Introducing Temperature as Variable Parameter into Kinetic Models for Anaerobic Fermentation of Coffee Husk, Pulp and Mucilage
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Inoculum
2.2. Chemical Analysis
2.3. Anaerobic Batch Digestion Tests
2.4. Data Fitting to Models
2.4.1. Modified Gompertz (GOM) Model
2.4.2. Modified Logistic (LOG) Model
2.5. Statistical Analysis
3. Results and Discussion
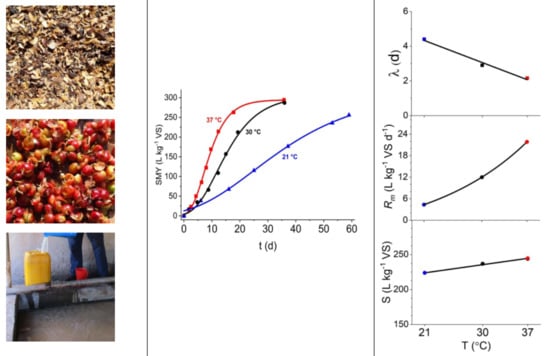
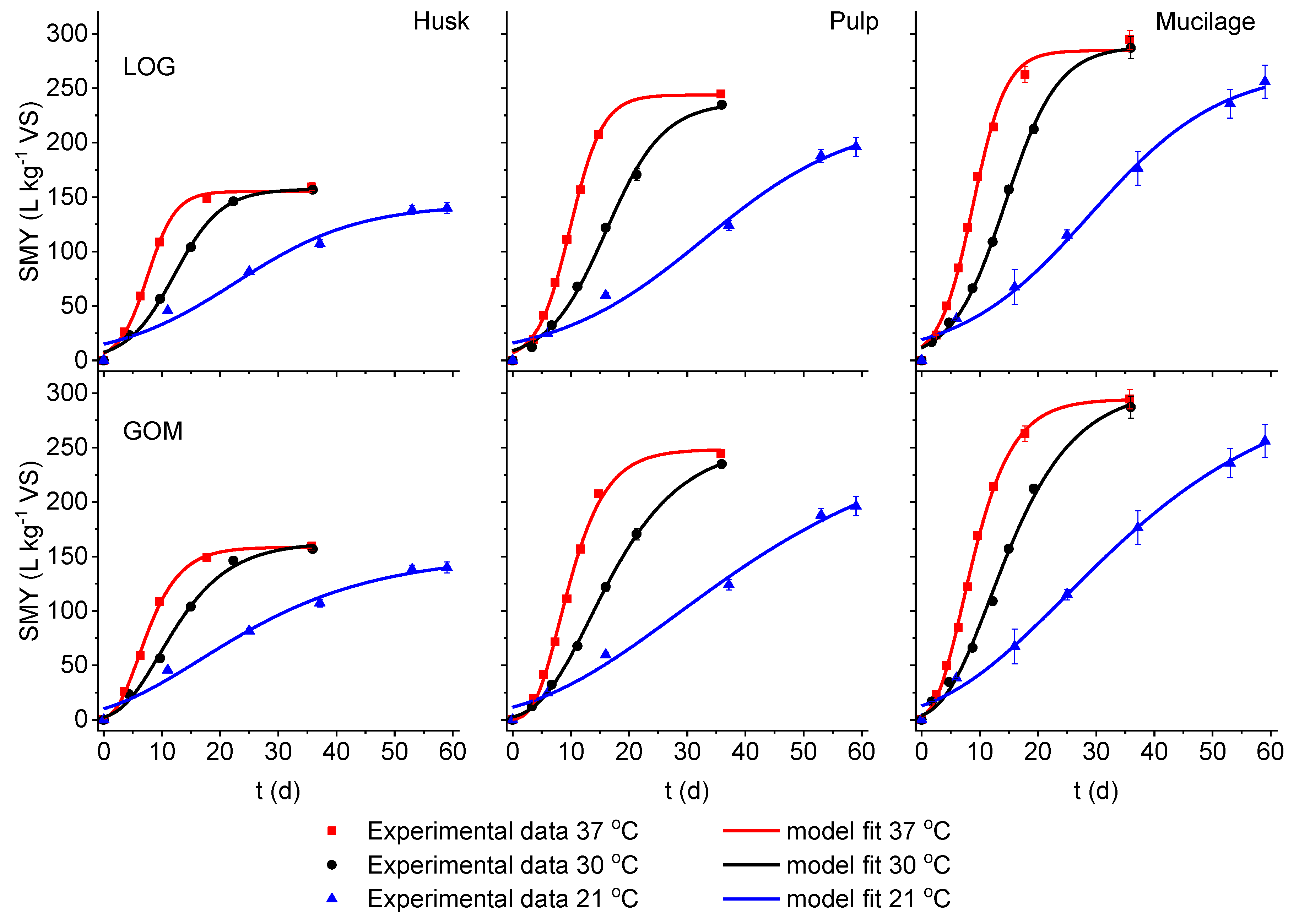
3.1. Model Fitting for Different Fermentation Temperatures
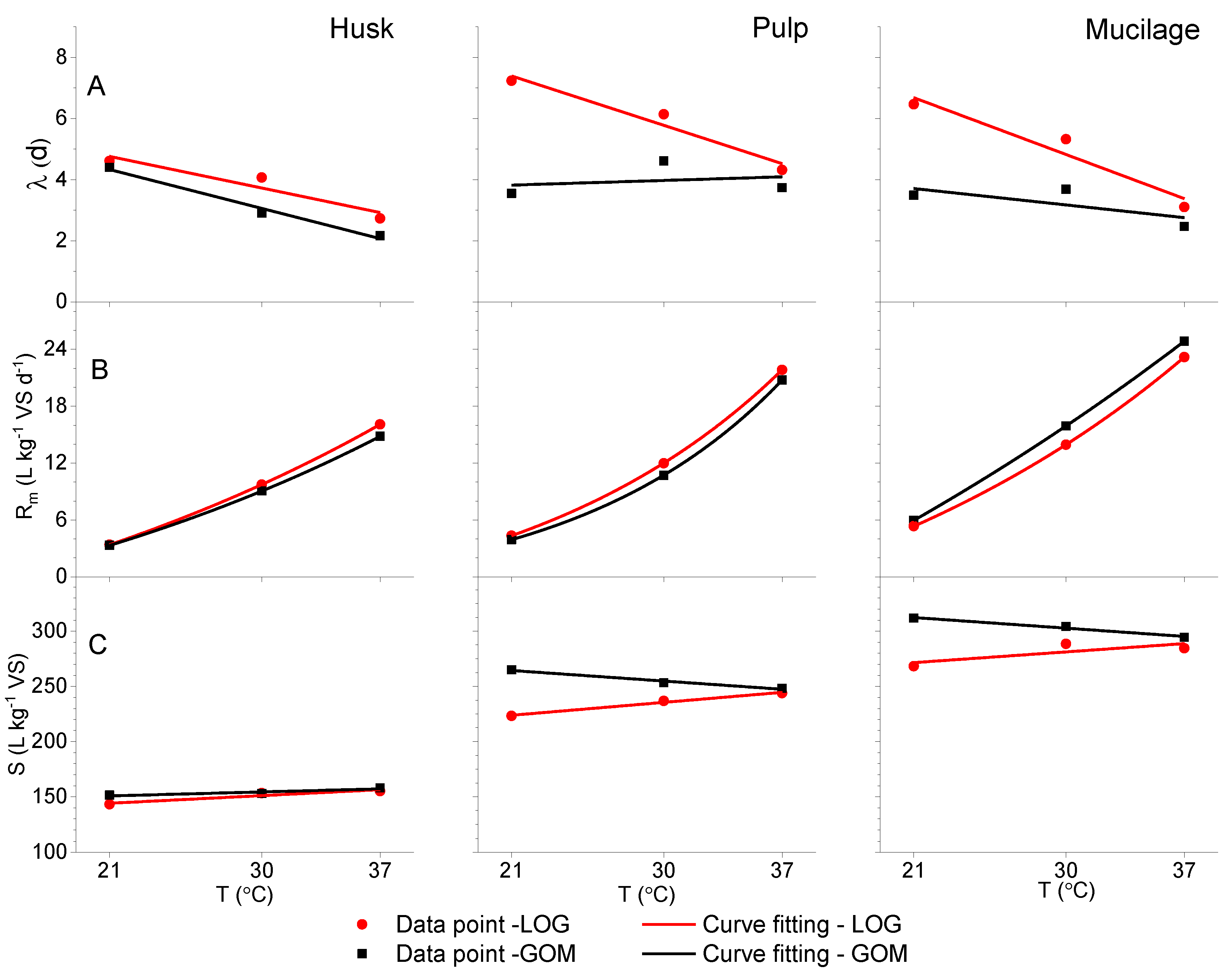
3.2. Model Fitting for Fermentation Temperature as an Input Variable
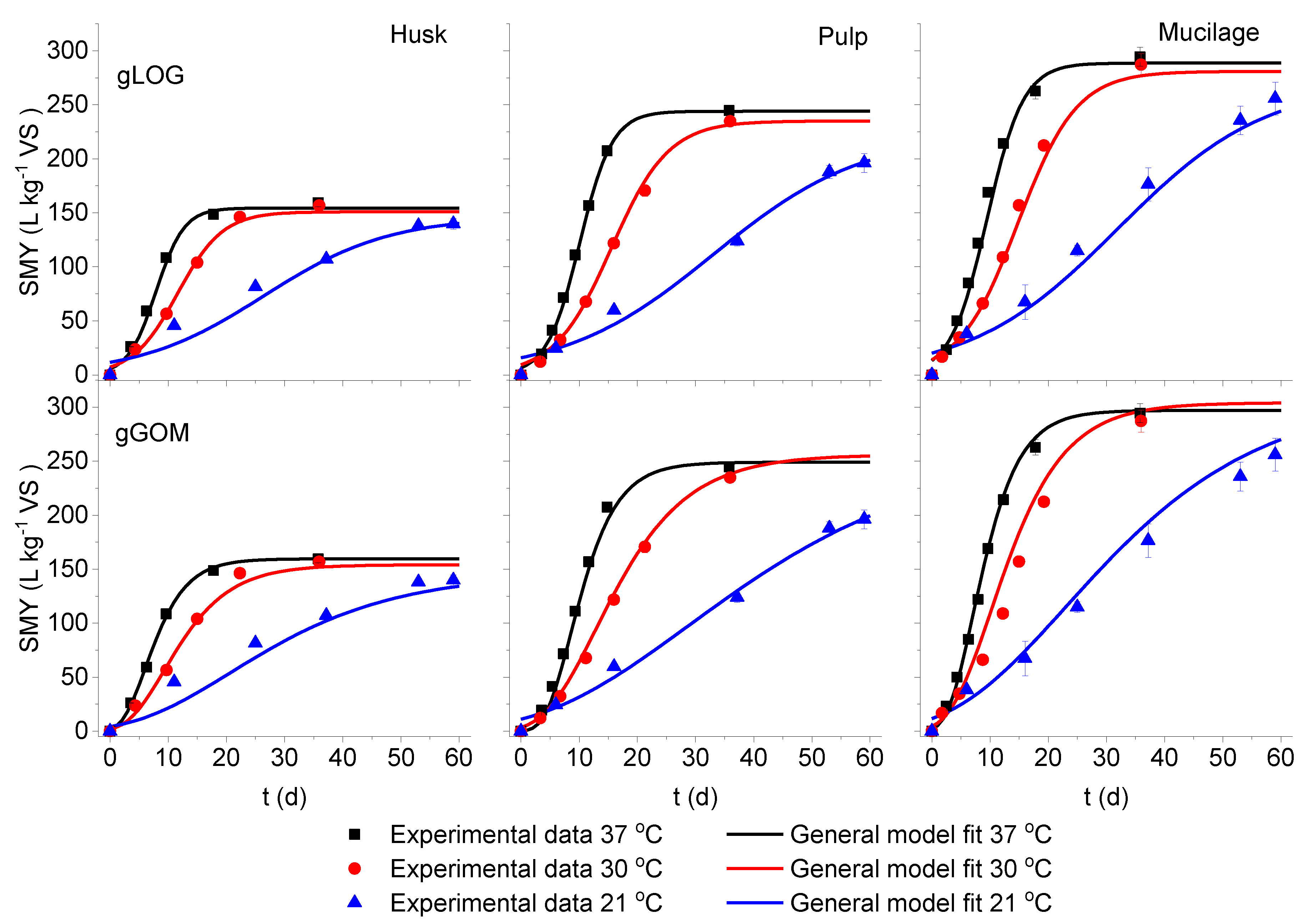
3.3. Model Validation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclatures
| Symbol | Definition |
| SMY | specific methane yield |
| BMP | bio-chemical methane potential |
| GOM | modified Gompertz model |
| LOG | modified logistic model |
| gLOG | generalized logistic model |
| gGOM | generalized Gompertz model |
| AIC | Akaike information criterion |
| ha | hectare (=10,000 m2) |
| a.s.l. | above sea level |
References
- Franca, A.S.; Oliveira, L.S. Coffee processing solid wastes: Current uses and future perspectives. In Agricultural Wastes; Ashworth, G.S., Azevedo, P., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; pp. 155–190. [Google Scholar]
- Gadhamshetty, V.; Arudchelvam, Y.; Nirmalakhandan, N.; Johnson, D.C. Modeling dark fermentation for biohydrogen production: ADM1-based model vs. Gompertz model. Int. J. Hydrogen Energy 2010, 35, 479–490. [Google Scholar] [CrossRef]
- Vincent, J.-C. Green Coffee Processing. In Coffee: Technology; Clarke, R., Macrae, R., Eds.; Elsevier Applied Science: London, UK, 1987; Volume 2. [Google Scholar]
- Oliveira, L.S.; Franca, A.S. An overview of the potential uses for coffee husks. In Coffee in health and disease prevention; Preedy, V.R., Ed.; Elsevier Inc.: New York, NY, USA, 2015; pp. 283–291. [Google Scholar] [CrossRef]
- Zayas Péerez, T.; Geissler, G.; Hernandez, F. Chemical oxygen demand reduction in coffee wastewater through chemical flocculation and advanced oxidation processes. J. Environ. Sci. 2007, 19, 300–305. [Google Scholar] [CrossRef]
- Devi, R.; Singh, V.; Kumar, A. COD and BOD reduction from coffee processing wastewater using Avacado peel carbon. Bioresour. Technol. 2008, 99, 1853–1860. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Mengistu, M.G.; Simane, B.; Eshete, G.; Workneh, T.S. A review on biogas technology and its contributions to sustainable rural livelihood in Ethiopia. Renew. Sust. Energy Rev. 2015, 48, 306–316. [Google Scholar] [CrossRef]
- Fischer, E.; Schmidt, T.; Hora, S.; Geirsdorf, J.; Stinner, W.; Scholwin, F. Agro-Industrial Biogas in Kenya; German International Cooperation (GIZ): Berlin, Germany, 2010. [Google Scholar]
- Ulsido, M.D.; Zeleke, G.; Li, M. Biogas potential assessment from a coffee husk: An option for solid waste management in Gidabo watershed of Ethiopia. In Proceedings of the 15th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 25–27 May 2016; pp. 1348–1354. [Google Scholar]
- Kivaisi, A.K.; Rubindamayugi, M.S.T. The potential of agro-industrial residues for production of biogas and electricity in Tanzania. Renew. Energy 1996, 9, 917–921. [Google Scholar] [CrossRef]
- Chala, B.; Oechsner, H.; Fritz, T.; Latif, S.; Müller, J. Increasing the loading rate of continuous stirred tank reactor for coffee husk and pulp: Effect of trace elements supplement. Eng. Life Sci. 2018, 18, 551–561. [Google Scholar] [CrossRef]
- Yadvika; Santosh; Sreekrishnan, T.R.; Kohli, S.; Rana, V. Enhancement of biogas production from solid substrates using different techniques––a review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar] [CrossRef]
- Tong, X.; Smith, L.H.; McCarty, P.L. Methane fermentation of selected lignocellulosic materials. Biomass 1990, 21, 239–255. [Google Scholar] [CrossRef]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Budiyono; Sumardiono, S. Predicting kinetic model of biogas production and biodegradability organic materials: Biogas production from vinasse at variation of COD/N ratio. Bioresour. Technol. 2013, 149, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Nyoman, W.I.; Seno, J.; Sunarso. The kinetic of biogas production rate from cattle manure in batch mode. Int. J. Chem. Biol. Eng. 2010, 3, 39–45. [Google Scholar]
- Zhu, B.; Gikas, P.; Zhang, R.; Lord, J.; Jenkins, B.; Li, X. Characteristics and biogas production potential of municipal solid wastes pretreated with a rotary drum reactor. Bioresour. Technol. 2009, 100, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.H.; Raj, M.A.; Muralidhara, P.L.; Desai, S.M.; Raju, G.K.M. Kinetics of Anaerobic Digestion of Water Hyacinth Using Poultry Litter as Inoculum. J. Environ. Dev. 2012, 3, 94–98. [Google Scholar]
- Adiga, S.; Ramya, R.; Shankar, B.B.; Patil, J.H.; Geetha, C.R. Kinetics of anaerobic digestion of water hyacinth, poultry litter, cow manure and primary sludge: A comparative study. Int. Conf. Biotechnol. Environ. Manag. 2012, 14, 73–78. [Google Scholar]
- Weissbach, F.; Parr, K.; Reinsdorf, U.; Warnke-Gurgel, C.; Losand, B. Losses of methane forming potential of pulpified sugar beets stored in open ground basins. LANDTECHNIK 2013, 68, 50–57. [Google Scholar]
- Chatterjee, T.; Chatterjee, B.K.; Majumdar, D.; Chakrabarti, P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochim. Biophys. Acta 2015, 1850, 299–306. [Google Scholar] [CrossRef]
- Franses, P.H. A method to select between Gompertz and logistic trend curves. Technol. Forecast. Soc. Chang. 1994, 45–49. [Google Scholar] [CrossRef]
- Wang, M.; Lee, E.; Dilbeck, M.P.; Liebelt, M.; Zhang, Q.; Ergas, S.J. Thermal pretreatment of microalgae for biomethane production: experimental studies, kinetics and energy analysis. J. Chem. Technol. Biotechnol. 2017, 92, 399–407. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; John Wiley & Sons: Weinheim, Germany, 2011. [Google Scholar]
- Ratkowsky, D.A.; Lowry, R.K.; McMeekin, T.A.; Stokes, A.N.; Chandler, R.E. Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J. Bacteriol. 1983, 154, 1222–1226. [Google Scholar] [PubMed]
- Jin, Y.; Hu, Z.; Wen, Z. Enhancing anaerobic digestibility and phosphorus recovery of dairy manure through microwave-based thermochemical pretreatment. Water Res. 2009, 43, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- Brulé, M.; Oechsner, H.; Jungbluth, T. Exponential model describing methane production kinetics in batch anaerobic digestion: A tool for evaluation of biochemical methane potential assays. Bioprocess Biosystems. Eng. 2014, 37, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van't Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [PubMed]
- Fazzini, M.; Bisci, C.; Billi, P. The climate of Ethiopia. In Landscapes and Landforms of Ethiopia; Springer: Dordrecht, The Netherlands, 2015; pp. 65–87. [Google Scholar]
- Mittweg, G.; Oechsner, H.; Hahn, V.; Lemmer, A.; Reinhardt-Hanisch, A. Repeatability of a laboratory batch method to determine the specific biogas and methane yields. Eng. Life Sci. 2012, 12, 270–278. [Google Scholar] [CrossRef]
- Muñoz, G.; Grieser, J. CLIMWAT 2.0 for CROPWAT: A Climatic Database for Irrigation Planning and Management. Available online: http://www.fao.org/land-water/databases-and-software/climwat-for-cropwat/en/ and http://www.webcitation.org/75aQLq1E1 (Archived by WebCite®) (accessed on 21 January 2019).
- Haag, N.L.; Nägele, H.J.; Fritz, T.; Oechsner, H. Effects of ensiling treatments on lactic acid production and supplementary methane formation of maize and amaranth - An advanced green biorefining approach. Bioresour. Technol. 2015, 178, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Chala, B.; Oechsner, H.; Latif, S.; Müller, J. Biogas Potential of Coffee Processing Waste in Ethiopia. Sustainability 2018, 10, 2678. [Google Scholar] [CrossRef]
- VDI. VDI 4630—Fermentation of Organic Materials : Characterisation of Substrate, Sampling, Collection of Material Data, Fermentation Tests; Verein Deutscher Ingenieure: Düsseldorf, Vietnam, 2006. [Google Scholar]
- Fischer, E.; Powrosnik, A.-M.; Beil, C. Assessment of process stability and biogas yield for the anaerobic digestion of horse dung in lab-scale. LANDTECHNIK 2013, 68, 248–251. [Google Scholar]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Khan, M.T.; Brulé, M.; Maurer, C.; Argyropoulos, D.; Müller, J.; Oechsner, H. Batch anaerobic digestion of banana waste-energy potential and modelling of methane production kinetics. Agric. Eng. Int. CIGR J. 2016, 18, 110–128. [Google Scholar]
- Shen, J.; Zhu, J. Development of General Gompertz Models and Their Simplified Two-Parameter Forms Based on Specific Microbial Growth Rate for Microbial Growth, Bio-Products and Substrate Consumption. Adv. Biotechnol. Microbiol. 2017, 4. [Google Scholar] [CrossRef]
- Pham, C.H.; Triolo, J.M.; Sommer, S.G. Predicting methane production in simple and unheated biogas digesters at low temperatures. Appl. Energy 2014, 136, 1–6. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Sarma, S.J.; Brar, S.K.; Le Bihan, Y.; Buelna, G.; Verma, M. Biohydrogen production by co-fermentation of crude glycerol and apple pomace hydrolysate using co-culture of Enterobacter aerogenes and Clostridium butyricum. Bioresour. Technol. 2015, 193, 297–306. [Google Scholar] [CrossRef]
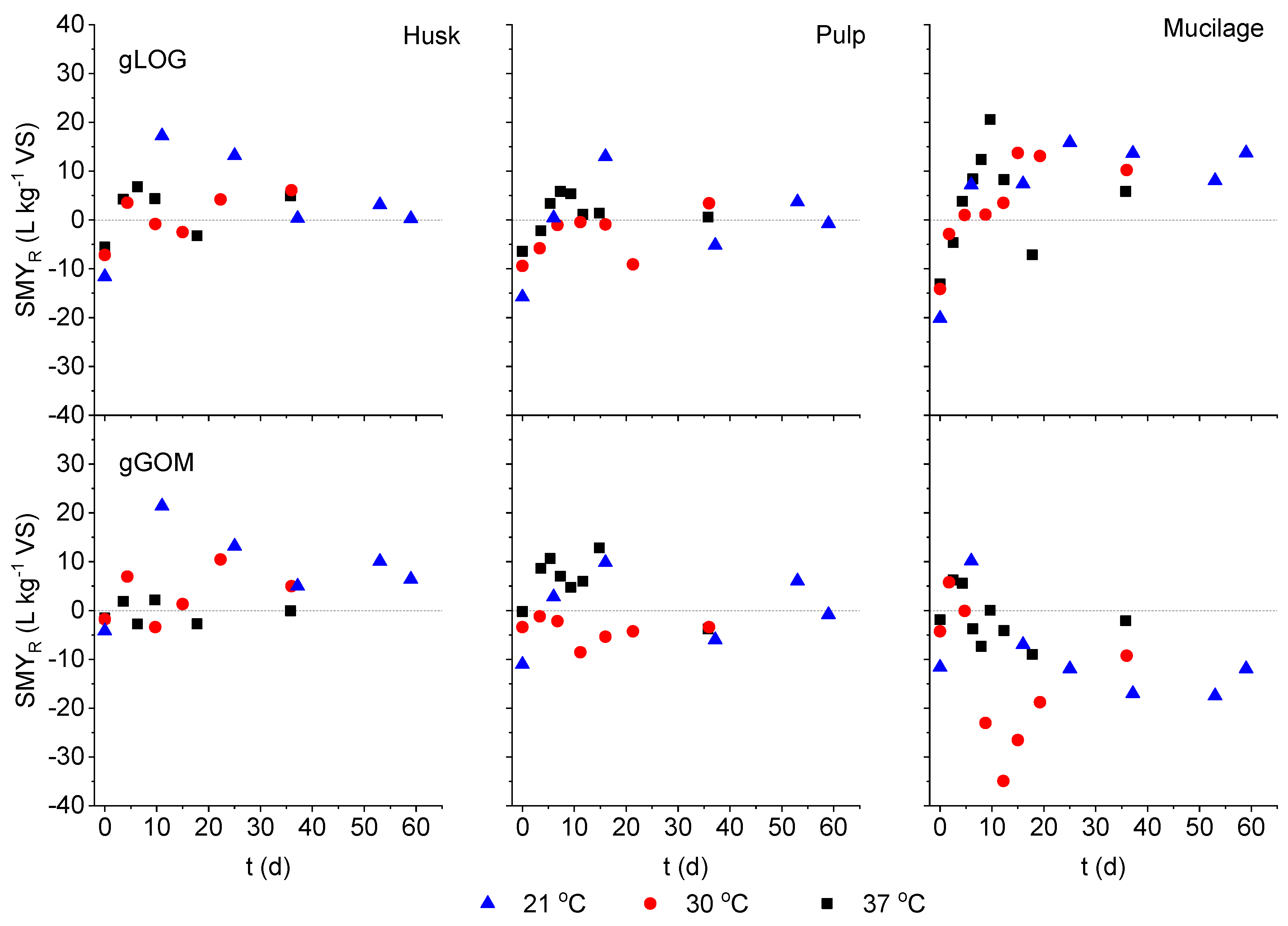
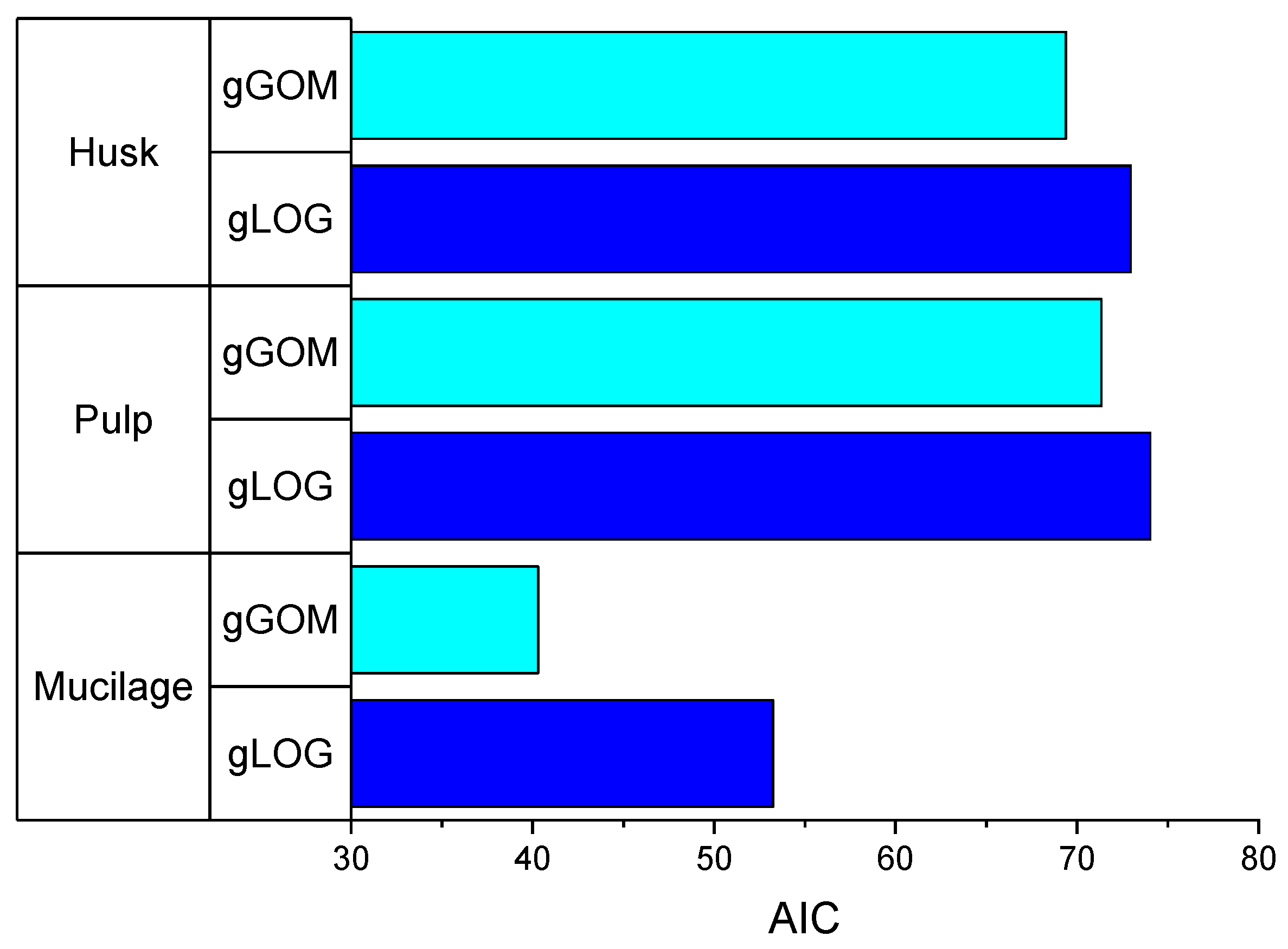
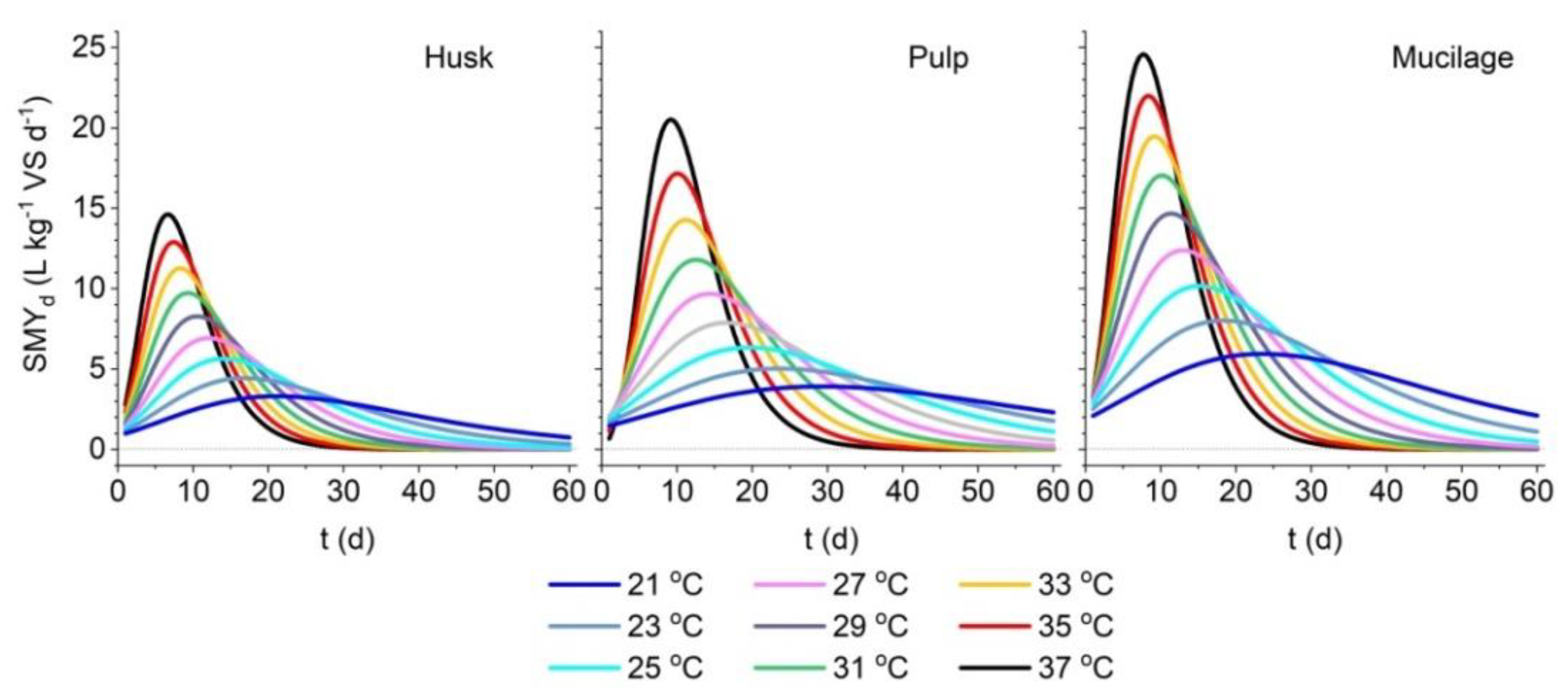
| Stations | H (m a.s.l.) | T min (°C) | T max (°C) | T mean (°C) |
|---|---|---|---|---|
| Gambella | 480 | 20.4 | 34.8 | 27.6 |
| Arbaminch | 1290 | 17.9 | 29.6 | 23.8 |
| Jinka | 1430 | 15.3 | 26.9 | 21.1 |
| Agaro | 1560 | 12.3 | 28.2 | 20.3 |
| Bonga | 1725 | 11.6 | 27.4 | 19.5 |
| Harer | 1856 | 13.8 | 24.4 | 19.1 |
| Gimbi | 1870 | 13.2 | 26.5 | 19.9 |
| Yirga chefe | 1925 | 10.3 | 24.7 | 17.5 |
| Gore | 2002 | 13.7 | 23.4 | 18.6 |
| Nekemte | 2080 | 11.9 | 24.0 | 18.0 |
| Substrate | Dig. Temp (°C) | SMY, measured (L kg−1 VS) | Model | S (L kg−1 VS) | Rm (L kg−1 VS d−1) | λ (d) | SMY, estimated (L kg−1 VS) | Goodness of fit | |
|---|---|---|---|---|---|---|---|---|---|
| R2 | MAE | ||||||||
| Husk | 37 | 159.4 ± 1.8 | LOG | 155.2 ± 3.9 | 16.1 ± 1.6 | 2.7 ± 0.5 | 155.2 | 0.997 | 2.888 |
| GOM | 158.5 ± 2.4 | 14.8 ± 0.8 | 2.1 ± 0.3 | 158.4 | 0.997 | 2.334 | |||
| 30 | 156.8 ± 2.6 | LOG | 157.3 ± 4.4 | 9.7 ± 0.8 | 4.1 ± 0.7 | 156.9 | 0.976 | 6.289 | |
| GOM | 163.0 ± 6.4 | 9.0 ± 0.9 | 3.0 ± 0.9 | 160.0 | 0.999 | 2.585 | |||
| 21 | 139.9 ± 6.8 | LOG | 143.2 ± 12.2 | 3.4 ± 0.7 | 1.6 ± 4.9 | 138.7 | 0.997 | 4.186 | |
| GOM | 151.4 ± 13.7 | 3.3 ± 0.6 | 0.1 ±3.3 | 139.6 | 0.987 | 6.485 | |||
| Pulp | 37 | 244.7 ± 6.4 | LOG | 243.8 ± 3.7 | 21.8 ± 0.8 | 4.3 ± 0.2 | 243.8 | 0.996 | 5.909 |
| GOM | 248.2 ± 5.3 | 20.7 ± 1.0 | 3.7 ± 0.3 | 247.8 | 0.998 | 3.230 | |||
| 30 | 234.8 ± 2.9 | LOG | 236.6 ± 7.3 | 12.0 ± 0.8 | 6.1 ± 0.7 | 232.7 | 0.992 | 6.749 | |
| GOM | 253.3 ± 3.8 | 10.7 ± 0.3 | 4.6 ± 0.2 | 235.1 | 0.999 | 1.924 | |||
| 21 | 196.2 ± 7.6 | LOG | 223.2 ± 29.5 | 4.3 ± 0.6 | 7.2 ± 3.7 | 197.3 | 0.995 | 3.607 | |
| GOM | 265.0 ± 52.6 | 3.9 ± 0.4 | 3.5 ± 2.7 | 197.9 | 0.987 | 4.660 | |||
| Mucilage | 37 | 294.5 ± 9.6 | LOG | 284.6 ± 7.0 | 24.8 ± 1.7 | 3.1 ± 0.4 | 284.5 | 0.998 | 3.317 |
| GOM | 294.4 ± 3.9 | 23.2 ± 0.8 | 2.5 ± 0.2 | 293.8 | 1.000 | 1.377 | |||
| 30 | 287.1 ± 11.1 | LOG | 288.5 ± 6.2 | 15.9 ± 0.8 | 5.3 ± 0.4 | 286.1 | 0.992 | 6.036 | |
| GOM | 304.2 ± 10.6 | 13.9 ± 0.9 | 3.8 ± 0.6 | 289.5 | 0.999 | 3.237 | |||
| 21 | 255.9 ± 16.6 | LOG | 268.3 ± 16.9 | 6.0 ± 0.6 | 6.5 ± 2.3 | 250.8 | 0.996 | 5.411 | |
| GOM | 311.7 ± 27.5 | 5.3 ± 0.4 | 3.5 ± 1.6 | 253.9 | 0.995 | 4.112 | |||
| Substrates | Model | S | Rm | λ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R² | R² | R² | |||||||||
| Husk | LOG | 137.0 | 0.5 | 0.640 | 9.43 | 0.01 | 2.40 | 1.000 | 7.17 | 0.1 | 0.905 |
| GOM | 130.3 | 0.8 | 0.999 | 6.42 | 0.58 | 5.93 | 1.000 | 7.3 | −0.1 | 0.985 | |
| Pulp | LOG | 196.6 | 1.3 | 0.987 | −5.68 | 10.00 | 15.9 | 1.000 | 11.2 | −0.2 | 0.954 |
| GOM | 286.7 | −1.0 | 0.977 | −2.44 | 6.36 | 12.4 | 0.999 | 3.47 | 0.02 | 0.570 | |
| Mucilage | LOG | 248.9 | 1.1 | 0.646 | −14.3 | 19.60 | 24.7 | 1.000 | 11 | -0.2 | 0.937 |
| GOM | 334.8 | -1.0 | 0.978 | -50.9 | 56.90 | 55.8 | 1.000 | 4.96 | -0.1 | 0.536 | |
| Substrate | Dig. Temp (°C) | Model | S (L kg−1 VS) | Rm (L kg−1 VS d−1) | λ (d) | SMY estimated (L kg−1 VS) | Goodness of fit | |
|---|---|---|---|---|---|---|---|---|
| R2 | MAE | |||||||
| Husk | 37 | gLOG | 155.5 | 17.29 | 3.5 | 154.5 | 0.993 | 4.86 |
| gGOM | 159.9 | 15.03 | 3.6 | 159.5 | 1.000 | 1.86 | ||
| 30 | gLOG | 152.0 | 9.86 | 4.2 | 150.7 | 0.994 | 4.05 | |
| gGOM | 154.3 | 9.07 | 4.3 | 151.8 | 0.997 | 4.82 | ||
| 21 | gLOG | 147.5 | 9.44 | 5.1 | 139.6 | 0.959 | 7.60 | |
| gGOM | 147.1 | 7.00 | 5.2 | 133.5 | 0.986 | 10.02 | ||
| Pulp | 37 | gLOG | 244.7 | 21.67 | 3.8 | 244.1 | 0.998 | 3.29 |
| gGOM | 249.7 | 20.67 | 4.2 | 248.5 | 0.992 | 6.72 | ||
| 30 | gLOG | 235.6 | 11.93 | 5.2 | 231.4 | 0.995 | 4.31 | |
| gGOM | 256.7 | 10.70 | 4.1 | 238.2 | 0.997 | 4.06 | ||
| 21 | gLOG | 223.9 | 4.32 | 7.0 | 197.0 | 0.987 | 6.48 | |
| gGOM | 265.7 | 3.92 | 3.9 | 197.1 | 0.991 | 6.08 | ||
| Mucilage | 37 | gLOG | 289.6 | 23.16 | 3.6 | 289.5 | 0.989 | 9.30 |
| gGOM | 297.8 | 24.90 | 1.3 | 293.7 | 0.998 | 4.06 | ||
| 30 | gLOG | 281.9 | 13.92 | 5.0 | 277.7 | 0.991 | 7.34 | |
| gGOM | 304.8 | 15.96 | 2.0 | 294.4 | 0.960 | 14.99 | ||
| 21 | gLOG | 272.0 | 5.30 | 6.8 | 242.6 | 0.980 | 12.15 | |
| gGOM | 313.8 | 6.00 | 2.9 | 267.2 | 0.981 | 12.27 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chala, B.; Oechsner, H.; Müller, J. Introducing Temperature as Variable Parameter into Kinetic Models for Anaerobic Fermentation of Coffee Husk, Pulp and Mucilage. Appl. Sci. 2019, 9, 412. https://doi.org/10.3390/app9030412
Chala B, Oechsner H, Müller J. Introducing Temperature as Variable Parameter into Kinetic Models for Anaerobic Fermentation of Coffee Husk, Pulp and Mucilage. Applied Sciences. 2019; 9(3):412. https://doi.org/10.3390/app9030412
Chicago/Turabian StyleChala, Bilhate, Hans Oechsner, and Joachim Müller. 2019. "Introducing Temperature as Variable Parameter into Kinetic Models for Anaerobic Fermentation of Coffee Husk, Pulp and Mucilage" Applied Sciences 9, no. 3: 412. https://doi.org/10.3390/app9030412
APA StyleChala, B., Oechsner, H., & Müller, J. (2019). Introducing Temperature as Variable Parameter into Kinetic Models for Anaerobic Fermentation of Coffee Husk, Pulp and Mucilage. Applied Sciences, 9(3), 412. https://doi.org/10.3390/app9030412