1. Introduction
Since industrial revolution, the massive utilization of fossil fuel has led to a significant increase of carbon dioxide in atmosphere and expedited climate change. Therefore, controlling and suppressing the emission of greenhouse gas are crucial issues for the development of a sustainable environment. Coal is still the most abundant source in traditional fossil fuels, and it is expected to be utilized for more than 110 years [
1]. Due to its low cost and stable supply, the USA and other industrial countries have proposed the concept of Clean Coal Technology (CCT). Coal gasification is an important means of CCT that can not only convert clean energy from conventional coal resources, but also produce various fuels and chemicals. Popular coal-derived fuels include gaseous fuels such as CO, H
2, synthetic (or substitute) natural gas (SNG), etc., and liquid fuels such as diesel, jet fuels, and methanol. The coal-derived chemicals, in turn, are as broad as petroleum-derived chemicals. The most popular chemicals are ammonia, urea, dimethyl ether, ethylene, and propylene.
Among various options of CCT, Integrated Gasification Combined-Cycle (IGCC) is an environmentally friendly coal utilization technology for power generation, which has been gradually commercialized, and more advanced developments continue globally. With a multi-fuel feeding feature, IGCC can be employed to produce power more efficiently with less pollution than traditional coal-fired power plants. Combining with carbon capture and storage (CCS) technology, it will also be an essential option for base-loaded power and sustainable development. If integrated with hydrogen technologies, i.e., hydrogen turbine and fuel cell, the said combination can achieve the vision of zero emission.
There have been five major IGCC demo plants globally since mid-1990s, i.e., Wabash River and Tampa in USA, Buggenum (Nuon) in Netherlands, Puertollano (Elcogas) in Spain, and CCP in Japan. Abundant operating experience has been accumulated from these five demo plants. All the gasifiers used in these demo plants are entrained-bed gasifiers, and each has its individual pros and cons. Besides the air-blown methods used by MHI, Japan, the oxygen-blown method is employed for the rest of the four plants. The gasifiers of Wabash River (E-GasTM gasifier) and Tampa (GE gasifier) employed coal-slurry feeding, while those of Buggenum (Shell gasifier), Puertollano (Prenflo gasifier) and CCP (MHI gasifier) all used dry-fed option. The downdraft flow condition is only employed in the GE gasifier in Tampa. The E-GasTM and MHI gasifiers feed coal in two-stages, while the Shell, GE, and Prenflo use only one-stage feed.
Coal gasification is an extremely complex process. It will take many years of continuous efforts to achieve meaningful and practical results, and for continuous improvements afterwards. The Institute of Nuclear Energy Research (INER) in Taiwan has developed Computational Fluid Dynamics (CFD) models for years to simulate gasification process. Previous studies have successfully established the CFD capability to analyze the gasification process in an E-Gas
TM type entrained-flow gasifier to produce syngas for power generation [
2]. To further investigate the different products that can be produced by coal gasification, the gasification model needs to be continuously expanded and improved by incorporating more complex and detailed sub-models.
It has been reported that a newly introduced Entrained Slagging Transport Reactor (E-STR) gasifier can reduce capital and operating costs, while improve operability [
3]. E-STR is a product modified from the existing E-Gasifier design, and the configuration can be seen in Slide 6 of Ref. [
3]. The major modifications include increased operating pressure and reduced temperature, with the aim of achieving a higher H
2/CO ratio of syngas for SNG production. Due to the reduced operating temperature, catalyst is utilized to convert tar or heavy volatiles to lighter gases. The major modifications of E-STR include the following features: (a) the horizontal cylinder in the E-Gas
TM gasifier is removed, (b) the oxidant is entirely fed with only the recycled char at the 1st stage (thus no coal slurry), (c) the coal/slurry mixture is fed at the 2nd stage entirely, (d) the operating pressure is much higher, while temperature is lower than the counterparts in E-Gas
TM gasifier, and (e) catalytic material is needed for tar/volatiles cracking (since the second-stage temperature is not high enough to sufficiently crack the tar or heavy volatiles). The main goal of E-STR is to produce syngas with a higher H
2/CO ratio suitable for SNG production. Compared to the cross-type configuration of E-Gas
TM, the design of E-STR excludes the first-stage cylinder. Therefore, several critical issues under high-pressure operation can be simplified, such as welding, sealing, maintenance and operation cost. The savings can be employed to compensate the cost of the catalyst for the low-temperature tar cracking process.
Choosing the E-STR gasifier in this study is beneficial in many folds. In our previous studies [
2,
4], the E-Gas
TM gasifier has been investigated and results have been compared with the reports issued by the Department of Energy/National Energy Technology Laboratory (DOE/NETL). After the model validation, the effects of the 2nd stage injection mode as well as the feedstock type were investigated comprehensively [
4]. It is more productive to continue follow-up studies of the new product derived from E-Gas
TM gasifier. E-STR is a new concept that is still in the development stage. Investigating this new concept is strategically important to keep INER in a lead position when it comes to understanding and applying E-STR. Both E- Gas
TM gasifier and E-STR gasifier have certain advantages in producing SNG over other commercial gasifiers.
The objective of this study is to develop a numerical model and simulate the low-temperature and high-pressure E-STR introduced by the E-Gas group [
3]. The CFD gasification model developed in the previous study [
2] will be modified to incorporated a new tar condensation and re-vaporization sub-model to simulate the E-STR gasifier under high-pressure and relatively low-temperature conditions without implementing a tar-cracking catalyst. The result of this study will pave the road for developing once-through SNG production in the gasifier for the future.
2. Literature Review
A mathematical model was developed by Wen et al. in 1979 [
5], to investigate the influence of input operating parameters for the Texaco (Now, GE) down-draft entrainment pilot plant gasifier. Furthermore, the gasification process and its performance have been studied comprehensively by CFD modeling for several years [
4,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. Chen et al. [
18] developed a numerical model to investigate a 200-ton, two-stage air-blown entrained type gasifier for an IGCC process. Results showed that the turbulent fluctuations influenced the interim reactions during gasification process, and significantly affected the temperature and outlet gas composition. It was also found that the effect of coal particle size is not significant on the carbon conversion; rather, the reaction kinetics turn out to be more sensitive [
19].
The research team of the Energy Conversion and Conservation Center (ECCC) at the University of New Orleans [
20,
21,
22,
23,
24,
25] has developed numerical models for the investigation of gasification process since 2005. In their work, the geometry and input data were adopted from literature data, e.g., Bockelie et al. [
26] and Chen et al. [
18]; furthermore, various operating conditions were studied, and the results were documented systematically. The water-gas-shift (WGS) reaction tunes the ratio of H
2 to CO, and thus plays an important role in predicting syngas composition. It is confirmed [
20] that the WGS rate proposed by Jones and Lindstedt [
27] is too fast, since the proposed rate was evaluated with catalyst. Another attempt to calibrate the WGS rate by Lu and Wang [
28,
29] showed that all of the proposed WGS reaction rates published in the literature were too fast for commercial entrained-flow gasifiers, because no catalyst is added in a typical gasification reactor.
Chyou et al. [
4] studied the effect of the 2nd stage design and coal type on the performance of E-Gasifier. Results showed that dual-injector design can minimize the circulation zone and improve the gasification performance; moreover, gasification of Lignite leads to lower performance than the counterpart of Illinois #6 coal. More comprehensive literature surveys have been conducted in previous works [
2,
4], from which further information can be cited.
3. Materials and Methods
The numerical model is developed via the commercial code ANSYS FLUENT V.12.0, which is a finite-volume based solver for fluid flow, chemical reaction, and heat transfer. The function of the unstructured meshes is also supported for complex geometry. The employed discretization scheme is second-order. The pressure-velocity coupling is iteratively solved by the SIMPLE algorithm. Under the Eulerian–Lagrangian frame of reference, the three-dimensional, steady-state Navier–Stokes equations are solved. Injected coal particles are considered as discrete and secondary phase. The Discrete Phase Model (DPM) is included for the interaction of coal with the continuous phase. Calculated results of the discrete phase are updated into the continuous phase every fifty iterations. The effect of radiation is considered by the P1 model, and the gravitational force is included in the calculation. The turbulence flow is described by the standard k-ε model. The Finite-Rate/Eddy-Dissipation Model is implemented for solving chemical reactions, for which the slower rate is selected by comparing the finite rate and the eddy-dissipation rate.
3.1. Governing Equations
The mass conservation equation is:
The added mass due to the devolatilization of coal particle and vaporization of water droplet from secondary phase into the continuous phase is considered as the source term
Sm.
The momentum conservation equation is:
where
p is the static pressure,
is the stress tensor, and
and
are the gravitational body force and external body forces (e.g., that arise from interaction with the dispersed phase), respectively. The stress tensor
is given by
where
µ is the molecular viscosity,
I is the unit tensor. The effect of volume dilation is considered in the second term on the RHS.
The energy conservation equation is:
where
Sph is the source term for particle-gas heat transfer, evaporation energy (latent heat), the radiation energy, and reaction heat.
3.2. Turbulence Model
The standard
k-
ε transport equations for the turbulence kinetic energy,
k, and its rate of dissipation,
ε, are:
and
where
Gk is the generation of turbulence kinetic energy due to the mean velocity gradients.
σk and
σε are the turbulent Prandtl numbers for
k and
ε. The turbulent (or eddy) viscosity,
μt, is evaluated as:
where the empirical model constants are
C1ε = 1.44,
C2ε = 1.92,
Cμ = 0.09,
σk = 1.0,
σε = 1.3 [
30].
3.3. Discrete Phase
In the discrete phase, each particle is tracked by the Lagrangian approach. The force balance on the particles is described as:
where
FD(
u − up) is the drag force per unit particle mass:
where
u is the fluid phase velocity,
up is the particle velocity,
μ is the molecular viscosity of the fluid,
ρ is the fluid density,
ρp is the particle density, and
dp is the diameter of the particle.
Re is the relative Reynolds number calculated from the slip velocity between the fluid phase velocity and the particle velocity.
The particles are tracked by the mean and fluctuating velocity as:
where the fluctuating velocity in Equation (11) is defined by the stochastic discrete random walk model.
The burning rate of the char particles is adopted from the study of Smith [
31]. The particle surface reaction rate is calculated as:
where
Ap is the particle surface area (m
2),
Y is the mass fraction of surface species in the particle,
η is the effectiveness factor (dimensionless),
R is the rate of particle surface species reaction per unit area (kg/m
2/s),
pn is the bulk concentration of the gas phase species (kg/m
3),
D is the diffusion rate coefficient for the reaction,
Rkin is the kinetic rate of reaction (units vary), and
N is the apparent order of reaction. In this study, D is 5E-12 and N is 0.5. The kinetic rate of reaction
r is defined as:
where the
n (temperature exponent) is 1. The Rosin–Rammler distribution [
32] is employed for the simulation.
The water within the coal slurry is assumed to atomized and evaporated when injecting into the gasifier. The rate of vaporization is of the droplet is calculated as:
where
kc is the mass transfer coefficient and
Cs is the concentration of the vapor at the particle’s surface.
C∞ is the vapor concentration of the bulk flow. The empirical correlation by [
33,
34] is employed to evaluated the value of
kc as:
where
Sh is the Sherwood number,
Sc is the Schmidt number (defined as
ν/D),
D is the diffusion coefficient of vapor in the bulk flow.
Red is the Reynolds number,
D is the droplet diameter.
When the droplet temperature reaches the boiling point, the evaporation rate [
35] is evaluated as:
The convection, radiation, devolatilization, and surface reactions are included in the energy equation as:
where
mp and
Tp are the particle mass and particle temperature, respectively.
hfg is the latent heat,
Hreac is the heat release due to surface reaction, and
fh is the fraction of due to the heat absorption of particle.
θR is the radiating temperature, defined as:
and
G is the incident radiation, which is related to the radiative intensity as:
The Pi model [
36] is employed for the radiation in the gasifier. The empirical relation by Ranz and Marshall [
33] is introduced to evaluate the convection coefficient as:
3.4. Devolatilization
The two-competing-rates model by Kobayashi [
37] is employed for the devolatilization reaction as:
These two kinetic rates are used as a weight function as:
where
α1 and
α2 are yield factors,
fω is the mass fraction of moisture,
mp is the mass of particle, and
ma is the mass of ash. The value of the constants are
A1 = 2 × 10
5,
A2 = 1.3 × 10
7,
E1 = 1.046 × 10
8 J/kg mol, and
E2 = 1.67 × 10
8 J/kg mol.
The heterogeneous reactions on the particle surface occur when the volatiles diffuse out of the coal particle. Those particle surface reactions are described by the implicit relations of Smith et al. [
31] as:
where
ηr is the effectiveness factor,
Yk is the mass fraction,
D0,r is the bulk diffusion coefficient,
Nr is the apparent reaction order,
Ar is the pre exponential factor.
3.5. Chemical Reaction
The general form for the species conservation equations is:
where
Ri is the net rate of production of species
i by chemical reaction and
Si is the rate of mass creation by addition from the dispersed phase sources. In addition,
is the diffusion flux of species
i, which arises due to the gradients in concentration and temperature. The source term for species
i due to all reactions is computed as the sum of the Arrhenius reaction sources over the
NR reactions that the species participate in:
where
Mw,i is the molecular weight of species
i and
is the Arrhenius molar rate of production/consumption of species
i in reaction
r. For the homogeneous reaction, the slower one between the finite-rate and eddy-dissipation-rate is selected, while only the finite-rate is used for the heterogeneous reactions.
3.6. Condensation and Re-Vaporization of Tar
As mentioned above, the second-stage temperature of E-STR reactor will not be sufficiently high enough to crack the tar, and a catalytic material is needed for tar cracking. Without including the actual catalytic kinetics, this study intends to implement sub-models of liquid tar (condensation) and its re-vaporization into the existing gasification simulation model. Specifically, it considers low-temperature operating conditions and a success of implementation of these models can pave the road for future incorporation of catalytic kinetics, if the information of the proprietary catalyst is released.
In the tar condensing sub-model, a threshold condensation temperature is specified. When the local temperature is lower than the designated condensation temperature (assumed as 780 K), the gaseous volatiles will condense to liquid form (liquid tar) and release the energy of latent heat. On the other hand, when the temperature of liquid tar is higher than the specified re-vaporization temperature (assumed as 810 K), it will crack into three species (CO, H2, C2H2) and absorb energy for cracking reaction.
The tar condensation and re-vaporization sub-models are described by the UDF (User-Defined Function), and interpreted into the E-STR model during the iteration.
3.7. Finite-Rate/Eddy-Dissipation-Rate Model
3.7.1. Arrhenius rate
where
are the reactant and product stoichiometric coefficients, respectively,
are the rate exponents for reactant
j, in reaction
r,
n is the temperature exponent of reaction
r,
Er is the activation energy,
R is the universal gas constant,
Ar is the pre-exponential factor, and
Cj is the molar concentration of species
j.
3.7.2. Eddy-Dissipation Rate
where
YR and
YP are the mass fractions of reactant and produce species, respectively.
A is the Magnussen constant for reactants (4.0),
B is the Magnussen constant for products (0.5),
M is the molecular weight, and the
R and
P subscripts are the reactants and products. The global gasification reaction rates used in this study are listed in
Table 1 [
22].
6. Summary and Conclusions
As mentioned before, the newly introduced E-STR gasifier [
3] features low-temperature and high-pressure operating conditions, aiming to realize a higher H
2/CO ratio of syngas for SNG production. However, information about the design characteristics or operation data has been barely available in public domain at present; hence, establishing a computational model for revealing the flow/thermal/chemical insights of the new gasifier can be a useful tool. Due to its high-pressure and low-temperature operation condition, tars will form inside the E-STR gasifier. Thus, the objective of this study is to modify the previously developed CFD model of E-Gas
TM gasifier, and implement additional mechanisms to systematically mimic related design characteristics, and further implement volatiles condensation and re-vaporization sub models into the modified CFD model to properly address the phenomena inside E-STR. For high operating pressure condition, different fuel and oxidant feeding distributions are compared. The results are summarized below.
There are different flow and thermal patterns that are observed in the vicinity of the second-stage injector, due to unlike injection arrangements; however, with sufficiently large volume of the gasifiers and resident times, similar syngas compositions and outlet temperatures are achieved between E-Gas and E-STR without char recycling. Higher interior temperature is obtained in E-STR because all coal slurry is fed at the 2nd stage rather than being separately injected as in an E-Gas gasifier. This also leads to longer particle residence time at the second stage for E-STR.
Since detailed operation data have not yet been available, the recycling and reaction rates of char were investigated to search for reasonable operating characteristics, based on the chemical reactions built in the present CFD model. It seems that reasonable performance characteristics can be achieved in the case of 15% char recycle, which was then selected for subsequent studies.
Incorporating the volatiles condensation-only sub-model results in the formation of about 6.47% liquid volatiles and an increase of outlet temperature about 135 K, due to the release of latent heat. When the re-vaporization model is incorporated together with the condensation model, under the test condition, the liquid volatiles re-vaporize with an amount depending on the set point of evaporation temperature. It is thus concluded that the volatiles condensation and re-vaporization sub-models have been successfully developed and implemented. These sub-models will be useful under the condition that the gasifier temperature is intentionally kept low, like in the E-STR gasifer.
Attempts have been made to reduce the reactor temperature by changing the fuel feeding mass distribution between the first and second stages, as well as changing the oxygen/coal ratio. By introducing 20% coal-slurry at the 1st stage and feeding the recycled char at the 2nd stage, the temperature is reduced near the 1st injector but is increased at the 2nd stage. Under the studied condition, the present model can’t predict reduced temperature inside the E-STR gasifier as claimed by the manufacturer [
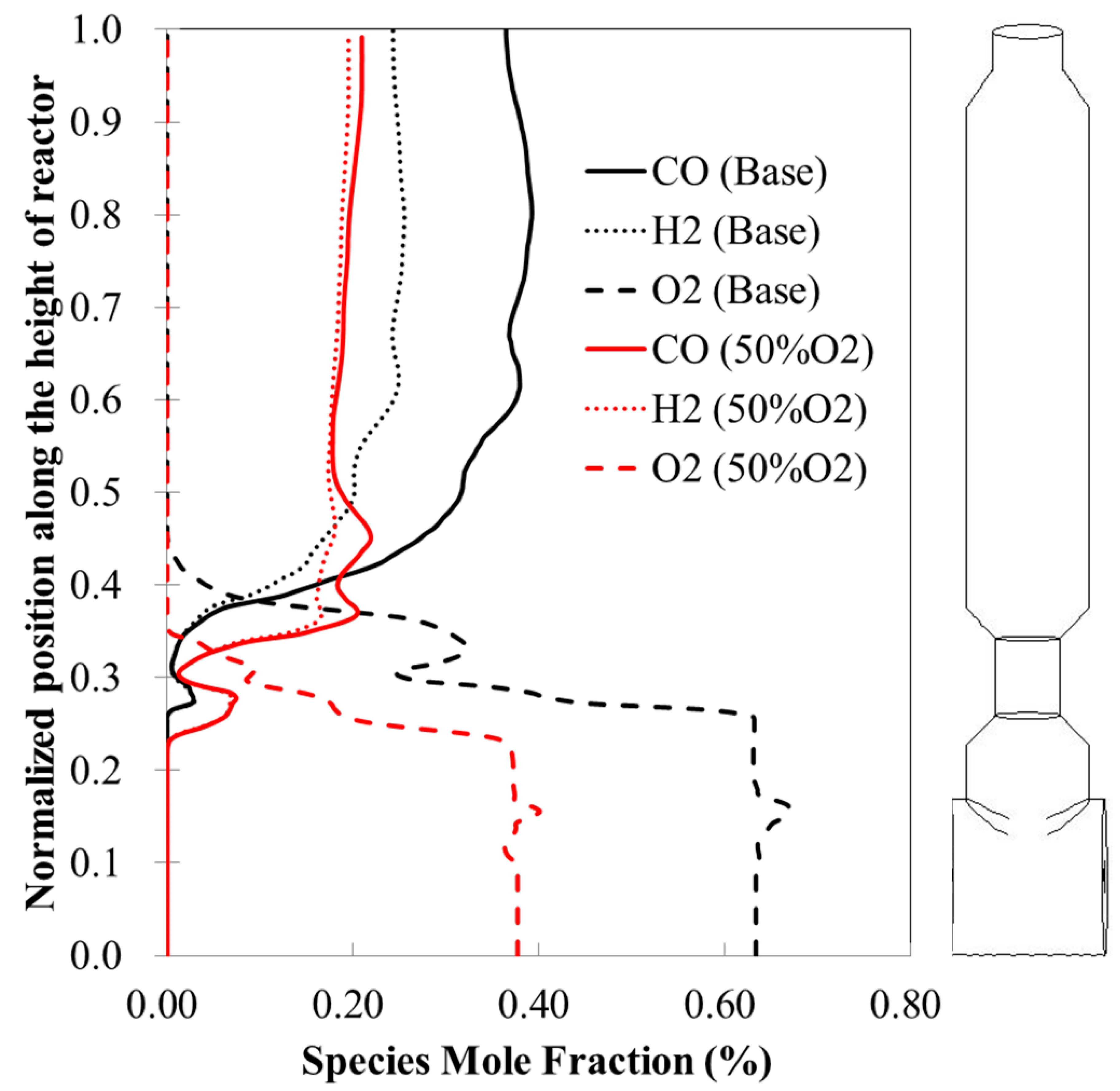
3]. By reducing the oxygen/coal ratio from 0.92 to 0.46 (i.e., with a 50% reduction), the temperature inside the gasifier increases near the 1st stage but reduces to a lower temperature level for the rest of the gasifier, with a reduction of exit temperature from 1463 K to 1100 K.
When the higher operational pressure is employed (70 atm), the temperature increases at the 2nd stage. The mole fractions of both H2 and CO in the exit syngas as well as HHV increase, but the outlet temperature maintains about the same.
In summary, it is shown that a CFD model has been modified from its precedent and implemented with the volatiles’ condensation and re-vaporization sub-models to study the gasification process in the newly developed E-STR gasifiers. The overall result indicates that operations with high pressure at 70 atm and low stoichiometric ratio lead to favorable higher H2/CO ratios suitable for producing synthetic natural gas (SNG). It is perceived that high char recycling rate and reduced O2/Coal ratio are effective to reduce the temperature at the 2nd stage of the gasifier, but this could compromise the gasification performance. Hence, more efforts should be devoted to optimizing the char recycling rate.