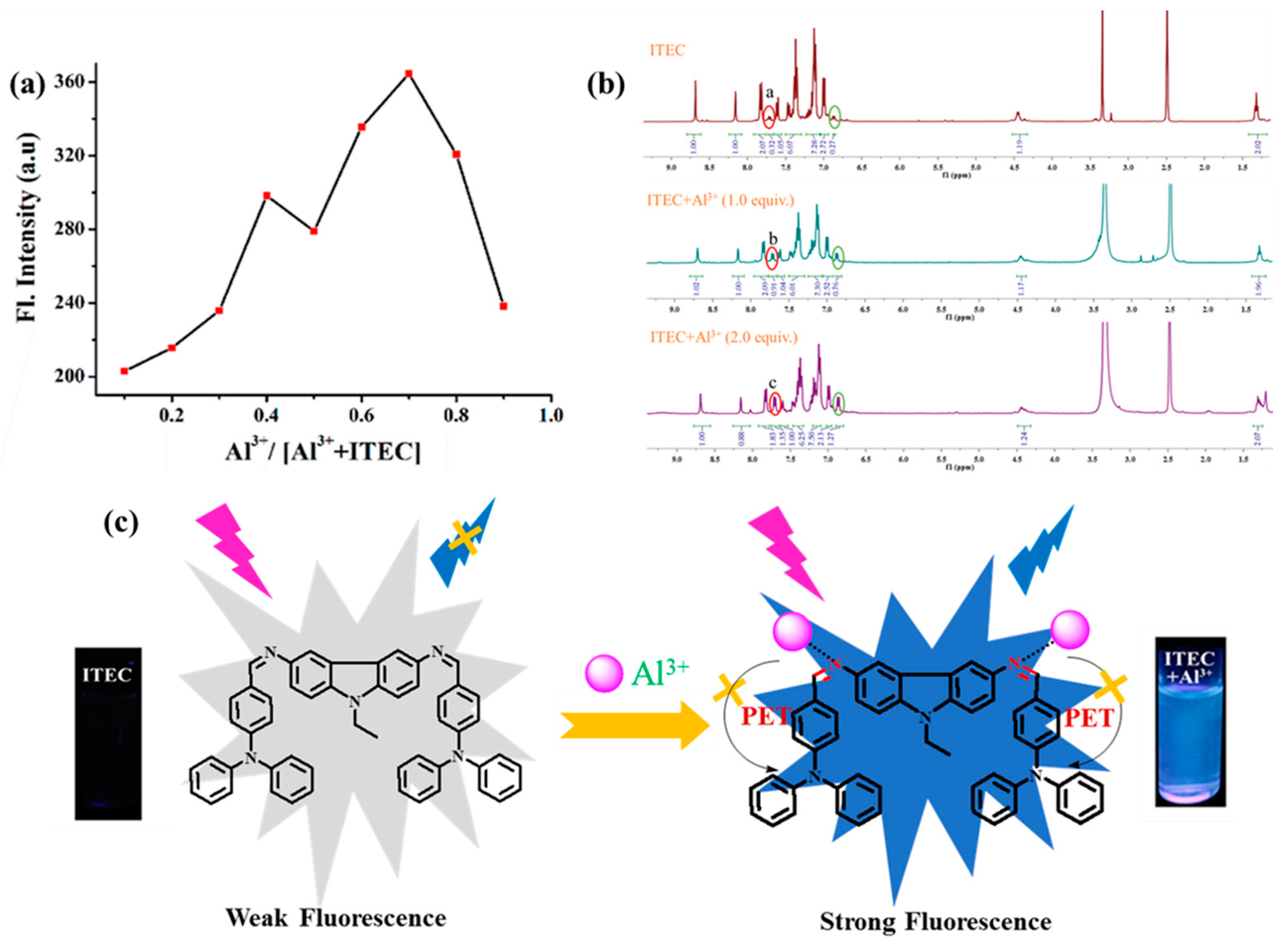
3. Results and Discussion
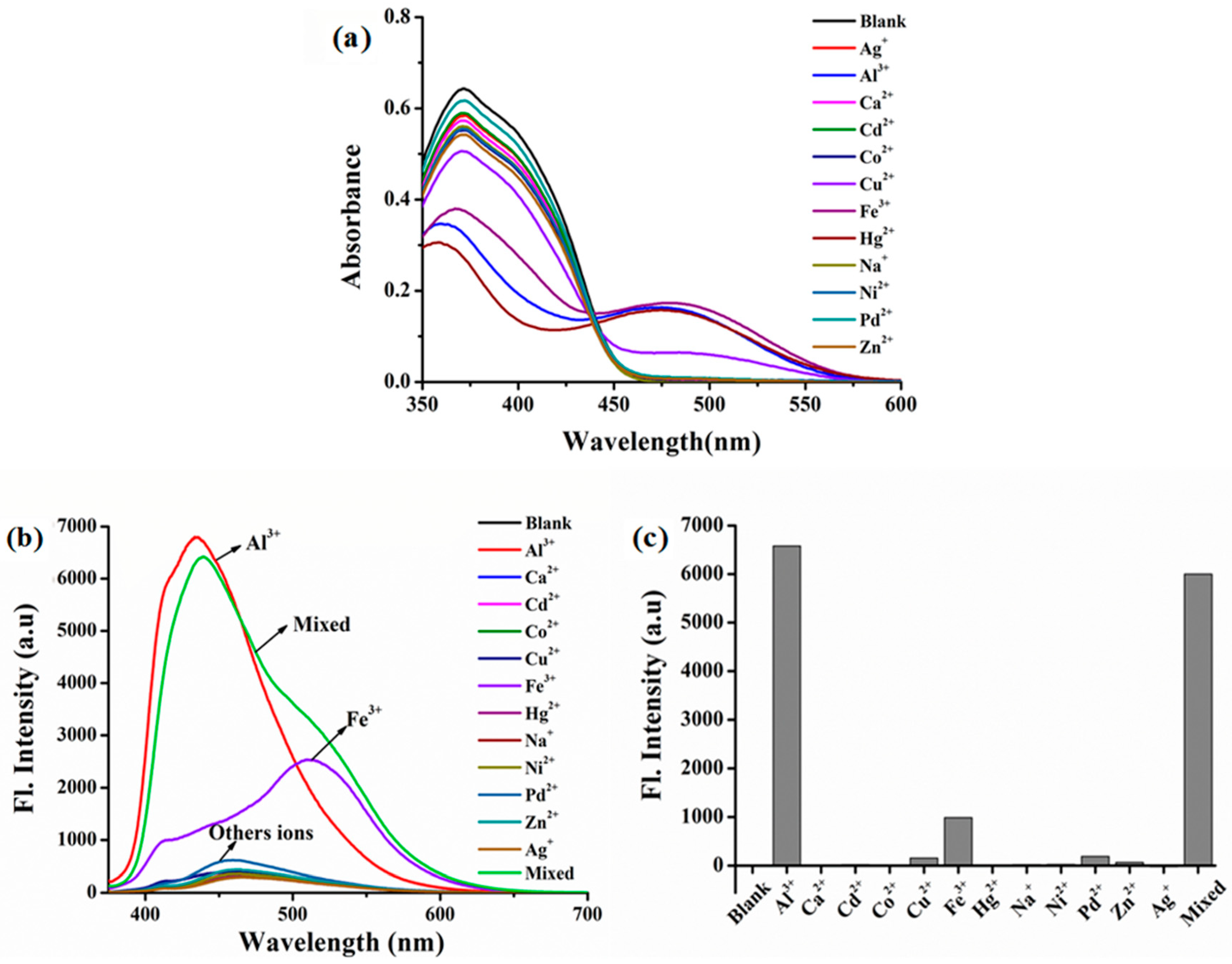
3.1. The Colorimetric Detection of ITEC to Al3+ by Naked Eyes
3.2. Selectivity of ITEC for Metal Ions
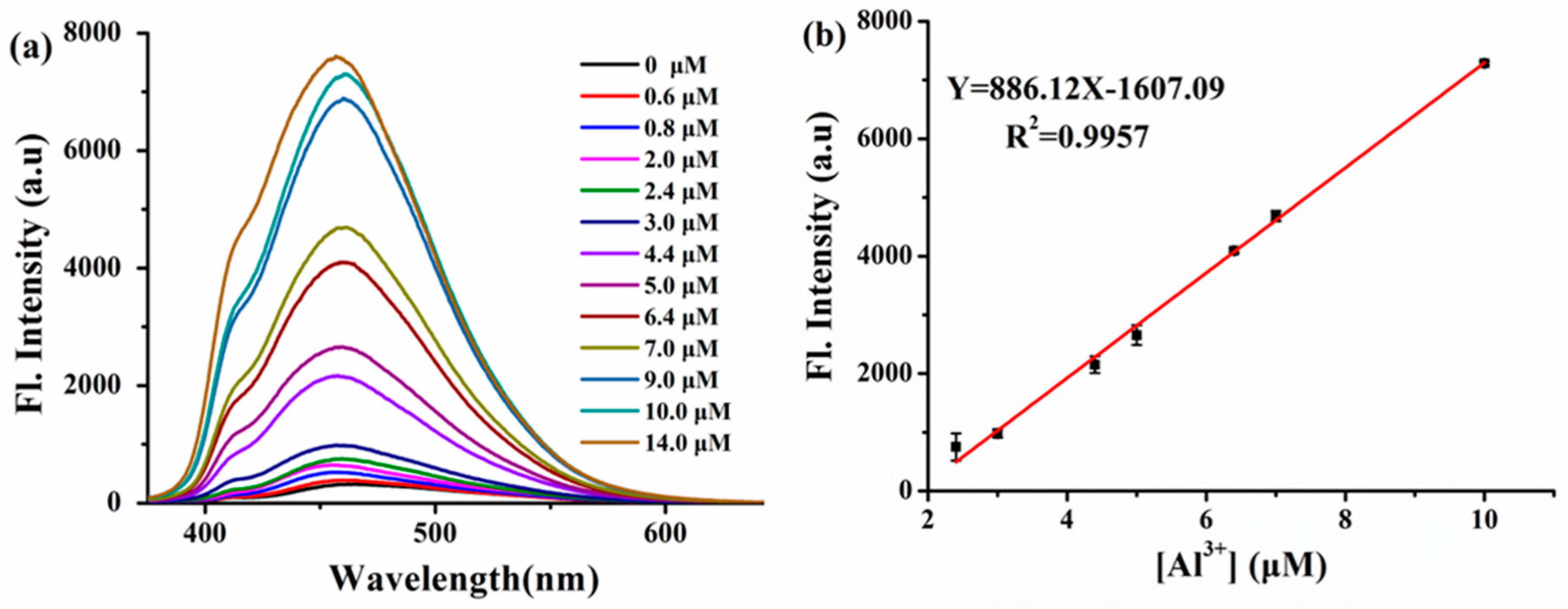
3.3. Sensitivity of ITEC to Al3+
3.4. The Interaction Mechanism of ITEC-Al3+
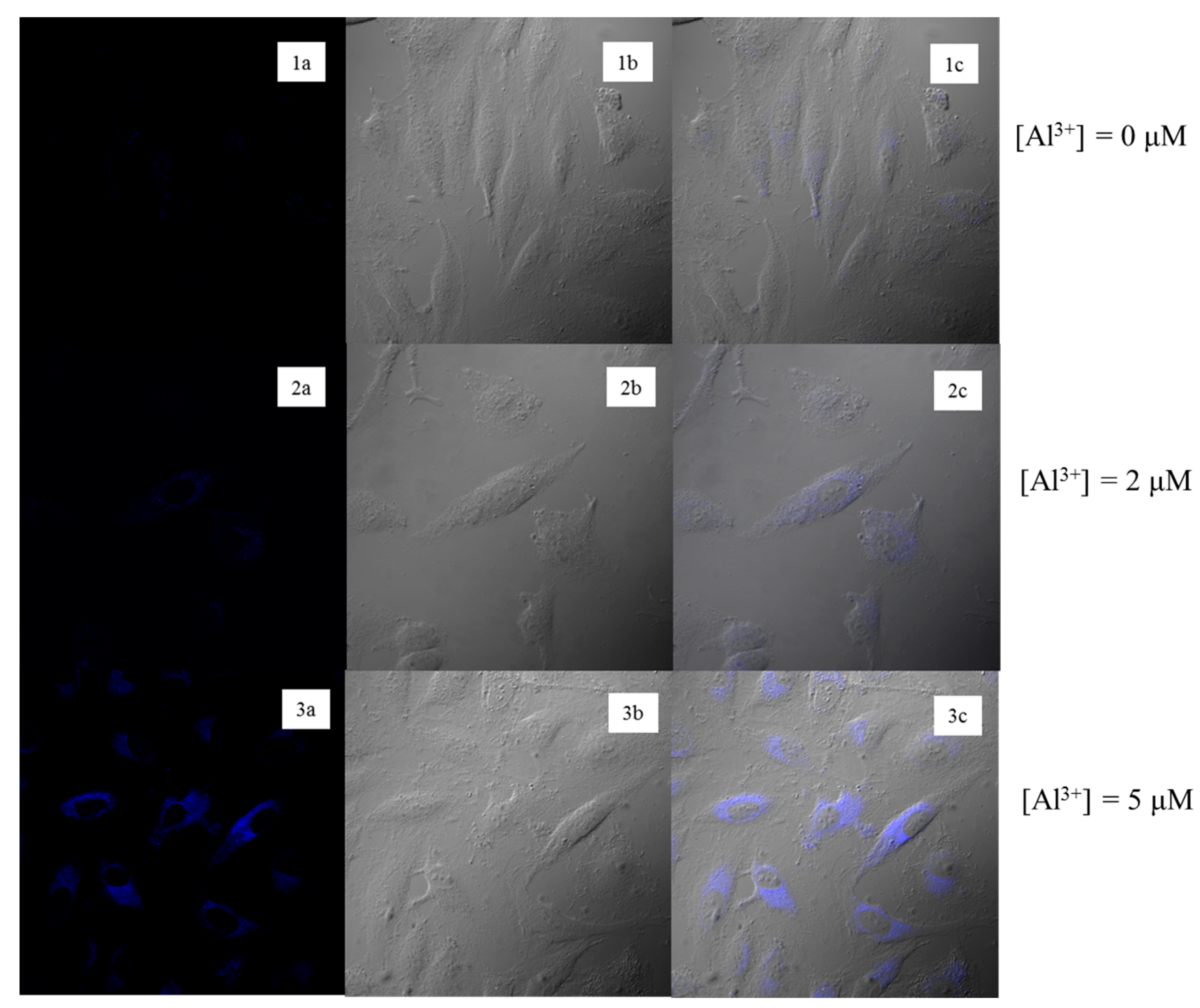
3.5. Fluorescence Imaging in Cells
3.6. The Test of the ITEC in Real Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.; Rao, B.A.; Jeong, J.W.; Mallick, S.; Kang, S.M.; Choi, J.S.; Lee, C.S.; Son, Y.A. A highly selective dual-channel Cu2+ and Al3+ chemodosimeter in aqueous systems: Sensing in living cells and microfluidic flows. Sens. Actuators B Chem. 2015, 210, 173–182. [Google Scholar] [CrossRef]
- Anand, T.; Sivaraman, G.; Mahesh, A.; Chellappa, D. Aminoquinoline based highly sensitive fluorescent sensor for lead(II) and aluminum(III) and its application in live cell imaging. Anal. Chim. Acta 2015, 853, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, R.; Hrishikesan, E.; Kannan, P. A selective colorimetric and fluorescent sensor for Al3+ ion and its application to cellular imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 140, 509–515. [Google Scholar] [PubMed]
- Dey, S.; Halder, S.; Mukherjee, A.; Ghosh, K.; Roy, P. Development of highly selective chemosensor for Al3+: Effect of substituent and biological application. Sens. Actuators B Chem. 2015, 215, 196–205. [Google Scholar] [CrossRef]
- Xie, J.Y.; Li, C.Y.; Li, Y.F.; Fu, Y.J.; Nie, S.X.; Tan, H.Y. A near-infrared chemosensor for determination of trivalent aluminum ions in living cells and tissues. Dyes Pigments 2017, 136, 817–824. [Google Scholar] [CrossRef]
- Asimov, I. The elementary composition of the earth. J. Chem. Educ. 1954, 33, 67. [Google Scholar] [CrossRef]
- Soni, M.G.; White, S.M.; Flamm, W.G.; Burdock, G.A. Safety Evaluation of Dietary Aluminum. Regul. Toxicol. Pharmacol. 2001, 33, 66–79. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Comparative efficacy and toxicity of desferrioxamine, deferiprone and other iron and aluminium chelating drugs. Toxicol. Lett. 1995, 80, 1–18. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Ding, Z.; Wang, X. Effects of advanced oxidation pretreatment on residual aluminum control in high humic acid water purification. J. Environ. Sci. 2011, 23, 1079–1085. [Google Scholar] [CrossRef]
- Liu, G.; Bangs, C.E.; Müller, D.B. Unearthing potentials for decarbonizing the U.S. aluminum cycle. Environ. Sci. Technol. 2011, 45, 9515. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R. Aluminum Toxicity and Tolerance in Plants. Plant Physiol. 1995, 107, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, O.; Yoshida, H. Effective recovery of harmful metal ions from squid wastes using subcritical and supercritical water treatments. Environ. Sci. Technol. 2005, 39, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Aimo, L.; Oteiza, P.I. Aluminium and lead: Molecular mechanisms of brain toxicity. Arch. Toxicol. 2008, 82, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Good, P.F.; Olanow, C.W.; Perl, D.P. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson’s disease: A LAMMA study. Brain Res. 1992, 593, 343–346. [Google Scholar] [CrossRef]
- Flaten, T.P. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res. Bull. 2001, 55, 187–196. [Google Scholar] [CrossRef]
- Shangguan, J.; Huang, J.; He, D.; He, X.; Wang, K.; Ye, R.; Yang, X.; Qing, T.; Tang, J. Highly Fe3+-selective fluorescent nanoprobe based on ultrabright N/P codoped carbon dots and its application in biological samples. Anal. Chem. 2017, 89, 7477–7484. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Mao, S.; Shi, X.; Wang, F.; Xu, Y.; Aderinto, S.O.; Wu, H. Characterization of a highly Al3+ selective fluorescence probe based on naphthalimide-Schiff base and its application to practical water samples. Luminescence 2018, 33, 54–63. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, X.J.; Zhu, Y.Y.; Zhou, B.J.; Zang, S.Q.; Tang, M.S.; Zhang, H.Y.; Mak, T.C. A new fluorescent probe for Al(3+) based on rhodamine 6G and its application to bioimaging. Dalton Trans. 2014, 43, 12624–12632. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, A.K.; Maheshwari, G. Aluminum(III) selective potentiometric sensor based on morin in poly(vinyl chloride) matrix. Talanta 2007, 72, 1469–1473. [Google Scholar] [CrossRef]
- Mergu, N.; Kumar Singh, A.; Kumar Gupta, V. Highly sensitive and selective colorimetric and off on fluorescent reversible chemosensors for Al3+ based on the rhodamine fluorophore. Sensors 2015, 15, 9097–9111. [Google Scholar] [CrossRef]
- Cassella, R.J.; Magalhaes, O.I.; Couto, M.T.; Lima, E.L.S.; Neves, M.A.F.; Coutinho, F.M.B. Synthesis and application of a functionalized resin for flow injection/F AAS copper determination in waters. Talanta 2005, 67, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Dong, Y.M.; Ma, T.H.; Wang, Y.W.; Peng, Y. A highly selective fluorescence-enhanced chemosensor for Al3+ in aqueous solution based on a hybrid ligand from BINOL scaffold and β-amino alcohol. Inorg. Chim. Acta 2012, 381, 137–142. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, A.K.; Kumawat, L.K. Thiazole Schiff base turn-on fluorescent chemosensor for Al3+ ion. Sens. Actuators B Chem. 2014, 195, 98–108. [Google Scholar] [CrossRef]
- Tian, J.; Yan, X.; Yang, H.; Tian, F. A novel turn-on Schiff-base fluorescent sensor for Aluminum (III) ions in living cells. RSC Adv. 2015, 5, 107012–107019. [Google Scholar] [CrossRef]
- Yong, S.K.; Lee, J.J.; Ye, W.C.; You, G.R.; Nguyen, L.T.; Noh, I.; Kim, C. Simultaneous bioimaging recognition of cation Al3+ and anion F- by a fluorogenic method. Dyes Pigments 2016, 129, 43–53. [Google Scholar]
- Azadbakht, R.; Almasi, T.; Keypour, H.; Rezaeivala, M. A new asymmetric Schiff base system as fluorescent chemosensor for Al3+ ion. Inorg. Chem. Commun. 2013, 33, 63–67. [Google Scholar] [CrossRef]
- Maity, S.B.; Bharadwaj, P.K. A Chemosensor Built with Rhodamine Derivatives Appended to an Aromatic Platform via 1,2,3-Triazoles: Dual Detection of Aluminum(III) and Fluoride/Acetate Ions. Inorg. Chem. 2013, 52, 1161–1163. [Google Scholar] [CrossRef]
- Boonkitpatarakul, K.; Wang, J.; Niamnont, N.; Liu, B.; Mcdonald, L.; Pang, Y.; Sukwattanasinitt, M. Novel Turn-On Fluorescent Sensors with Mega Stokes Shifts for Dual Detection of Al3+ and Zn2+. ACS Sens. 2015. [Google Scholar] [CrossRef]
- Samanta, S.; Nath, B.; Baruah, J.B. Hydrolytically stable Schiff base as highly sensitive aluminium sensor. Inorg. Chem. Commun. 2012, 22, 98–100. [Google Scholar] [CrossRef]
- Oliveira, E.; Santos, H.M.; Capelo, J.L.; Lodeiro, C. New emissive dopamine derivatives as fluorescent chemosensors for metal ions: A CHEF effect for Al(III) interaction. Inorg. Chim. Acta 2012, 381, 203–211. [Google Scholar] [CrossRef]
- Sen, B.; Pal, S.; Banerjee, S.; Lohar, S.; Chattopadhyay, P. Al3+-Ion-Triggered Conformational Isomerization of a Rhodamine B Derivative Evidenced by a Fluorescence Signal—A Crystallographic Proof. Eur. J. Inorg. Chem. 2015, 2015, 1383–1389. [Google Scholar] [CrossRef]
- Kaya, S.; Koyuncu, S. Synthesis and characterization of imine-coupled polyphenols containing carbazole units. J. Appl. Poly. Sci. 2009, 113, 1975–1985. [Google Scholar] [CrossRef]
- Monçalves, M.; Rampon, D.D.S.; Schneider, P.H.; Rodembusch, F.S.; Silveira, C.D.C. Divinyl sulfides/sulfones-based D-π-A-π-D dyes as efficient non-aromatic bridges for π-conjugated compounds. Dyes Pigments 2014, 102, 71–78. [Google Scholar] [CrossRef]
- Han, T.; Feng, X.; Tong, B.; Shi, J.; Chen, L.; Zhi, J.; Dong, Y. A novel “turn-on” fluorescent chemosensor for the selective detection of Al3+ based on aggregation-induced emission. Chem. Commun. 2012, 48, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Zhu, R.; Ren, Y.; Dong, J.; Feng, L. A “turn-on” fluorescent chemosensor for aluminum ion and cell imaging application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Sun, C.; Chen, C.; Cui, X.; Li, W. Research of a highly selective fluorescent chemosensor for aluminum(III) ions based on photoinduced electron transfer. J. Mol. Struct. 2015, 1079, 315–320. [Google Scholar] [CrossRef]
- Orlovskii, Y.V.; Popov, A.V.; Orlovskaya, E.O.; Vanetsev, A.S.; Vagapova, E.A.; Rähn, M.; Sammelselg, V.; Sildos, I.; Baranchikov, A.E.; Grachev, P.V. Comparison of concentration dependence of relative fluorescence quantum yield and brightness in first biological window of wavelengths for aqueous colloidal solutions of Nd3+: LaF3 and Nd3+: Ky3 F10 nanocrystals synthesized by microwave-hydrothermal. J. Alloys Compd. 2018. [Google Scholar] [CrossRef]
- Li, C.R.; Qin, J.C.; Wang, B.D.; Fan, L.; Yan, J.; Yang, Z.Y. A Chromone-Derived Schiff-Base Ligand as Al3+ “Turn on” Fluorescent Sensor: Synthesis and Spectroscopic Properties. J. Fluoresc. 2016, 26, 345–353. [Google Scholar] [CrossRef]
- Peng, H.; Shen, K.; Mao, S.; Shi, X.; Xu, Y.; Aderinto, S.O.; Wu, H. A Highly Selective and Sensitive Fluorescent Turn-on Probe for Al(3+) Based on Naphthalimide Schiff Base. J. Fluoresc. 2017, 27, 1191–1200. [Google Scholar] [CrossRef]
- Qin, J.C.; Yang, Z.Y. Bis-schiff base as a donor-acceptor fluorescent probe: Recognition of Al3+ ions in near 100% aqueous solution. J. Photoch. Photobiol. A 2015, 303–304, 99–104. [Google Scholar] [CrossRef]
- Shoora, S.K.; Jain, A.K.; Gupta, V.K. A simple schiff base based novel optical probe for aluminium (iii) ions. Sens. Actuators B Chem. 2015, 216, 86–104. [Google Scholar] [CrossRef]
- Wang, G.; Qin, J.; Li, C.; Yang, Z. A highly selective fluorescent probe for Al3+ based on quinoline derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liu, Y.T.; Li, N.N.; Dang, Q.X.; Xing, Z.Y.; Li, J.L.; Zhang, Y. A schiff-base receptor based naphthalimide derivative: Highly selective and colorimetric fluorescent turn-on sensor for Al3+. J. Luminesc. 2017, 186, 48–52. [Google Scholar] [CrossRef]
- Mu, X.; Li, Q.; Qiao, J.; Ma, H. One-pot synthesis of tyrosine-stabilized fluorescent gold nanoclusters and their application as turn-on sensors for Al3+ ions and turn-off sensors for Fe3+ ions. Anal. Methods 2014, 6, 6445–6451. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yan, B. A novel covalent post-synthetically modified mof hybrid as a sensitive and selective fluorescent probe for Al3+ detection in aqueous media. Dalton Trans. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.Y.; Li, C.; Song, Y.Y.; Tan, F.; Cao, Y.C.; Zhao, Y.D. Nitrogen-doped graphene quantum dot for direct fluorescence detection of Al3+ in aqueous media and living cells. Biosens. Bioelectron. 2017, 100, S0956566317305894. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-L.; Zhang, X.; Yao, J.-S.; Zhang, J.-J.; Ding, B.-Y. Fluorescence and fluorescence imaging of two Schiff derivatives sensitive to Fe3+ induced by single- and two-photon excitation. Sens. Actuators B Chem. 2013, 176, 488–496. [Google Scholar] [CrossRef]
- Li, C.R.; Qin, J.C.; Wang, G.Q.; Wang, B.D.; Fu, A.K.; Yang, Z.Y. Development of a simple pyrazine-derived “turn on” Al3+ fluorescent sensor with high selectivity and sensitivity. Inorg. Chim. Acta 2015, 430, 91–95. [Google Scholar] [CrossRef]
- Lashgari, N.; Badiei, A.; Ziarani, G.M. A Fluorescent Sensor for Al(III) and Colorimetric Sensor for Fe(III) and Fe(II) Based on a Novel 8-Hydroxyquinoline Derivative. J. Fluoresc. 2016, 26, 1885–1894. [Google Scholar] [CrossRef]


| Structure of Probes | Detection Limit (mol/L) | Modes (probe:Al3+) | Application | Ref. |
|---|---|---|---|---|
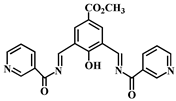 | 1.0 × 10−7 | 1:1 | - | [40] |
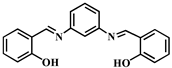 | 4.79 × 10−8 | 1:1 | - | [41] |
 | 1.0 × 10−7 | 1:1 | - | [42] |
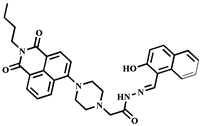 | 7.4 × 10−9 | 1:1 | Real samples | [43] |
| Tyrosine-stabilized fluorescent gold nanoclusters | 3.0 × 10−7 | - | Real samples | [44] |
| Salicylaldehyde modified MOF (UiO-66-NH2-SA) | 6.98 × 10−6 | - | - | [45] |
| Nitrogen-doped graphene quantum dot | 1.3 × 10−6 | 1:1 | Cell imaging | [46] |
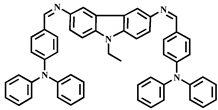 | 7.4 × 10−9 | 1:2 | Cell imaging | This work |
| Sample | Added (μmol/L) | Measured (μmol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| River water 1 | 3 | 2.99 | 99.7 | 0.40 |
| River water 2 | 5 | 5.27 | 105.4 | 0.48 |
| River water 3 | 10 | 10.3 | 103.3 | 0.60 |
| Tap water 1 | 3 | 3.17 | 105.5 | 0.50 |
| Tap water 2 | 5 | 5.25 | 104.9 | 0.12 |
| Tap water 3 | 10 | 9.76 | 97.6 | 0.36 |
| Bottle water 1 | 3 | 2.99 | 99.8 | 0.88 |
| Bottle water 2 | 5 | 5.46 | 109.3 | 0.23 |
| Bottle water 3 | 10 | 9.88 | 98.8 | 0.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.-Y.; Zhang, C.-J.; Zhang, X.; Xing, S.; Yao, J.-S.; Qiao, C.-D.; Liu, W.-L. A New “Turn-On” Fluorescence Probe for Al3+ Detection and Application Exploring in Living Cell and Real Samples. Appl. Sci. 2019, 9, 577. https://doi.org/10.3390/app9030577
Gao Z-Y, Zhang C-J, Zhang X, Xing S, Yao J-S, Qiao C-D, Liu W-L. A New “Turn-On” Fluorescence Probe for Al3+ Detection and Application Exploring in Living Cell and Real Samples. Applied Sciences. 2019; 9(3):577. https://doi.org/10.3390/app9030577
Chicago/Turabian StyleGao, Zhi-Yan, Cui-Jiao Zhang, Xian Zhang, Shu Xing, Jin-Shui Yao, Cong-De Qiao, and Wei-Liang Liu. 2019. "A New “Turn-On” Fluorescence Probe for Al3+ Detection and Application Exploring in Living Cell and Real Samples" Applied Sciences 9, no. 3: 577. https://doi.org/10.3390/app9030577
APA StyleGao, Z.-Y., Zhang, C.-J., Zhang, X., Xing, S., Yao, J.-S., Qiao, C.-D., & Liu, W.-L. (2019). A New “Turn-On” Fluorescence Probe for Al3+ Detection and Application Exploring in Living Cell and Real Samples. Applied Sciences, 9(3), 577. https://doi.org/10.3390/app9030577