Ultrasonic Parametrization of Arterial Wall Movements in Low- and High-Risk CVD Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of In Vivo Data
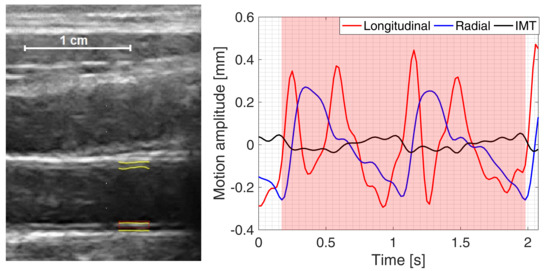
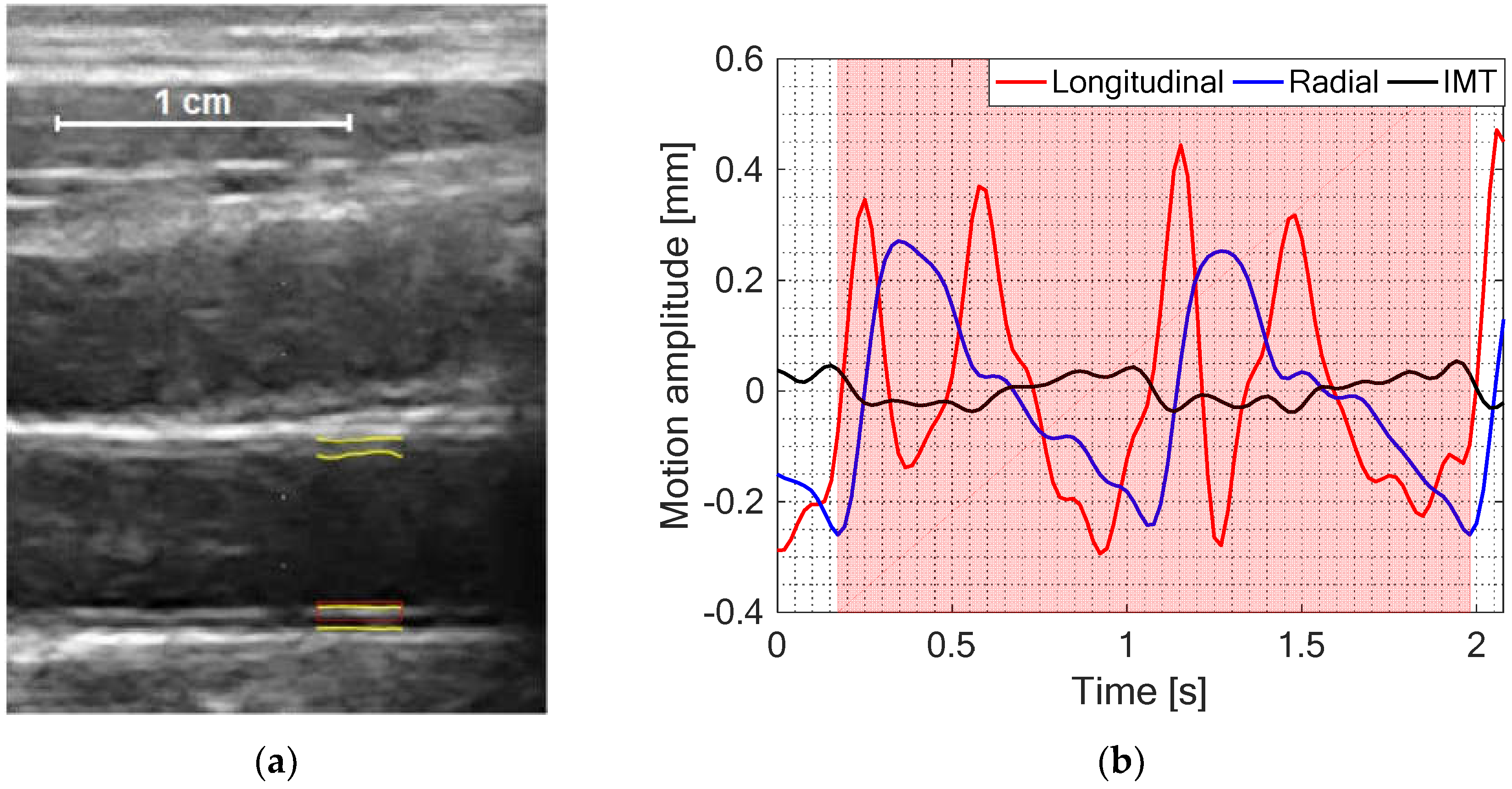
2.3. Estimation and Post-Processing of IMT, Longitudinal and Radial Motion Signals of CCA
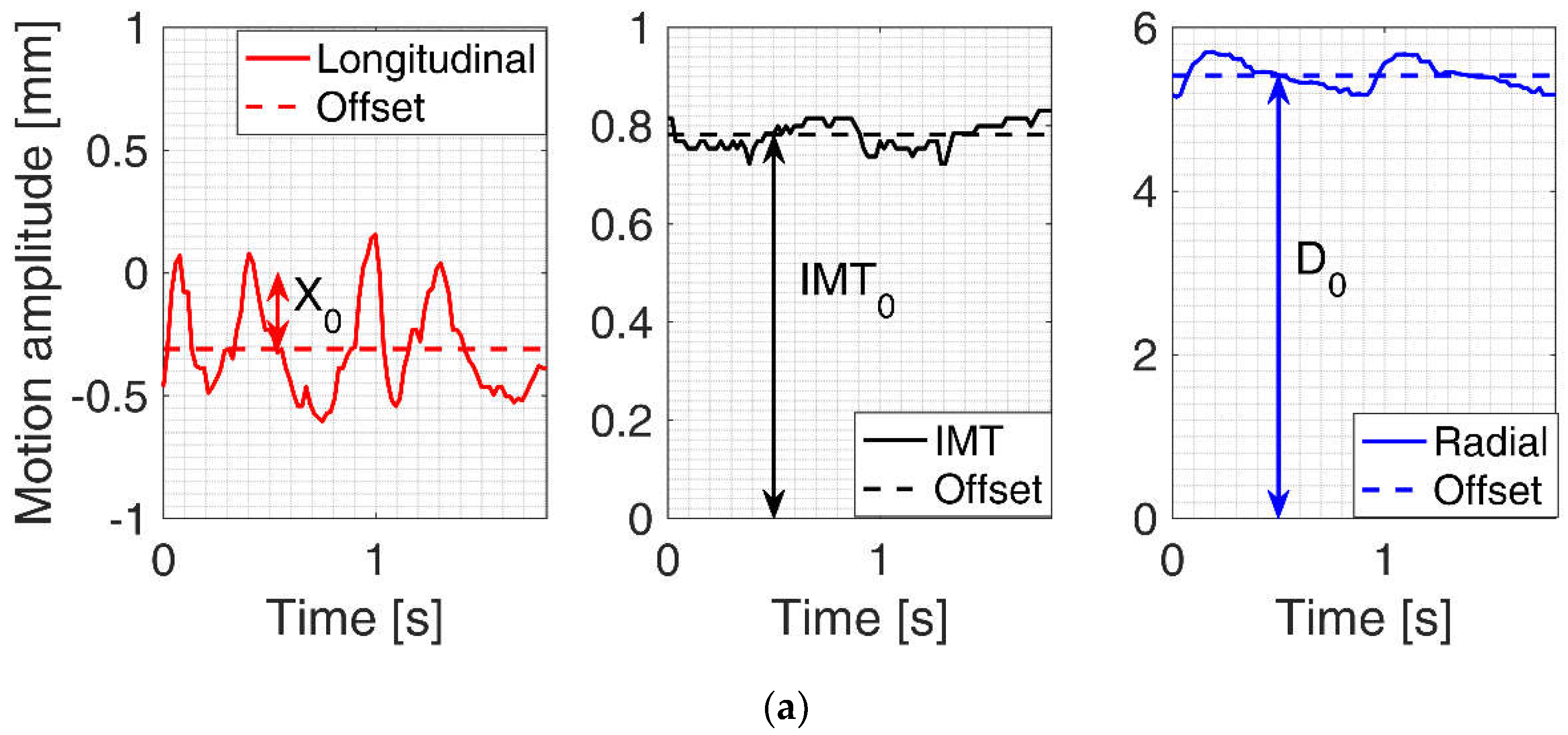
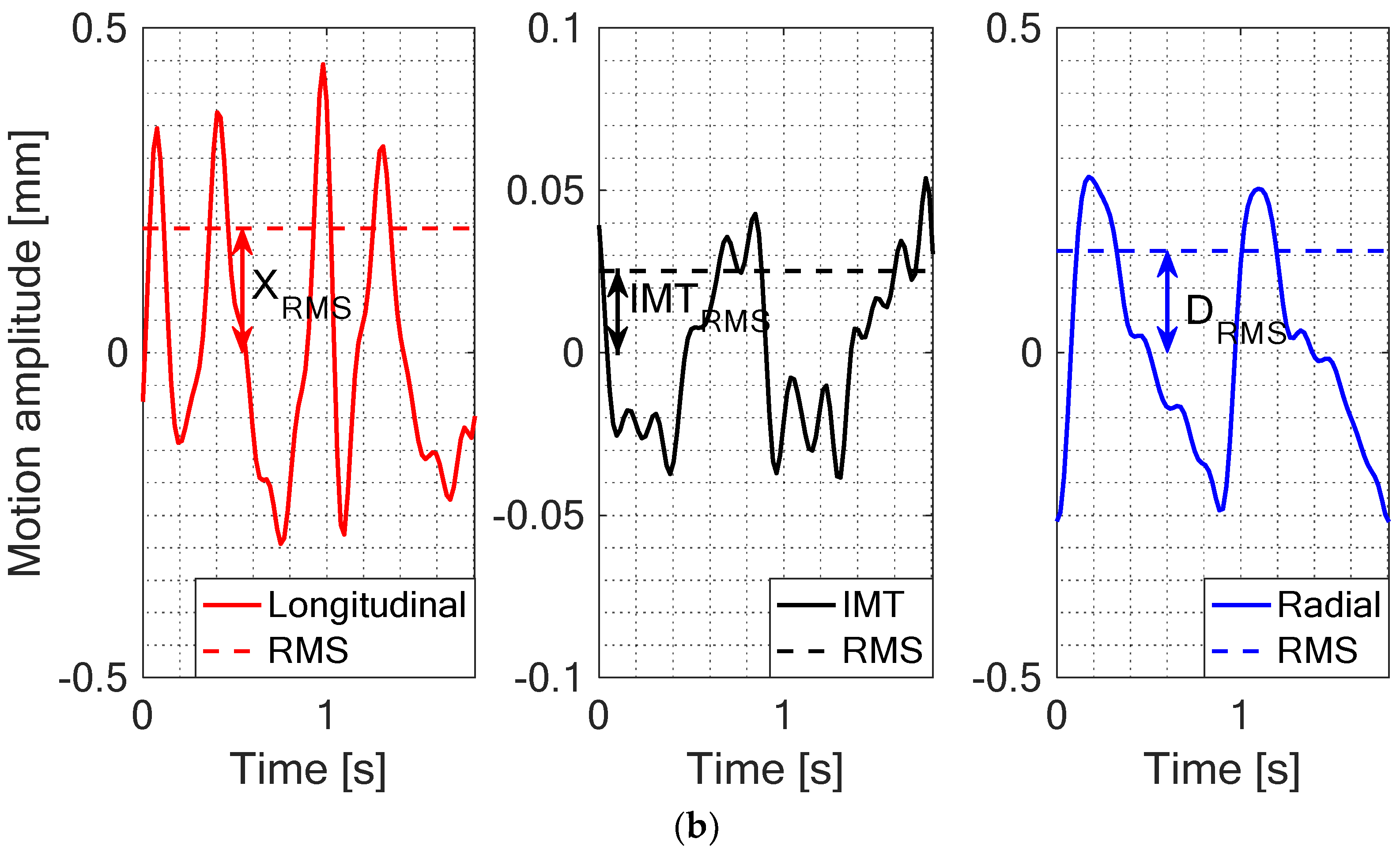
2.4. Quantitative Parametrization of IMT, Longitudinal and Radial Motion Signals of CCA
2.5. Statistical Analysis
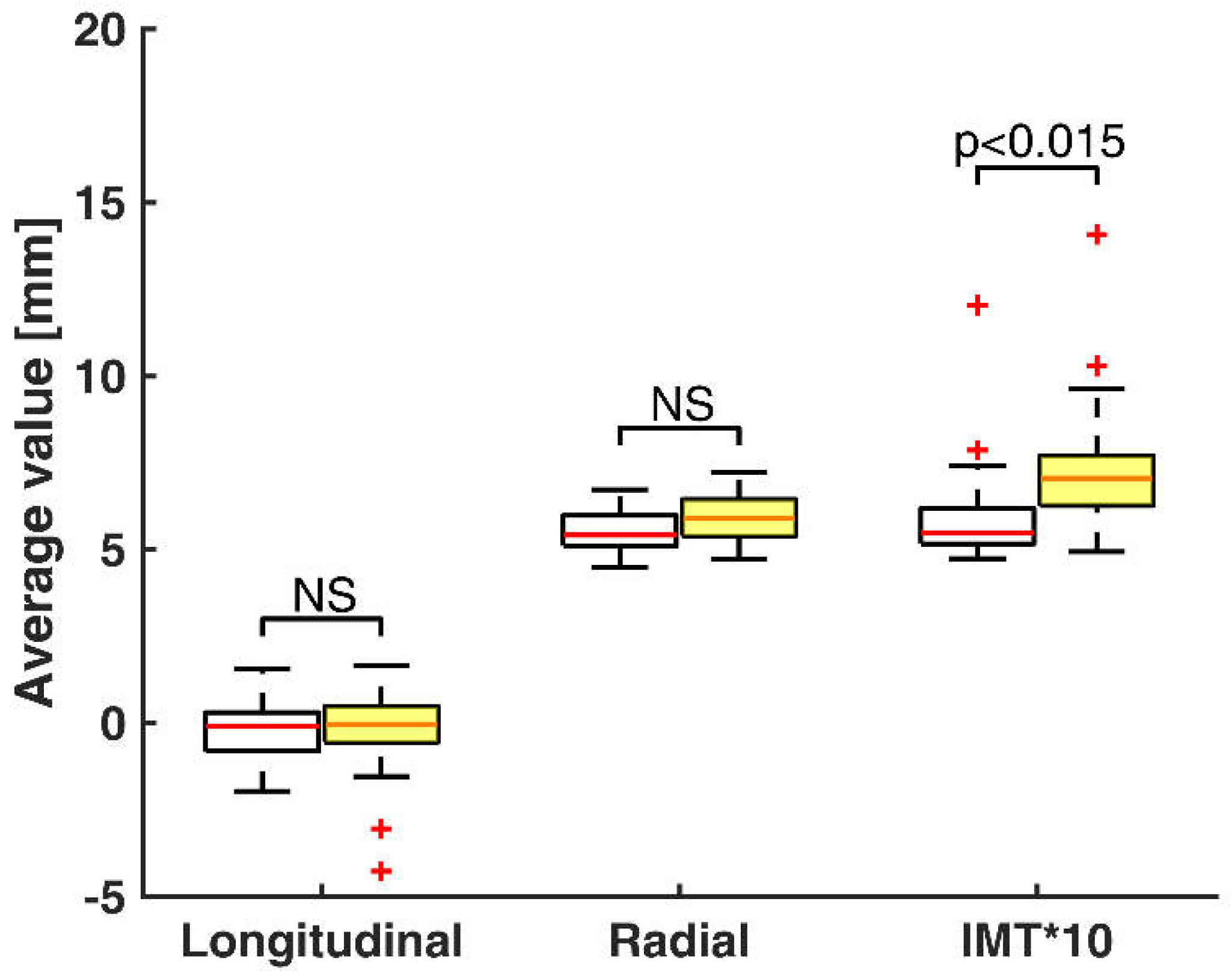
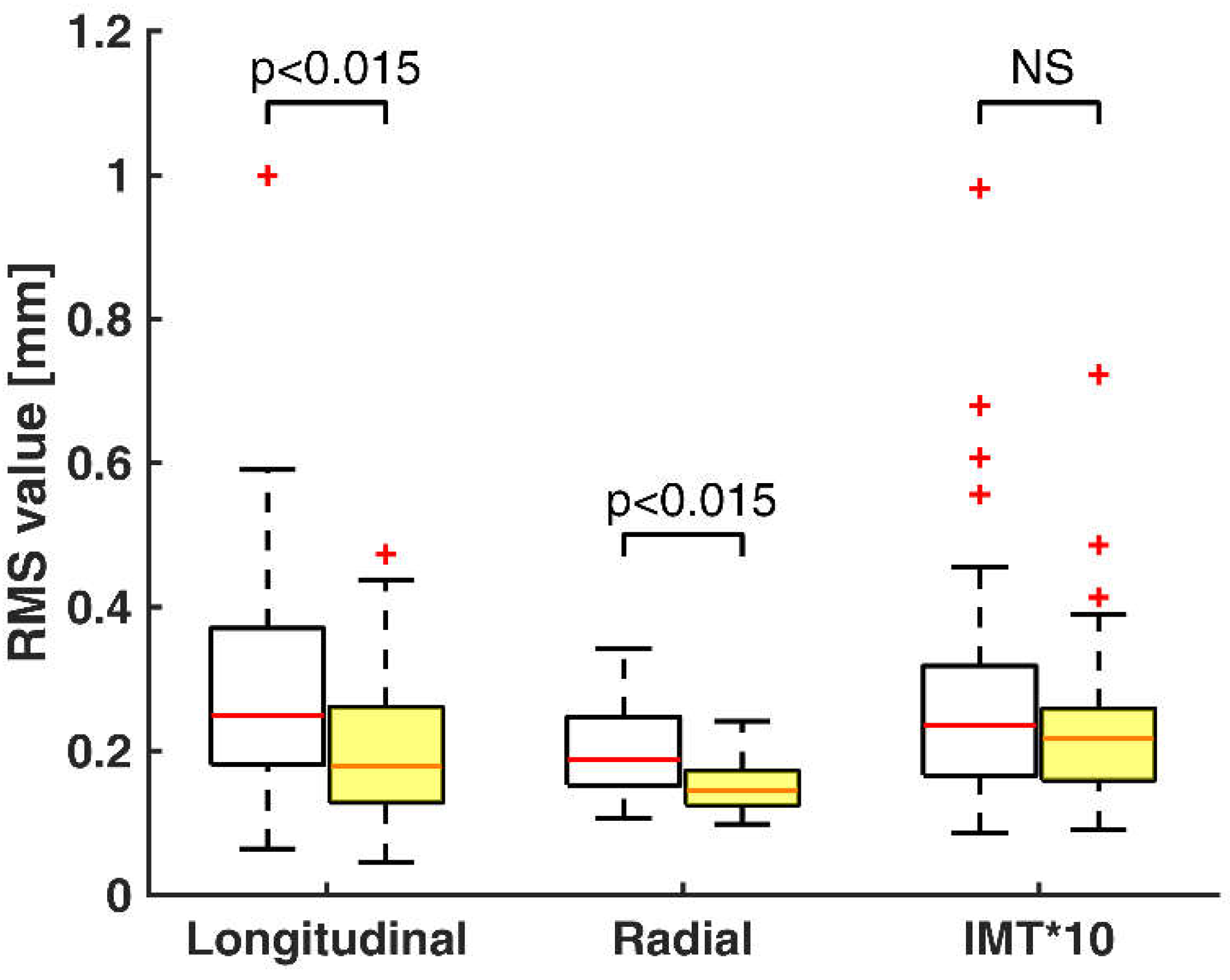
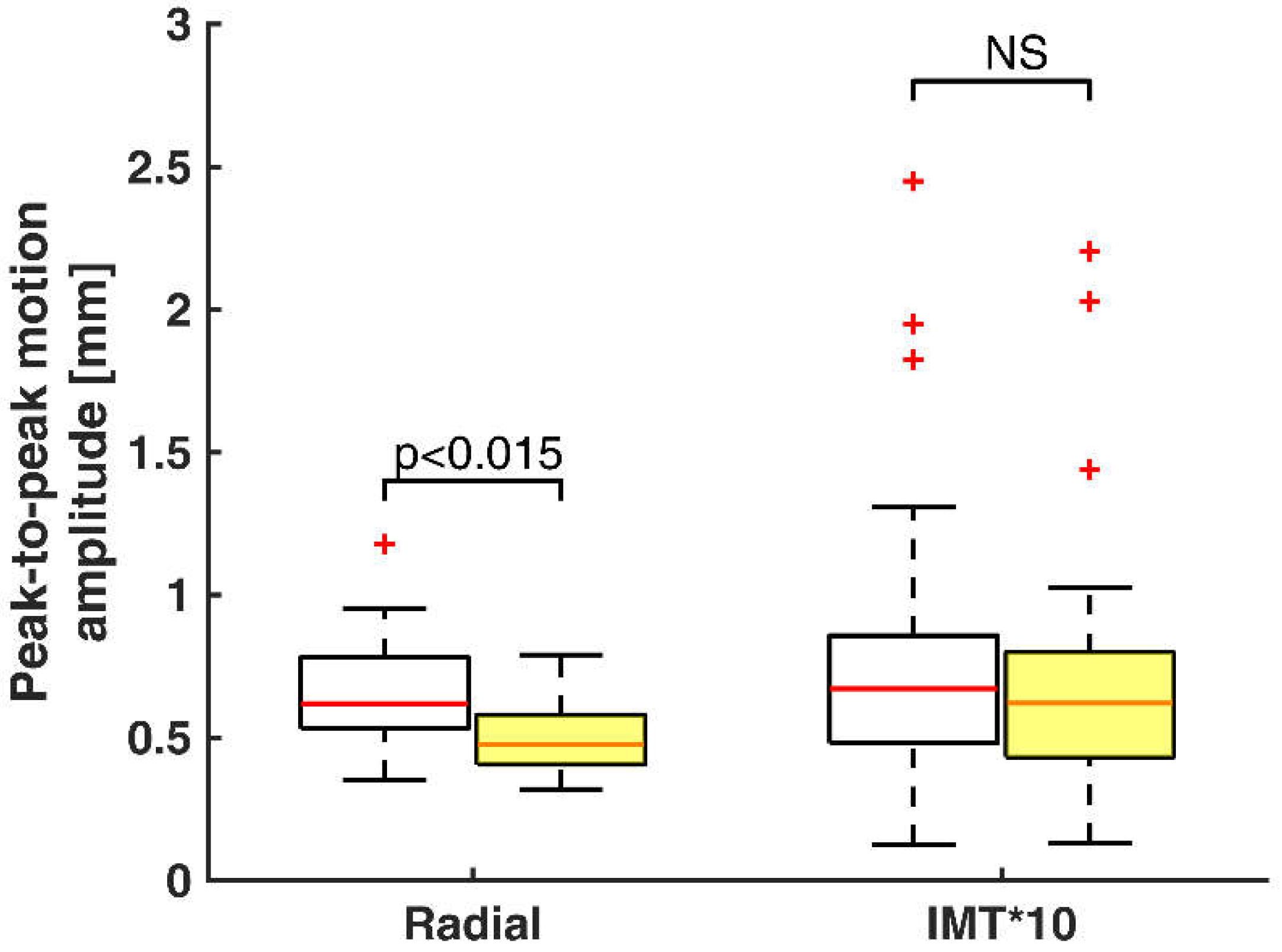
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 December 2018).
- Cardiovascular Diseases (CVDs)–Global Facts and Figures. Available online: https://www.world-heart-federation.org/resources/cardiovascular-diseases-cvds-global-facts-figures/ (accessed on 17 December 2018).
- Zahnd, G.; Boussel, L.; Sérusclat, A.; Vray, D. Intramural Shear Strain can Highlight the Presence of Atherosclerosis: A Clinical in Vivo Study. In Proceedings of the 2011 IEEE International Ultrasonics Symposium, Orlando, FL, USA, 18–21 October 2011; pp. 1770–1773. [Google Scholar]
- Molinari, F.; Suri, J.S. Automated Measurement of Carotid Artery Intima-Media Thickness. In Ultrasound and Carotid Bifurcation Atherosclerosis; Nicolaides, A., Beach, K.W., Kyriacou, E., Pattichis, C.S., Eds.; Springer: London, UK; Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA, 2012; pp. 177–192. ISBN 978-1-84882-687-8. [Google Scholar]
- Hobbs, F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Zahnd, G.; Orkisz, M.; Sérusclat, A.; Vray, D. Minimal-Path Contours Combined with Speckle Tracking to Estimate 2D Displacements of the Carotid Artery Wall in B-Mode Imaging. In Proceedings of the 2011 IEEE International Ultrasonics Symposium, Orlando, FL, USA, 18–21 October 2011; pp. 732–735. [Google Scholar]
- Ahlgren, A.R.; Cinthio, M.; Steen, S.; Persson, H.W.; Sjöberg, T.; Lindström, K. Effects of Adrenaline on Longitudinal Arterial Wall Movements and Resulting Intramural Shear Strain: A First Report. Clin. Physiol. Funct. Imaging 2009, 29, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, S.; Gan, L. Longitudinal Wall Motion of the Common Carotid Artery can be Assessed by Velocity Vector Imaging. Clin. Physiol. Funct. Imaging 2011, 31, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Au, J.S.; Ditor, D.S.; Macdonald, M.J.; Stöhr, E.J. Carotid Artery Longitudinal Wall Motion is Associated with Local Blood Velocity and Left Ventricular Rotational, but Not Longitudinal, Mechanics. Physiol. Rep. 2016, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, G.; Orkisz, M.; Sérusclat, A.; Moulin, P.; Vray, D. Evaluation of a Kalman-Based Block Matching Method to Assess the Bi-Dimensional Motion of the Carotid Artery Wall in B-Mode Ultrasound Sequences. Med. Image Anal. 2013, 17, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Caviezel, S.; Teynor, A.; Erbel, R.; Mahabadi, A.A.; Schmidt-Trucksäss, A. Carotid Intima-Media Thickness as a Biomarker of Subclinical Atherosclerosis. Swiss Med. Wkly. 2012, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, G.; Salles, S.; Liebgott, H.; Vray, D.; Sérusclat, A.; Moulin, P. Real-time Ultrasound-tagging to Track the 2D Motion of the Common Carotid Artery Wall in Vivo. Med. Phys. 2015, 42, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Ahlgren, A.R.; Jansson, T.; Eriksson, A.; Persson, H.W.; Lindström, K. A New Non-invasive Ultrasonic Method for Simultaneous Measurements of Longitudinal and Radial Arterial Wall Movements: First in Vivo Trial. Wiley Online Libr. 2003, 23, 247–251. [Google Scholar] [CrossRef]
- Cinthio, M.; Ahlgren, A.R.; Bergkvist, J.; Jansson, T.; Persson, H.W.; Lindström, K. Longitudinal Movements and Resulting Shear Strain of the Arterial Wall. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Cinthio, M.; Ahlgren, A.R.; Jansson, T.; Eriksson, A.; Persson, H.W.; Lindstrom, K. Evaluation of an Ultrasonic Echo-Tracking Method for Measurements of Arterial Wall Movements in Two Dimensions. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, S. Carotid Artery Longitudinal Displacement Predicts 1-Year Cardiovascular Outcome in Patients with Suspected Coronary Artery Disease Arteriosclerosis. Thromb. Vasc. Biol. 2011, 31, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, G.; Boussel, L.; Marion, A.; Durand, M.; Moulin, P.; Sérusclat, A.; Vray, D. Measurement of Two-Dimensional Movement Parameters of the Carotid Artery Wall for Early Detection of Arteriosclerosis: A Preliminary Clinical Study. Ultrasound Med. Biol. 2011, 37, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Taivainen, S.H.; Yli-Ollila, H.; Juonala, M.; Kähönen, M.; Raitakari, O.T.; Laitinen, T.M.; Laitinen, T.P. Interrelationships between Indices of Longitudinal Movement of the Common Carotid Artery Wall and the Conventional Measures of Subclinical Arteriosclerosis. Clin. Physiol. Funct. Imaging 2017, 37, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Yli-Ollila, H.; Laitinen, T.; Weckström, M.; Laitinen, T.M. New Indices of Arterial Stiffness Measured from Longitudinal Motion of Common Carotid Artery in Relation to Reference Methods, a Pilot Study. Clin. Physiol. Funct. Imaging 2016, 36, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Qorchi, S. Extraction of Characteristic Patterns from Carotid Longitudinal Kinetics. In Proceedings of the Recherche en Imagerie et Technologies pour la Santé (RITS) 2017, Lyon, France, 27–29 March 2017. [Google Scholar]
- Cinthio, M.; Albinsson, J.; Erlöv, T.; Bjarnegård, N.; Länne, T.; Ahlgren, Å.R. Longitudinal Movement of the Common Carotid Artery Wall: New Information on Cardiovascular Aging. Ultrasound Med. Biol. 2018, 44, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, G.; Orkisz, M.; Vray, D. Imaging-Based Computational Biomedicine Lab; Graduate School of Information Science, Nara Institute of Science and Technology (NAIST): Nara, Japan, 2017. [Google Scholar] [CrossRef]
- Zahnd, G.; Kapellas, K.; van Hattem, M.; van Dijk, A.; Sérusclat, A.; Moulin, P.; van der Lugt, A.; Skilton, M.; Orkisz, M. A Fully-Automatic Method to Segment the Carotid Artery Layers in Ultrasound Imaging: Application to Quantify the Compression-Decompression Pattern of the Intima-Media Complex during the Cardiac Cycle. Ultrasound Med. Biol. 2017, 43, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Wells, P.N.T.; Liang, H.-D. Medical ultrasound: Imaging of soft tissue strain and elasticity. J. R. Soc. Interface 2011, 8, 1521–1549. [Google Scholar] [CrossRef] [PubMed]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
- Zambacevičienė, M. RF Ultrasound Based Estimation of Pulsatile Flow Induced Microdisplacements in Phantom. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering 2018, Prague, Czech Republic, 3–8 June 2018; pp. 601–605. [Google Scholar]
- Au, J.S.; Yli-Ollila, H.; Macdonald, M.J. An Assessment of Intra-Individual Variability in Carotid Artery Longitudinal Wall Motion: Recommendations for Data Acquisition. Physiol. Meas. 2018, 39, 09NT01. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makūnaitė, M.; Jurkonis, R.; Rodríguez-Martínez, A.; Jurgaitienė, R.; Semaška, V.; Mėlinytė, K.; Kubilius, R. Ultrasonic Parametrization of Arterial Wall Movements in Low- and High-Risk CVD Subjects. Appl. Sci. 2019, 9, 465. https://doi.org/10.3390/app9030465
Makūnaitė M, Jurkonis R, Rodríguez-Martínez A, Jurgaitienė R, Semaška V, Mėlinytė K, Kubilius R. Ultrasonic Parametrization of Arterial Wall Movements in Low- and High-Risk CVD Subjects. Applied Sciences. 2019; 9(3):465. https://doi.org/10.3390/app9030465
Chicago/Turabian StyleMakūnaitė, Monika, Rytis Jurkonis, Alberto Rodríguez-Martínez, Rūta Jurgaitienė, Vytenis Semaška, Karolina Mėlinytė, and Raimondas Kubilius. 2019. "Ultrasonic Parametrization of Arterial Wall Movements in Low- and High-Risk CVD Subjects" Applied Sciences 9, no. 3: 465. https://doi.org/10.3390/app9030465
APA StyleMakūnaitė, M., Jurkonis, R., Rodríguez-Martínez, A., Jurgaitienė, R., Semaška, V., Mėlinytė, K., & Kubilius, R. (2019). Ultrasonic Parametrization of Arterial Wall Movements in Low- and High-Risk CVD Subjects. Applied Sciences, 9(3), 465. https://doi.org/10.3390/app9030465