Heavy Metal Ion Detection Platforms Based on a Glutathione Probe: A Mini Review
Abstract
:Featured Application
Abstract
1. Introduction
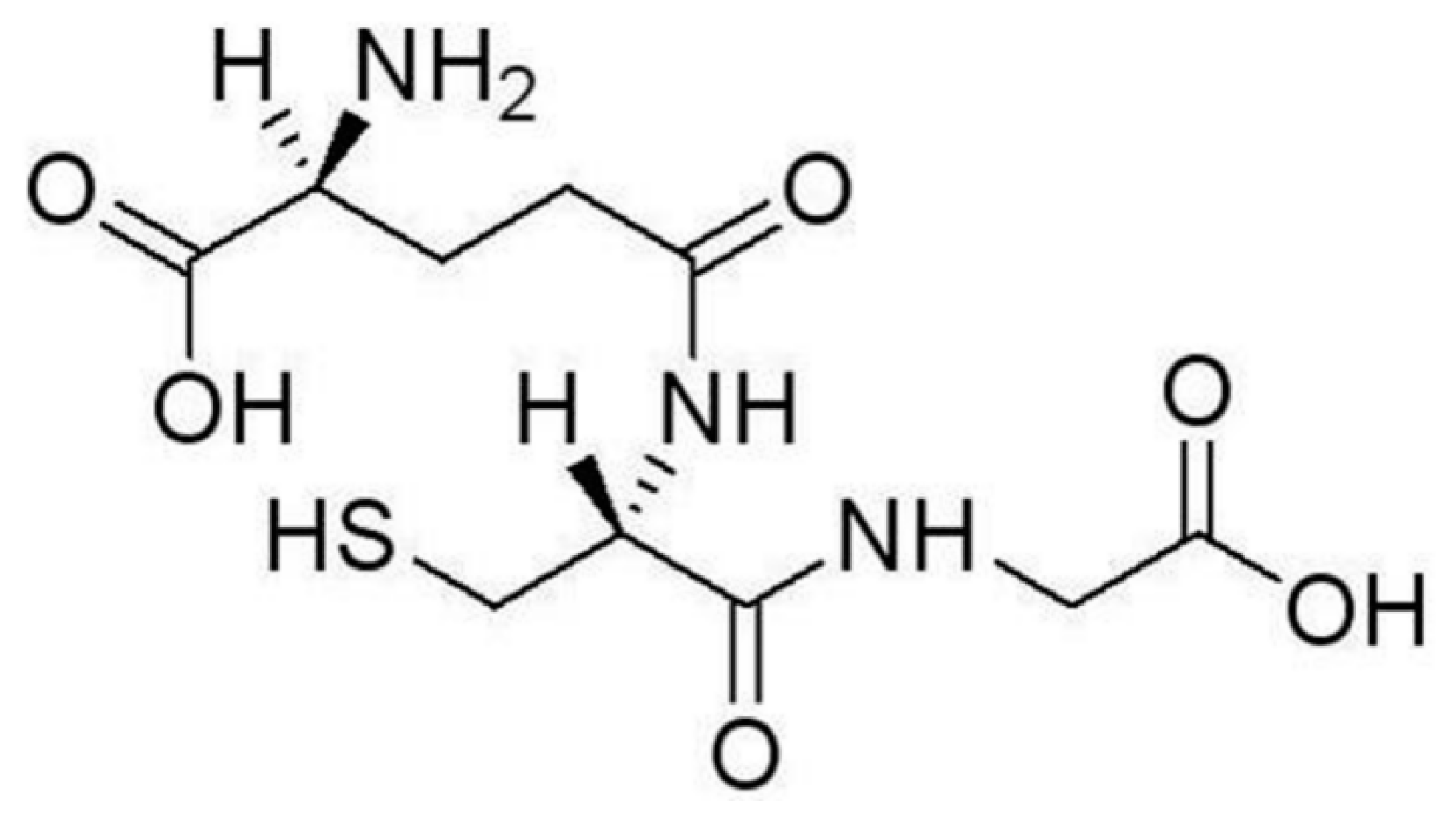
2. Recognition of HMIs by GSH
3. Heavy Metal Ion Detection Platforms Using Glutathione
3.1. Optical Techniques
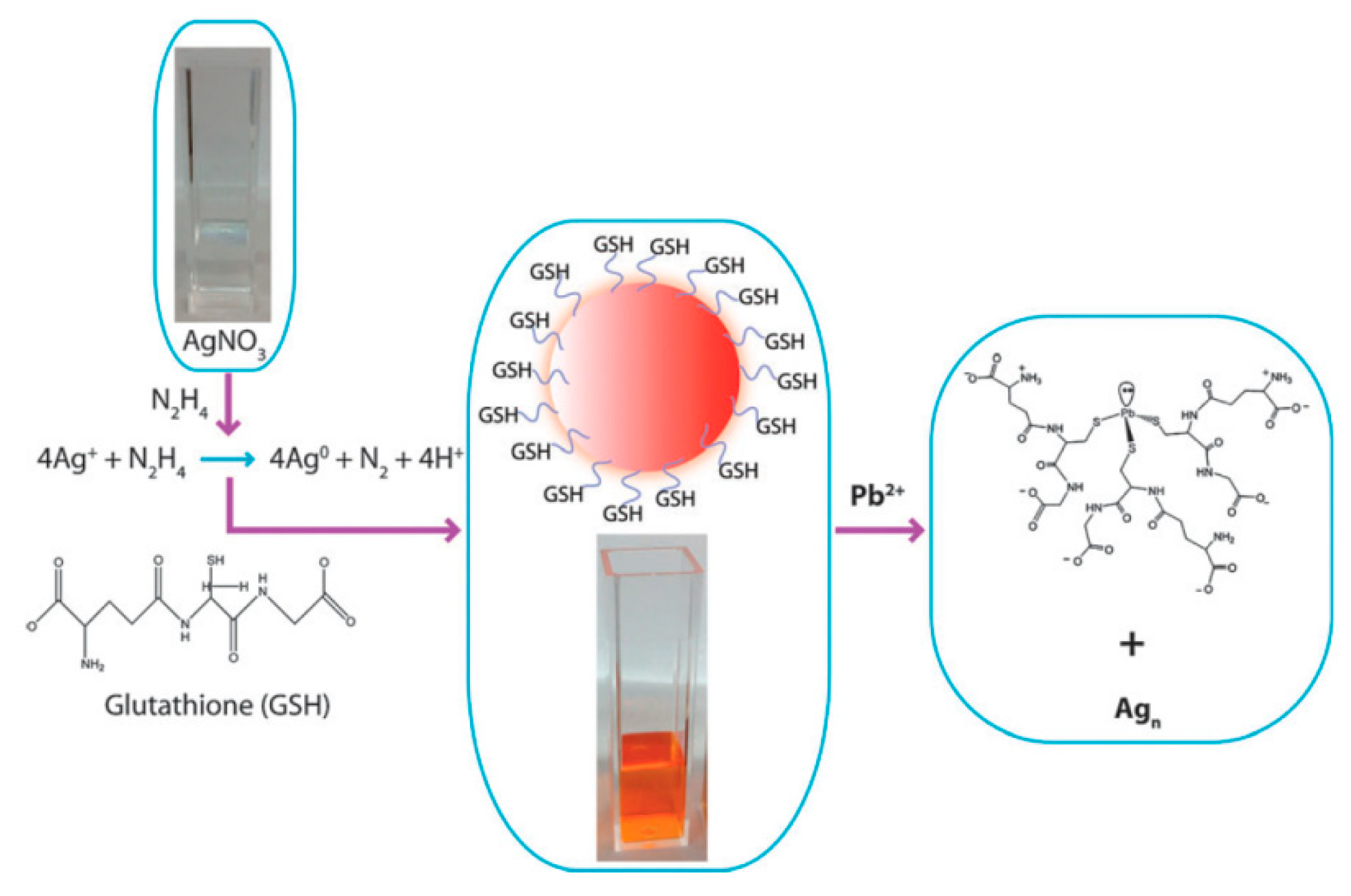
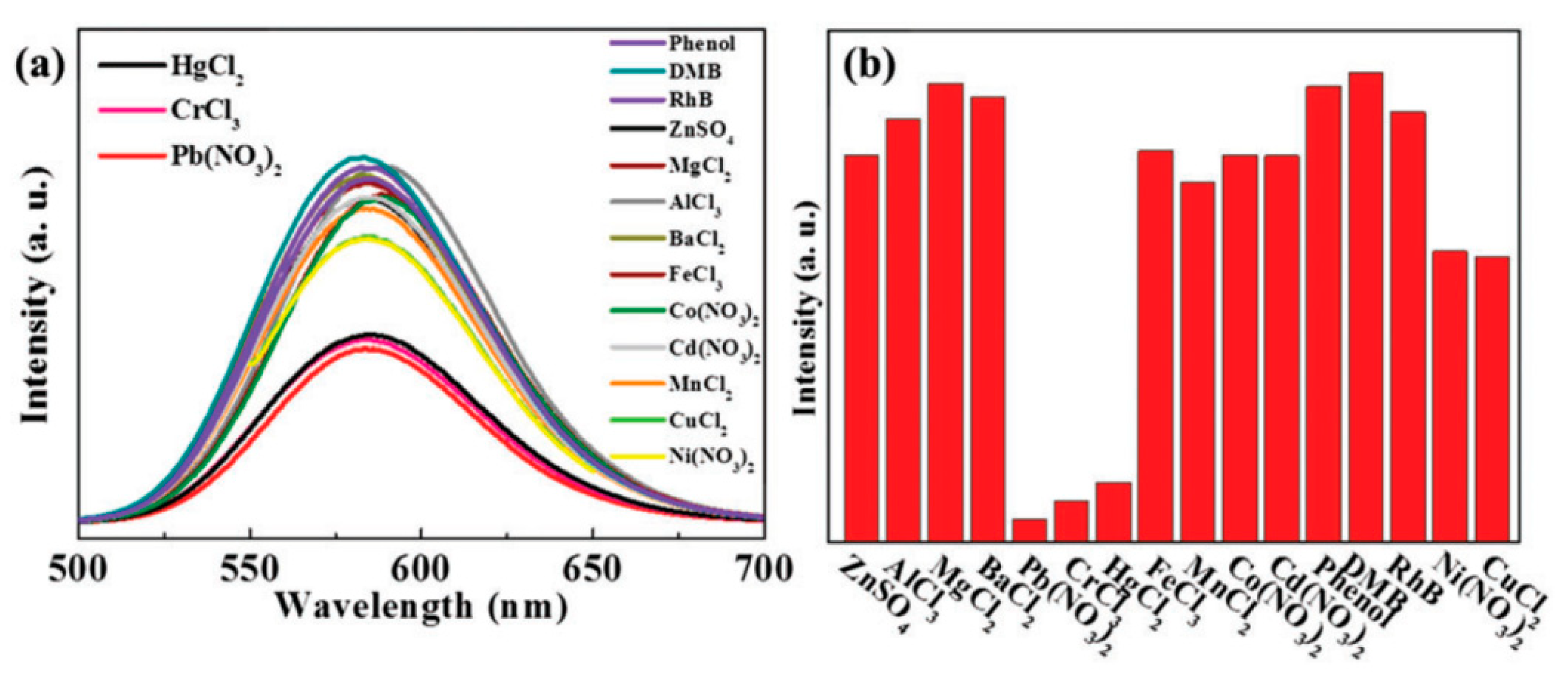
3.1.1. Metallic-Nanoparticle-Based Platforms
3.1.2. Semiconductor Quantum-Dot-Based Platforms
3.2. HMI Detection Combined with Electrochemical Techniques
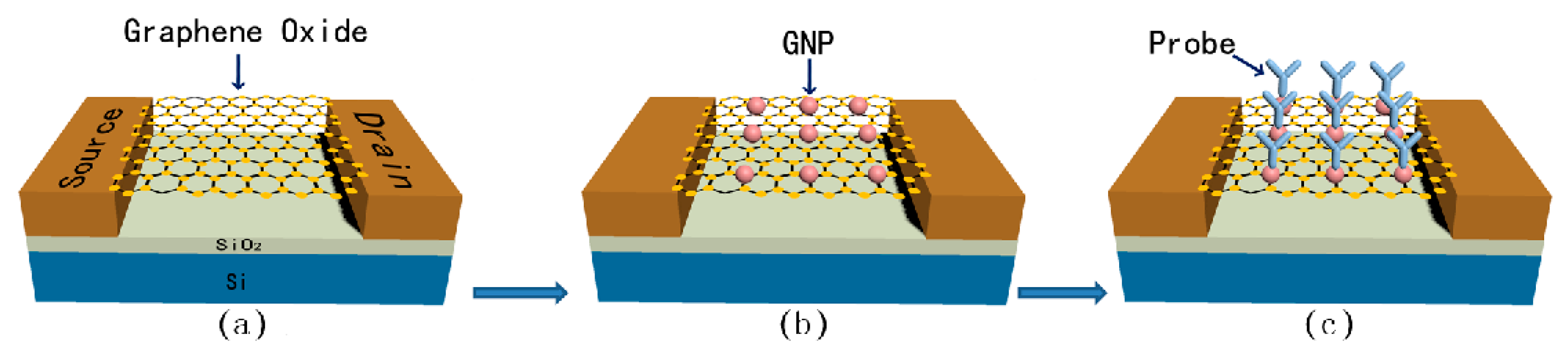
3.2.1. Field Effect Transistor-Based Platforms
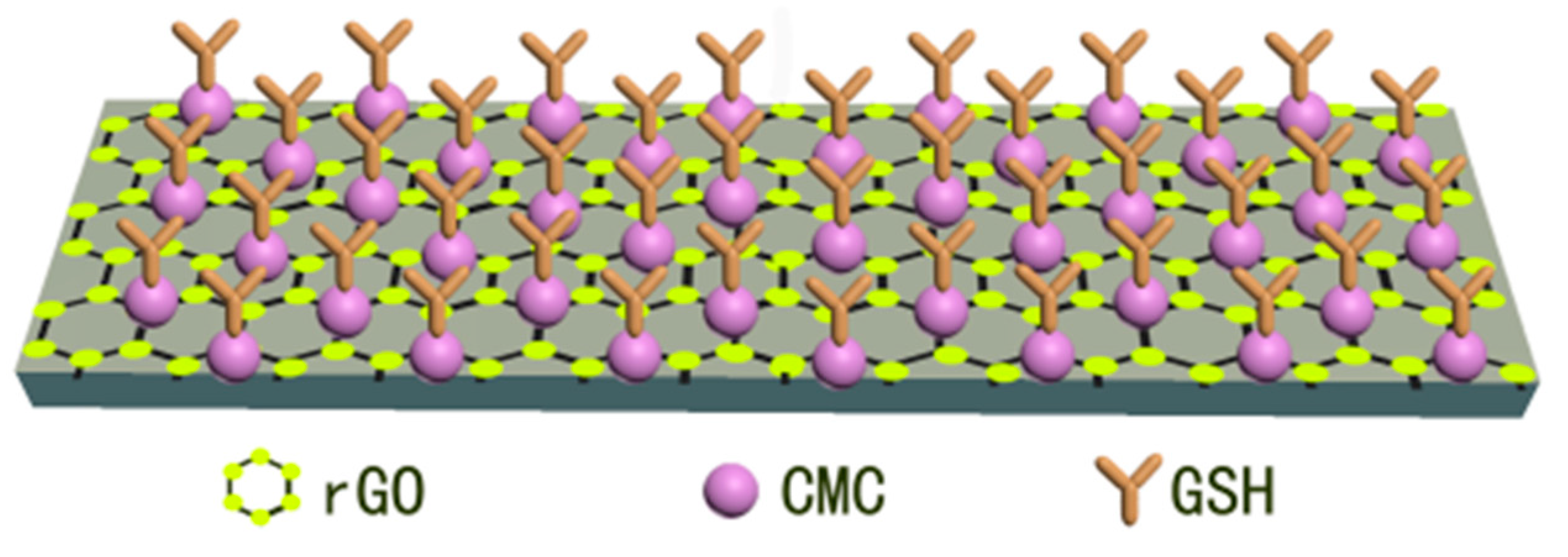
3.2.2. Working-Electrode-Based Platforms
3.3. Summary of HMI Detection Platforms Using GSH
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Bansod, B.K.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 2015, 63, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Pujol, L.; Evrard, D.; Serrano, K.G.; Freyssinier, M.; Cizsak, A.R.; Gros, P. Electrochemical sensors and devices for electrochemical assay in water: The French groups’ contribution. Front. Chem. 2014, 2, 19. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Carrillo-Carrion, C. Analytical strategies based on quantum dots for heavy metal ions detection. J. Biomed. Opt. 2014, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ginya, M.A.; Idris, M.B.; Zakariyya, U.A.; Singh, D.; Yakasai, H.; Sani, N. The role of some antioxidants on absorption, desorption and elimination of lead and iron: An in-vivo study. Eur. J. Biomed. Pharm. Sci. 2016, 3, 528–531. [Google Scholar]
- Afkhami, A.; Soltanifelehgari, F.; Madrakian, T.; Ghaedi, H.; Rezaeivala, M. Fabrication and application of a new modified electrochemical sensor using nano-silica and a newly synthesized schiff base for simultaneous determination of Cd2+, Cu2+ and Hg2+ ions in water and some foodstuff samples. Anal. Chim. Acta 2013, 771, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, S.; Siddiqi, N.J. Biomedical implications of heavy metals induced imbalances in redox systems. BioMed. Res. Int. 2014, 2014, 640754. [Google Scholar] [CrossRef]
- Gong, T.; Liu, J.; Liu, X.; Liu, J.; Xiang, J.; Wu, Y. A sensitive and selective platform based on CdTe QDs in the presence of L-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem. 2016, 213, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Turdean, G.L. Design and development of biosensors for the detection of heavy metal toxicity. Int. J. Electrochem. 2011, 2011, 343125. [Google Scholar] [CrossRef]
- Gao, C.; Yu, X.Y.; Xiong, S.Q.; Liu, J.H.; Huang, X.J. Electrochemical detection of arsenic(III) completely free from noble metal: Fe3O4 microspheres-roomtemperature ionic liquid composite showing better performance than gold. Anal. Chem. 2013, 85, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Array, G.; Merkoci, A. Nanomaterials application in electrochemical detection of heavy metals. Electrochim. Acta 2012, 84, 49–61. [Google Scholar] [CrossRef]
- Sitko, R.; Janik, P.; Zawisza, B.; Talik, E.; Margui, E.; Queralt, I. Green approach for ultra trace determination of divalent metal ions and arsenic species using totalreflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Anal. Chem. 2015, 87, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, Z.; Li, H.; He, D.; Li, Y.; Zheng, H.; Gan, Y.; Li, Y.; Belshaw, N.S.; Hu, S. Liquid spray dielectric barrier discharge induced plasma-chemical vapor generation for the determination of lead by ICPMS. Anal. Chem. 2017, 89, 6827–6833. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xue, X.; Liu, X. One-step, room-temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J. Am. Chem. Soc. 2008, 130, 3244–3245. [Google Scholar]
- Li, S.; Xu, L.; Ma, W.; Kuang, H.; Wang, L.; Xu, C. Triple raman label-encoded gold nanoparticle trimers for simultaneous heavy metal ion detection. Small 2015, 11, 3435–3439. [Google Scholar] [CrossRef]
- Skotadis, E.; Tsekenis, G.; Chatzipetrou, M.; Patsiouras, L.; Madianos, L.; Bousoulas, P.; Zergioti, I.; Tsoukalas, D. Heavy metal ion detection using dnazyme-modified platinum nanoparticle networks. Sens. Actuators B Chem. 2017, 239, 962–969. [Google Scholar] [CrossRef]
- Kullick, T.; Quack, R.; Röhrkasten, C.; Pekeler, T.; Scheper, T.; Schügerl, H.C.K. Pbs-field-effect-transistor for heavy metal concentration monitoring. Chem. Eng. Technol. 2010, 18, 225–228. [Google Scholar] [CrossRef]
- Maity, A.; Sui, X.; Tarman, C.R.; Pu, H.; Chang, J.; Zhou, G.; Ren, R.; Mao, S.; Chen, J. Pulse-driven capacitive lead ion detection with reduced graphene oxide field-effect transistor integrated with an analyzing device for rapid water quality monitoring. ACS Sens. 2017, 2, 1653–1661. [Google Scholar] [CrossRef]
- Hang, R.; Kang, Y.; Gladwin, E.; Claus, R.O. Selective detection of heavy metal ions by self assembled chemical field effect transistors. Appl. Phys. Lett. 2015, 106, 402647. [Google Scholar]
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef]
- Blake, D.A.; Jones, R.M.; Ii, R.C.B.; Pavlov, A.R.; Darwish, I.A.; Yu, H. Antibody-based sensors for heavy metal ions. Biosens. Bioelectron. 2001, 16, 799–809. [Google Scholar] [CrossRef]
- Saidur, M.R.; Aziz, A.R.; Basirun, W.J. Recent advances in dna-based electrochemical biosensors for heavy metal ion detection: A review. Biosens. Bioelectron. 2017, 90, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Wu, Y.; Wang, L.; Zhan, X.; Zhou, P. A mini-review on functional nucleic acids-based heavy metal ion detection. Biosens. Bioelectron. 2016, 86, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.M.C.; Kissick, K.E.; Bachas, L.G.; Sikdar, S.K.; Parikh, C.; Bhattacharyya, D. Polycysteine and other polyamino acid functionalized microfiltration membranes for heavy metal capture. Environ. Sci. Technol. 2001, 35, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Neupane, L.N.; Oh, E.T.; Park, H.J.; Lee, K.H. Selectively and sensitively detection of heavy metal ions in 100% aqueous solution and cells with a fluorescence chemosensor based on peptide using aggregation induced emission. Anal. Chem. 2016, 88, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Shamsipur, M.; Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef]
- Oroval, M.; Coll, C.; Bernardos, A.; Marcos, M.D.; Martínez-Máñez, R.; Shchukin, D.G.; Sancenón, F. Selective fluorogenic sensing of As(III) using aptamer-capped nanomaterials. ACS Appl. Mater. Interfaces 2017, 9, 11332–11336. [Google Scholar] [CrossRef]
- Zhou, S.F.; Wang, J.J.; Gan, L.; Han, X.J.; Fan, H.L.; Mei, L.Y.; Huang, J.; Liu, Y.Q. Individual and simultaneous electrochemical detection toward heavy metal ions based on l-cysteine modified mesoporous MnFe2O4 nanocrystal clusters. J. Alloys Compd. 2017, 721, 492–500. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Li, B. Simple and sensitive detection method for chromium(VI) in water using glutathione-capped CdTe quantum dots as fluorescent probes. Microchim. Acta 2009, 166, 61–68. [Google Scholar] [CrossRef]
- Mah, V.; Jalilehvand, F. Lead(II) complex formation with glutathione. Inorg. Chem. 2012, 51, 6285–6298. [Google Scholar] [CrossRef] [PubMed]
- Anambiga, I.V.; Suganthan, V.; Arunai Nambi Raj, N.; Buvaneswari, G.; Sampath Kumar, T.S. Colorimetric Detection of lead ions using glutathione stabilized silver nanoparticles. Int. J. Sci. Eng. Res. 2013, 4, 710–715. [Google Scholar]
- Feng, B.; Zhu, R.; Xu, S.; Chen, Y.; Di, J. A sensitive LSPR sensor based on glutathione-functionalized gold nanoparticles on a substrate for the detection of Pb2+ ions. RSC Adv. 2018, 8, 4049–4056. [Google Scholar] [CrossRef]
- Zhou, G.; Chang, J.; Cui, S.; Pu, H.; Wen, Z.; Chen, J. Real-time, selective detection of Pb2+ in water using a reduced graphene oxide/gold nanoparticle field-effect transistor device. ACS Appl. Mater. Inter. 2014, 6, 19235–19241. [Google Scholar] [CrossRef] [PubMed]
- Panich, S.; Wilson, K.A.; Nuttall, P.; Wood, C.K.; Albrecht, T.; Edel, J.B. Label-free Pb(II) whispering gallery mode sensing using self-assembled glutathione-modified gold nanoparticles on an optical microcavity. Anal. Chem. 2014, 86, 6299–6306. [Google Scholar] [CrossRef] [PubMed]
- Beqa, L.; Singh, A.K.; Khan, S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water Samples. ACS Appl. Mater. Interfaces 2011, 3, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.B.; Dunkhorst, A.; Gilbert, J.; Bunz, U.H.F. Sensing of lead ions by a carboxylate-substituted PPE: Multivalency effects. Macromolecules 2005, 38, 4560–4562. [Google Scholar] [CrossRef]
- Vila-Viçosa, D.; Teixeira, V.H.; Santos, H.A.F.; Machuqueiro, M. Conformational Study of GSH and GSSG Using Constant-pH Molecular Dynamics Simulations. J. Phys. Chem. B 2013, 117, 7507–7517. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.C.; Huang, H.; Wang, A.J.; Chen, J.R.; Feng, J.J. Solvent-free synthesis of sulfur- and nitrogen-co-doped fluorescent carbon nanoparticles from glutathione for highly selective and sensitive detection of mercury(II) ions. Sens. Actuators B Chem. 2014, 202, 741–747. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Maleki, B.; Alizadeh, Z.; Reiser, O. A simple approach for simultaneous detection of cadmium(II) and lead(II) based on glutathione coated magnetic nanoparticles as a highly selective electrochemical probe. Sens. Actuators B Chem. 2018, 273, 1442–1450. [Google Scholar] [CrossRef]
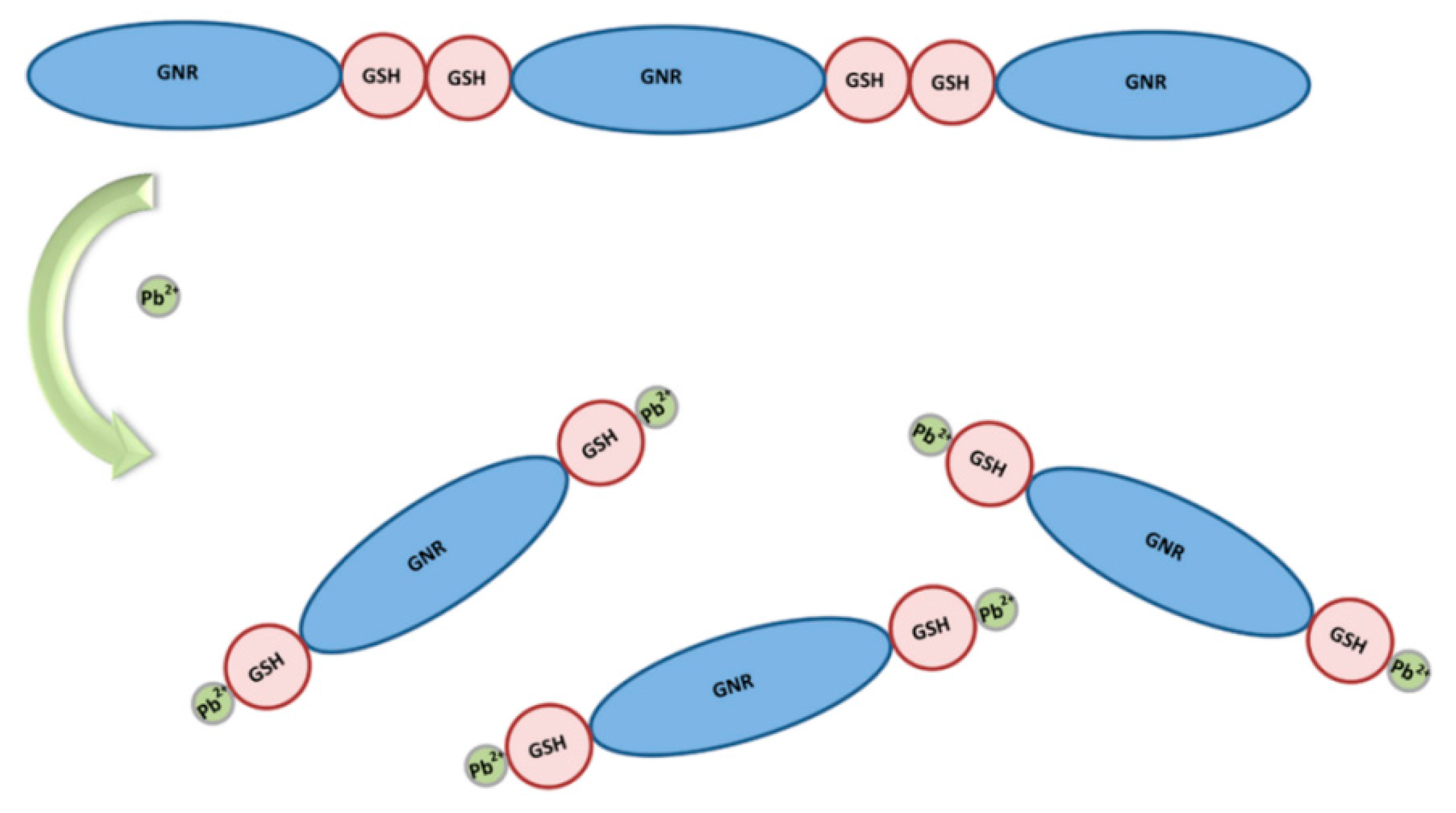
- Durgadas, C.V.; Lakshmi, V.N.; Sharma, C.P.; Sreenivasan, K. Sensing of lead ions using glutathione mediated end to end assembled gold nanorod chains. Sens. Actuators B Chem. 2011, 156, 791–797. [Google Scholar] [CrossRef]
- D’Agostino, A.; Taglietti, A.; Bassi, B.; Donà, A.; Pallavicini, P. A naked eye aggregation assay for Pb2+ detection based on glutathione-coated gold nanostars. J. Nanopart. Res. 2014, 16, 2683. [Google Scholar] [CrossRef]
- Chai, F.; Wang, C.; Wang, T.; Li, L.; Su, Z. Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1466–1470. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Chen, Z.; Ge, P.; Jia, R.; Xiao, E.; Zeng, W. A turn-on fluorescent nanoprobe for lead(II) based on the aggregation of weakly associated gold(I)-glutathione nanoparticles. Microchim. Acta 2017, 184, 4209–4215. [Google Scholar] [CrossRef]
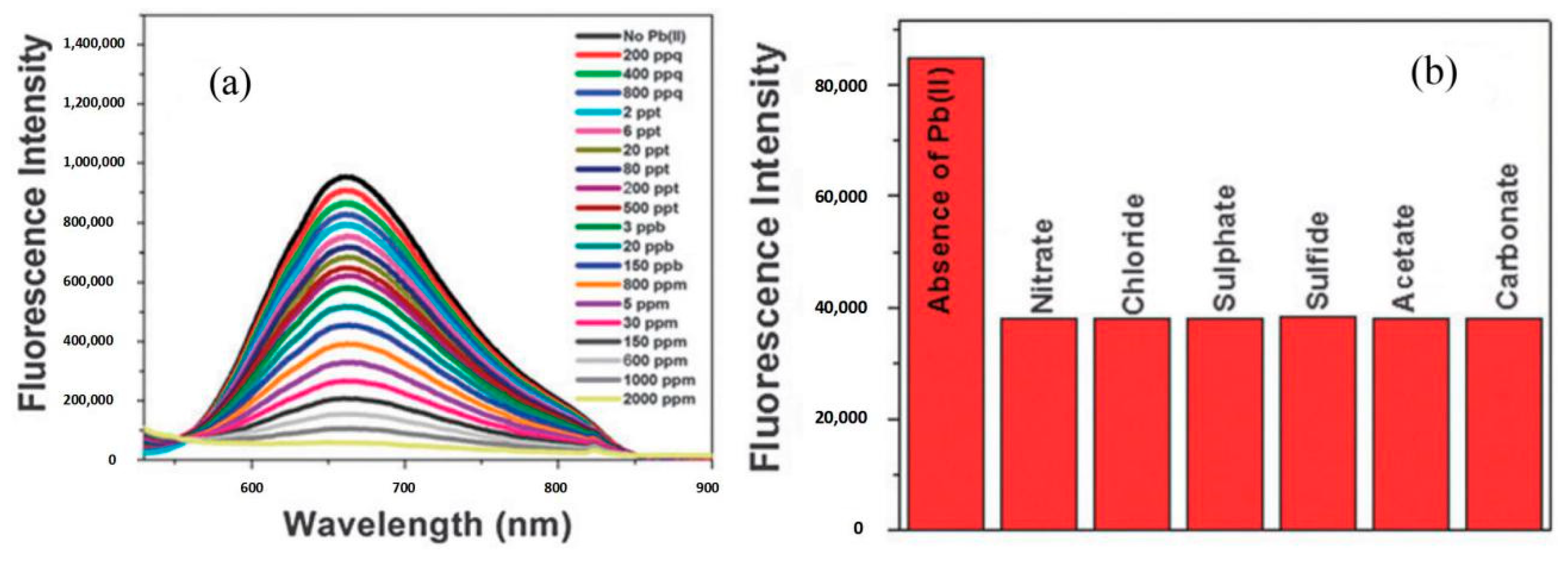
- Singh, A.K.; Kanchanapally, R.; Fan, Z.; Senapati, D.; Ray, P.C. Synthesis of highly fluorescent water-soluble silver nanoparticles for selective detection of Pb(II) at the parts per quadrillion (PPQ) level. Chem. Commun. 2012, 48, 9047–9049. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Lou, T.; Wang, Y. Highly sensitive SERS detection of As3+ ions in aqueous media using glutathione functionalized silver nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 3936–3941. [Google Scholar] [CrossRef]
- Fu, R.; Li, J.; Yang, W. Aggregation of glutathione-functionalized Au nanoparticles induced by Ni2+ ions. J. Nanopart. Res. 2012, 14, 929. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Han, C. Glutathione-stabilized silver nanoparticles as colorimetric sensor for Ni. Sens. Actuators B Chem. 2009, 143, 87–92. [Google Scholar] [CrossRef]
- Li, L.; Liu, B.; Chen, Z. Colorimetric and dark-field microscopic determination of cadmium(II) using unmodified gold nanoparticles and based on the formation of glutathione-cadmium(II) complexes. Microchim. Acta 2019, 186, 37. [Google Scholar] [CrossRef]
- Nightingale, A.M.; deMelloa, J.C. Improving the ensemble optical properties of InP quantum dots by indium precursor modification. J. Mater. Chem. C 2016, 4, 8454–8458. [Google Scholar] [CrossRef]
- Choi, D.B.; Kim, S.; Yoon, H.C.; Ko, M.; Yang, H.; Do, Y.R. Color-tunable Ag-In-Zn-S quantum-dot light-emitting devices realizing green, yellow and amber emissions. J. Mater. Chem. C 2017, 5, 953–959. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Zhang, Y. Glutathione-capped Mn-doped ZnS quantum dots as a room-temperature phosphorescence sensor for the detection of pb2+ ions. Spectrochim. Acta A 2016, 164, 98–102. [Google Scholar] [CrossRef]
- Liu, J.; Lv, G.; Gu, W.; Li, Z.; Tang, A.; Mei, L. A novel luminescence probe based on layered double hydroxides loaded with quantum dots for simultaneous detection of heavy metal ions in water. J. Mater. Chem. C 2017, 5, 5024–5030. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Tan, Z.; Yin, X.; Lun, W. Electrochemiluminescence of CdTe quantum dots capped with glutathione and thioglycolic acid and its sensing of Pb2+. Electrochim. Acta 2012, 72, 28–31. [Google Scholar] [CrossRef]
- Tian, J.; Liu, R.; Zhao, Y.; Xu, Q.; Zhao, S. Controllable synthesis and cell-imaging studies on CdTe quantum dots together capped by glutathione and thioglycolic acid. J. Colloid Interfaces Sci. 2009, 336, 504–509. [Google Scholar] [CrossRef]
- Priya, T.; Dhanalakshmi, N.; Thennarasu, S.; Thinakaran, N. Ultra sensitive detection of Cd (II) using reduced graphene oxide/carboxymethyl cellulose/glutathione modified electrode. Carbohyd. Polym. 2018, 197, 366–374. [Google Scholar]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Glutathione modified screen-printed carbon nanofiber electrode for the voltammetric determination of metal ions in natural samples. Talanta 2016, 155, 8–13. [Google Scholar] [CrossRef]
- Kaabi, R.; Abderrabba, M.; Gómez-Ruiz, S.; Hierro, I.D. Bioinspired materials based on glutathione-functionalized SBA-15 for electrochemical Cd(II) detection. Microporous Mesoporous Mater. 2016, 234, 336–346. [Google Scholar] [CrossRef]
| Signal Transduction | Sensor Structure | HMI Target | Detection Limit | Linear Range | Response Time | References |
|---|---|---|---|---|---|---|
| Optical methods | ||||||
| Colorimetry | Gold NPs | Pb | 0.1 μM | 0.1~50 μM | 20 ~ 25 min | [43] |
| Silver NPs | Pb | 1 nM | Not mentioned | At least 10 min | [32] | |
| Gold NPs | Cd | 4.3 pM | 17 pM~16.67 nM | About 17 min | [49] | |
| Localized surface plasmon resonance | Gold NPs | Pb | 50 pM | 0.1 nM~10 μM | 15 min | [33] |
| Fluorescence | Gold NPs | Pb | 0.1 μM | 2~350 μM | About 1 min | [44] |
| Silver NPs | Pb | 0.6 pM (200 ppq) | 60 pM~2.4 nM (20~800 ppt estimated) | Less than 20 min | [45] | |
| Surface-enhanced Raman scattering | Silver NPs | As3+ | 10.2 nM (0.76 ppb) | 53.7 nM~4.0 μM (4~300 ppb) | At least 2 min | [46] |
| Whispering gallery mode | Gold NPs | Pb | 0.05 nM | 2.40~48.26 nM | About 40 min | [35] |
| Spectrophotometry | Mn-doped ZnS QDs | Pb | 2.2 nM (0.45 μg/L) | 4.9 nM~0.49 μM (1.0~100 μg/L) | Not mentioned | [52] |
| Mn-doped ZnS QDs | Pb, Cr, Hg | 0.93 μM for mixed HMIs | 1 μM~1 mM | Not mentioned | [53] | |
| CdTe QDs | Pb | 0.26 nM | 0.8~15 nM | About 15 s | [54] | |
| Carbon QDs | Hg | 0.05 nM | 1 nM~50 μM | At least 20 min | [39] | |
| Silver NPs | Ni2+ | 75 μM | About 75 μM~1 mM | Not mentioned | [48] | |
| Gold Nanostars | Pb | 0.5 μM | About 0.5~4 μM | About 30 min | [42] | |
| Dynamic Light Scattering | Gold Nanorod Chains | Pb | 0.025 mM | Not mentioned | Not mentioned | [41] |
| Electrochemical methods | ||||||
| Square Wave Anodic Stripping Voltammetry, SWASV | Magnetic NPs | Cd, Pb | 1.6 nM (0.182 μg/L); 0.8 nM (0.172 μg/L) | 4.4~879 nM (0.5~100 μg/L), 2.3~460 nM (0.5~100 μg/L) | 210 s 210 s | [40] |
| Glassy-Carbon Electrode | Cd | 0.05 nM | 2~20 nM | 120 s | [56] | |
| Carbon Paste Electrode | Cd | 8.5 nM (2 ppb) | 4.2~420 nM (1~100 ppb) | Longer than 7 min | [58] | |
| Differtial pulsed anodic striping voltammetry, DPASV | Screen-Printed Carbon Nanofiber Electrode | Pb, Cd | ~0.1 nM (~3 μg/L) | About 0.3~4.5 nM (about 10~150 μg/L) | 120 s | [57] |
| FET (Drain current) | Field effect Transistor | Pb | 10 nM | 10 nM~10 μM | 1–2 s | [34] |
| FET (Pulse-driven capacitance) | Gate Capacitance | Pb | <4.8 nM (<1 ppb) | 24~96 nM (5~20 ppb) | 1–2 s | [19] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Sun, X.; Wu, J. Heavy Metal Ion Detection Platforms Based on a Glutathione Probe: A Mini Review. Appl. Sci. 2019, 9, 489. https://doi.org/10.3390/app9030489
Zhang J, Sun X, Wu J. Heavy Metal Ion Detection Platforms Based on a Glutathione Probe: A Mini Review. Applied Sciences. 2019; 9(3):489. https://doi.org/10.3390/app9030489
Chicago/Turabian StyleZhang, Jian, Xuan Sun, and Jayne Wu. 2019. "Heavy Metal Ion Detection Platforms Based on a Glutathione Probe: A Mini Review" Applied Sciences 9, no. 3: 489. https://doi.org/10.3390/app9030489
APA StyleZhang, J., Sun, X., & Wu, J. (2019). Heavy Metal Ion Detection Platforms Based on a Glutathione Probe: A Mini Review. Applied Sciences, 9(3), 489. https://doi.org/10.3390/app9030489






