Review of Solvents Based on Biomass for Mitigation of Wax Paraffin in Indonesian Oilfield
Abstract
1. Introduction
2. Availability of Raw Materials
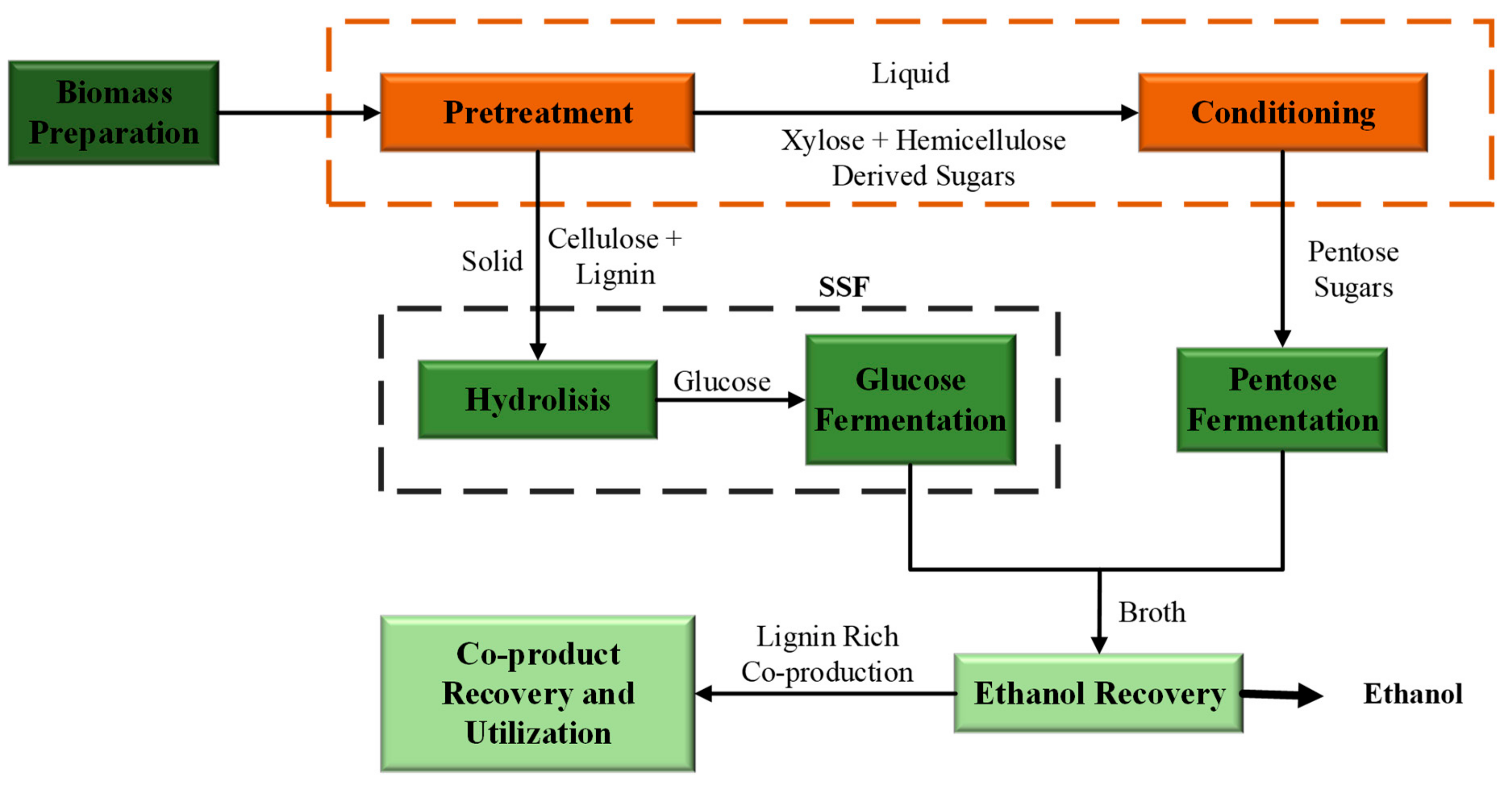
3. Bioethanol Production Process
3.1. Pretreatment
3.2. Hydrolysis
3.3. Fermentation
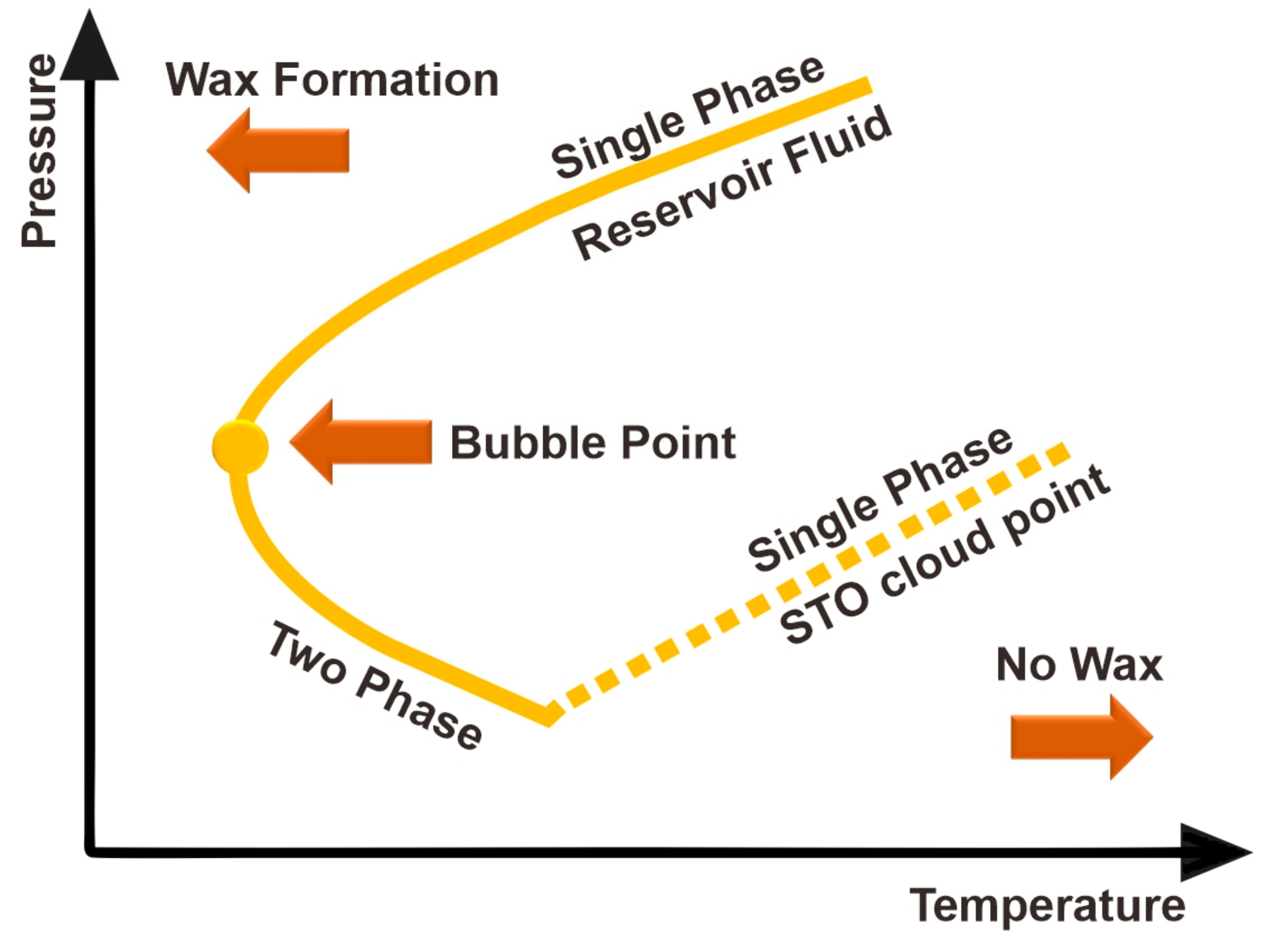
4. Wax Problems and Use of Solvents in the Oil Industry
- (1)
- Thermodynamic wax inhibitor (TWI)—pressing cloud point, reducing viscosity and pour point, requires a high volume;
- (2)
- Depressant/pour point—modifies the wax crystal structure, reduces viscosity, and produces stress, but does not reduce the rate of wax deposition;
- (3)
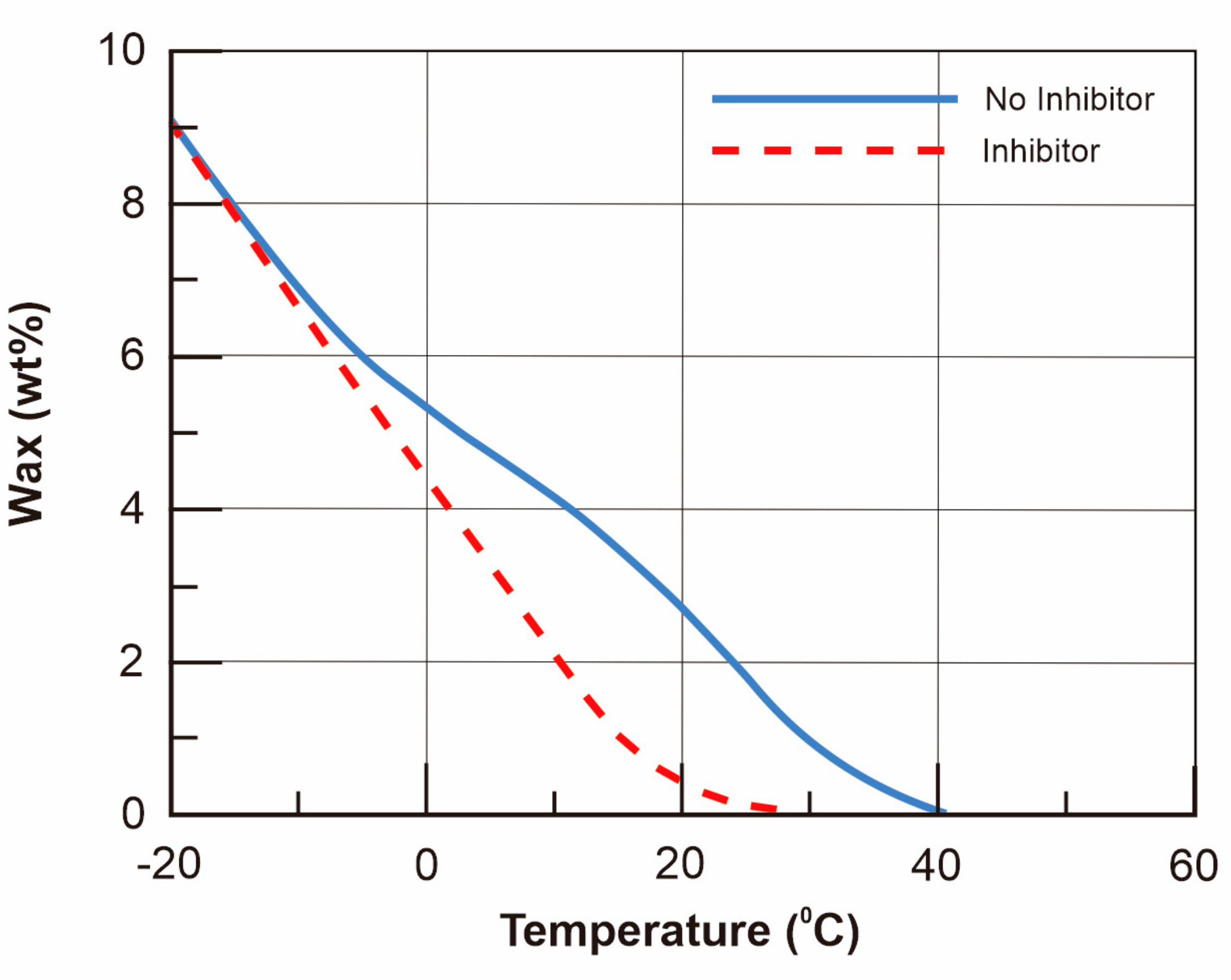
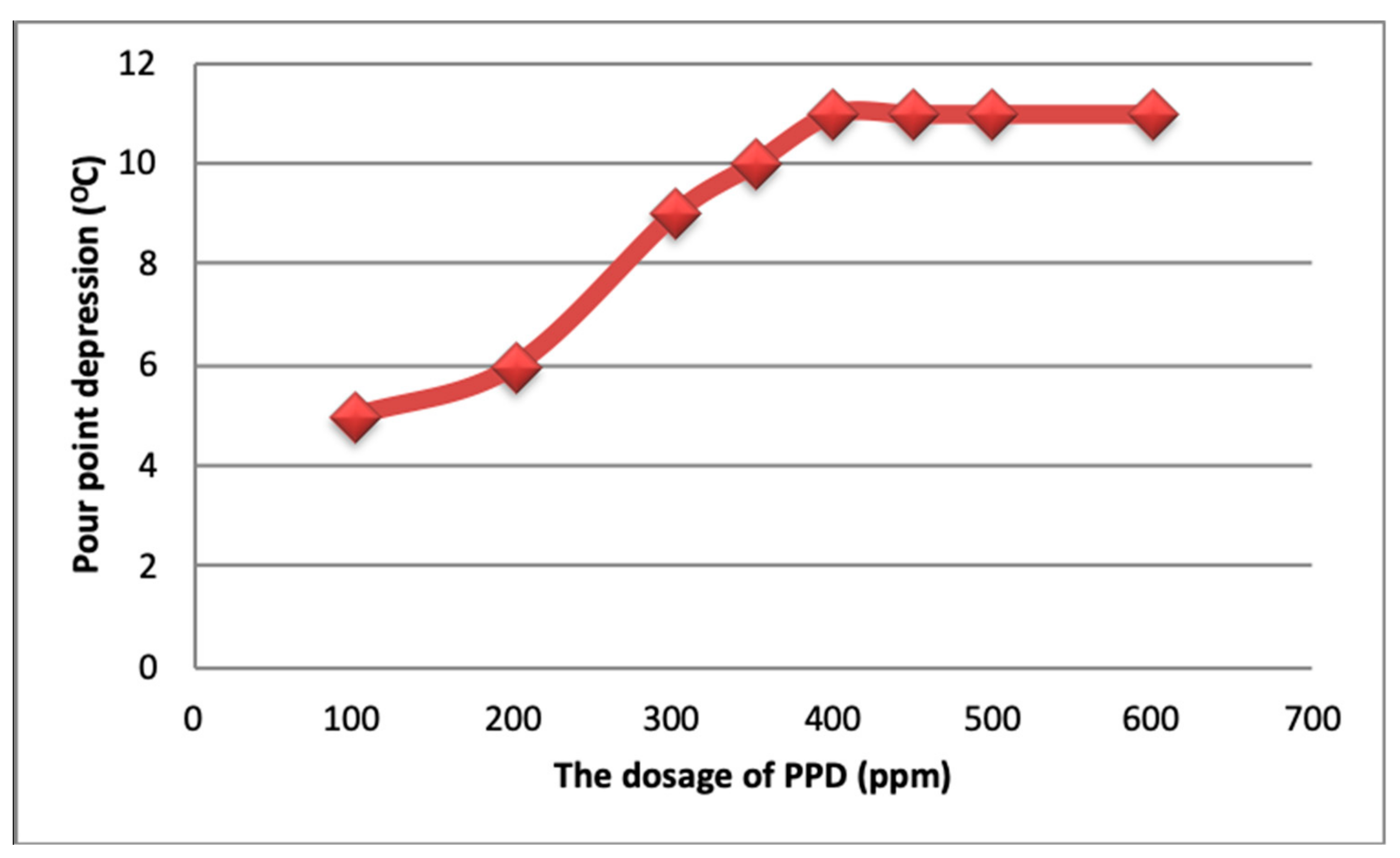
4.1. Pour Point Depressants (PPDs)
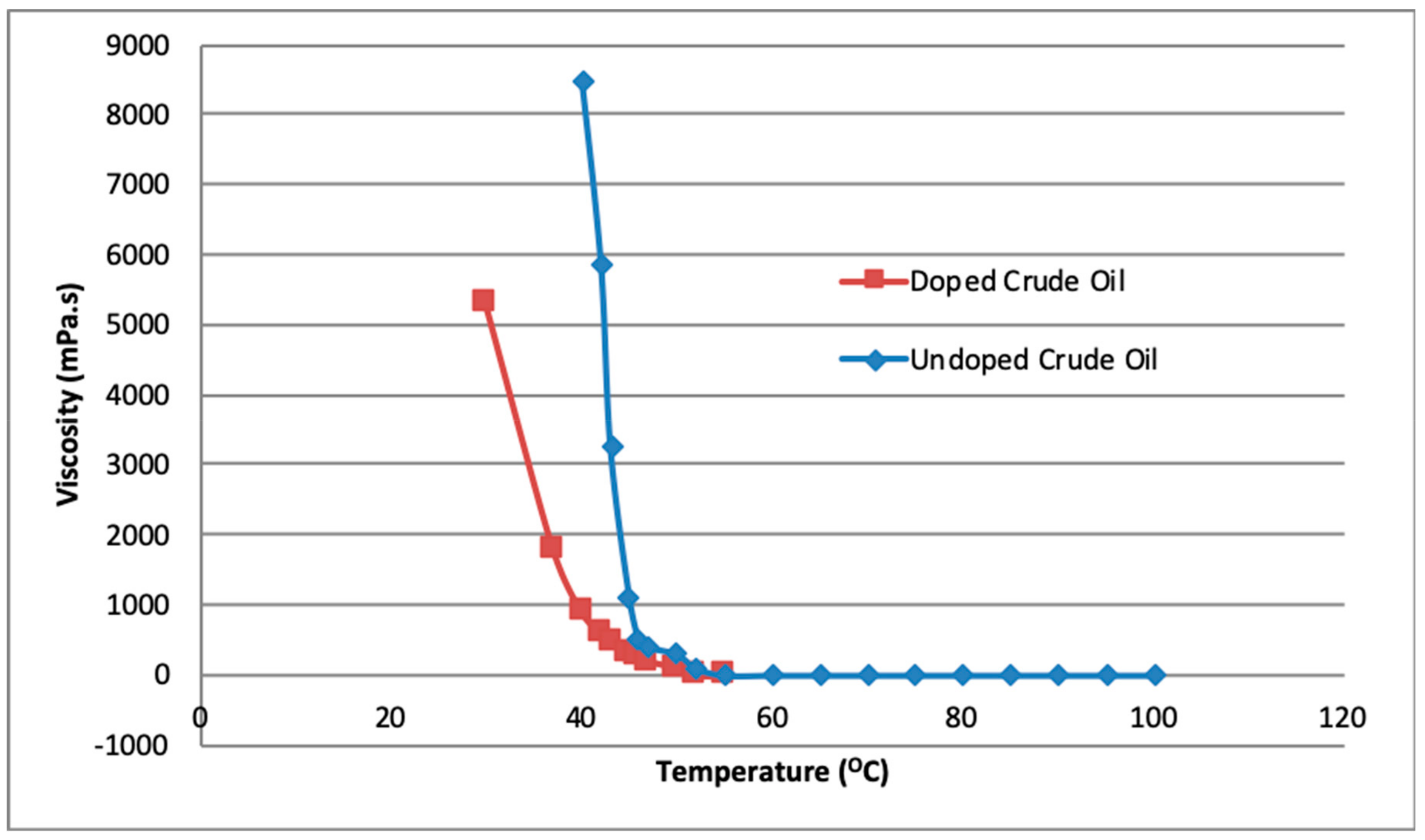
4.2. Rheological Studies
4.3. Evaluation of Paraffin Inhibition Efficiency
WAT Determination (ASTM Standard D5773-04, 2005)
5. Discussion
5.1. Production of Solvents for the Oil Industry
5.2. Solvent Application in the Oil Industry
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| TWI | Thermodynamic wax inhibitor |
| WAT | Wax appearance temperature |
| WDT | Wax disappearance temperature |
| WTE | Waste to energy |
| PIE | Paraffin inhibition efficiency |
References
- Tukenov, D. Technology update: Nanochemistry drives new method for removal and control of wax. J. Pet. Technol. 2014, 66, 30–33. [Google Scholar] [CrossRef]
- Taraneh, J.B.; Rahmatollah, G.; Hassan, A.; Alireza, D. Effect of wax inhibitors on pour point and rheological properties of Iranian waxy crude oil. Fuel Process. Technol. 2008, 89, 973–977. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, Q. Wax and asphaltenes. In Subsea Engineering Handbook; Gulf Professional Publishing: Houston, TX, USA, 2018; pp. 435–453. ISBN 978-0-12-812622-6. [Google Scholar]
- Bello, O.; Fasesan, S.; Teodoriu, C.; Reinicke, K. An evaluation of the performance of selected wax inhibitors on paraffin deposition of Nigerian crude oils. Pet. Sci. Technol. 2006, 24, 195–206. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Yang, F.; Zhang, Y.; Xiao, Z.; Sun, G. Structural properties of gelled Changqing waxy crude oil benefitted with nanocomposite pour point depressant. Fuel 2016, 184, 544–554. [Google Scholar] [CrossRef]
- Admiral, A.; Abdullah, M.K.; Ariffin, A. Evaluation of emulsified acrylate polymer and its pour point depressant performance. Procedia Chem. 2016, 19, 319–326. [Google Scholar] [CrossRef]
- Merino-Garcia, D.; Correra, S. Cold flow: A review of a technology to avoid wax deposition. Pet. Sci. Technol. 2008, 26, 446–459. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, B.; Fu, X.; Wang, R.; Lei, J. Preparation of biodegradable microcapsules through an organic solvent-free interfacial polymerization method. Polym. Adv. Technol. 2019, 30, 483–488. [Google Scholar] [CrossRef]
- Yuliusman; Nasruddin; Afdhol, M.K.; Haris, F.; Amiliana, R.A.; Hanafi, A.; Ramadhan, I.T. Production of activated carbon from coffee grounds using chemical and physical activation method. Adv. Sci. Lett. 2017, 23, 5751–5755. [Google Scholar] [CrossRef]
- Yuliusman; Afdhol, M.K.; Sanal, A.; Nasruddin. CFD modelling of adsorption behaviour in AGN tank with polyethylene terephthalate plastic waste based activated carbon. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012015. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic solvent effects in biomass conversion reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Pang, S.; Su, C.; Lv, M.; An, N.; Wang, K.; Cai, D.; Qin, P. Importance of redefinition of corn stover harvest time to enhancing non-food bio-ethanol production. Renew. Energy 2020, 146, 1444–1450. [Google Scholar] [CrossRef]
- Alencar, B.R.A.; Dutra, E.D.; Sampaio, E.V.d.S.B.; Menezes, R.S.C.; Morais, M.A. Enzymatic hydrolysis of cactus pear varieties with high solids loading for bioethanol production. Bioresour. Technol. 2018, 250, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, M.; Sandoval, M.; Hernáiz, M.J. Bio-solvents change regioselectivity in the synthesis of disaccharides using Biolacta β-galactosidase. Tetrahedron 2012, 68, 2141–2145. [Google Scholar] [CrossRef]
- Tobiszewski, M. Analytical chemistry with biosolvents. Anal. Bioanal. Chem. 2019, 411, 4359–4364. [Google Scholar] [CrossRef]
- Bergez-Lacoste, M.; Thiebaud-Roux, S.; De Caro, P.; Fabre, J.-F.; Gerbaud, V.; Mouloungui, Z. From chemical platform molecules to new biosolvents: Design engineering as a substitution methodology. Biofuels Bioprod. Biorefin. 2014, 8, 438–451. [Google Scholar] [CrossRef]
- Katrib, F.A.; Chambat, G.; Joseleau, J.-P. Organic solvent pretreatment to enhance enzymic saccharification of straw. J. Sci. Food Agric. 1988, 43, 309–317. [Google Scholar] [CrossRef]
- Bar, R.; Gainer, J.L. Acid fermentation in water-organic solvent two-liquid phase systems. Biotechnol. Prog. 1987, 3, 109–114. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Potential of bioethanol production waste for methane recovery. Energy 2019, 133–139. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, J.; Bhaskar, T. Advanced hydrothermal liquefaction of biomass for bio-oil production. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 245–266. ISBN 978-0-12-816856-1. [Google Scholar]
- Breeden, S.W.; Clark, J.H.; Macquarrie, D.J.; Sherwood, J. Green solvents. In Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W., Cue, B.W., Jr., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 241–261. [Google Scholar]
- Soccol, C.R.; de Souza Vandenberghe, L.P.; Medeiros, A.B.P.; Karp, S.G.; Buckeridge, M.; Ramos, L.P.; Pitarelo, A.P.; Ferreira-Leitão, V.; Gottschalk, L.M.F.; Ferrara, M.A.; et al. Bioethanol from lignocelluloses: Status and perspectives in Brazil. Bioresour. Technol. 2010, 101, 4820–4825. [Google Scholar] [CrossRef]
- Martín, M.; Grossmann, I.E. Superstructure optimization of Lignocellulosic Bioethanol plants. In 20th European Symposium on Computer Aided Process Engineering; Computer Aided Chemical Engineering Volume 28; Pierucci, S., Ferraris, G.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 943–948. ISBN 1570-7946. [Google Scholar]
- Camargos, C.H.M.; Silva, R.A.P.; Csordas, Y.; Silva, L.L.; Rezende, C.A. Experimentally designed corn biomass fractionation to obtain lignin nanoparticles and fermentable sugars. Ind. Crop. Prod. 2019, 140, 111649. [Google Scholar] [CrossRef]
- Abaide, E.R.; Dotto, G.L.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Adsorption of 2–nitrophenol using rice straw and rice husks hydrolyzed by subcritical water. Bioresour. Technol. 2019, 284, 25–35. [Google Scholar] [CrossRef]
- Romo, J.E.; Bollar, N.V.; Zimmermann, C.J.; Wettstein, S.G. Conversion of sugars and biomass to furans using heterogeneous catalysts in biphasic solvent systems. ChemCatChem 2018, 10, 4805–4816. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Liu, H.; Hui, L.; Wang, H.; Liu, H. Characterization of liquefied products from corn stalk and its biomass components by polyhydric alcohols with phosphoric acid. Carbohydr. Polym. 2019, 215, 170–178. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. Sustainable waste management: Waste to energy plant as an alternative to landfill. Energy Convers. Manag. 2017, 131, 18–31. [Google Scholar] [CrossRef]
- Yuliusman; Nasruddin; Afdhol, M.K.; Amiliana, R.A.; Hanafi, A. Preparation of activated carbon from palm shells using KOH and ZnCl2 as the activating agent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 180, 012282. [Google Scholar] [CrossRef]
- Yuliusman; Afdhol, M.K.; Sanal, A. Carbon monoxide and methane adsorption of crude oil refinery using activated carbon from palm shells as biosorbent. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012016. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Nikolić, S.; Mojović, L.; Rakin, M.; Pejin, D. Bioethanol production from corn meal by simultaneous enzymatic saccharification and fermentation with immobilized cells of Saccharomyces cerevisiae var. ellipsoideus. Fuel 2009, 88, 1602–1607. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Zhao, Y.; Damgaard, A.; Christensen, T.H. Bioethanol from corn stover—A review and technical assessment of alternative biotechnologies. Prog. Energy Combust. Sci. 2018, 67, 275–291. [Google Scholar] [CrossRef]
- Mabee, W.E.; Saddler, J.N. Bioethanol from lignocellulosics: Status and perspectives in Canada. Bioresour. Technol. 2010, 101, 4806–4813. [Google Scholar] [CrossRef]
- Nikolić, S.; Mojović, L.; Pejin, D.; Rakin, M.; Vukašinović, M. Production of bioethanol from corn meal hydrolyzates by free and immobilized cells of Saccharomyces cerevisiae var. ellipsoideus. Biomass Bioenergy 2010, 34, 1449–1456. [Google Scholar] [CrossRef]
- Saha, K.; Maheswari, U.R.; Sikder, J.; Chakraborty, S.; da Silva, S.S.; dos Santos, J.C. Membranes as a tool to support biorefineries: Applications in enzymatic hydrolysis, fermentation and dehydration for bioethanol production. Renew. Sustain. Energy Rev. 2017, 74, 873–890. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Rajkumar, T.; Pandi, D.K.; Ahmed, A.S.; Ani, F.N. Microwave assisted acid hydrolysis for bioethanol fuel production from sago pith waste. Waste Manag. 2019, 86, 80–86. [Google Scholar] [CrossRef]
- Gnansounou, E. Production and use of lignocellulosic bioethanol in Europe: Current situation and perspectives. Bioresour. Technol. 2010, 101, 4842–4850. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, V. Bioethanol production from corn. In Corn, 3rd ed.; Chemistry and Technology; Serna-Saldiva, S.O., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 615–631. [Google Scholar]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Chen, H.; Fu, X. Industrial technologies for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2016, 57, 468–478. [Google Scholar] [CrossRef]
- Bhatt, S.M. Shilpa Lignocellulosic feedstock conversion, inhibitor detoxification and cellulosic hydrolysis—A review. Biofuels 2014, 5, 633–649. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Sun, J.; Liao, W. Effects of liquefaction parameters of cellulose in supercritical solvents of methanol, ethanol and acetone on products yield and compositions. Bioresour. Technol. 2019, 275, 123–129. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Tiryaki, O.N.; Irmak, S.; Ramchandran, D.; Subbiah, J.; Morton, M. Utilization of excess corn kernels for hydrogen gas biofuel production. Int. J. Hydrog. Energy 2019, 44, 29956–29963. [Google Scholar] [CrossRef]
- Zhao, L.; Ou, X.; Chang, S. Life-cycle greenhouse gas emission and energy use of bioethanol produced from corn stover in China: Current perspectives and future prospectives. Energy 2016, 115, 303–313. [Google Scholar] [CrossRef]
- Gomes, J.; Batra, J.; Chopda, V.R.; Kathiresan, P.; Rathore, A.S. Monitoring and control of bioethanol production from lignocellulosic biomass. In Waste Biorefinery: Potential and Perspectives; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 727–749. [Google Scholar]
- Pino, M.S.; Rodríguez-Jasso, R.M.; Michelin, M.; Flores-Gallegos, A.C.; Morales-Rodriguez, R.; Teixeira, J.A.; Ruiz, H.A. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem. Eng. J. 2018, 347, 119–136. [Google Scholar] [CrossRef]
- Li, X.; Kim, T.H.; Nghiem, N.P. Bioethanol production from corn stover using aqueous ammonia pretreatment and two-phase simultaneous saccharification and fermentation (TPSSF). Bioresour. Technol. 2010, 101, 5910–5916. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Nandal, P.; Sharma, S.; Arora, A. Bioprospecting non-conventional yeasts for ethanol production from rice straw hydrolysate and their inhibitor tolerance. Renew. Energy 2020, 147, 1694–1703. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Yan, X.-H.; Zhang, J.-L.; Wang, L.-Y.; Xue, H.; Jiang, G.-C.; Ma, X.-T.; Liu, X.-J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef]
- Zou, W.; Li, J.; Vinogradov, E.; Cox, A. Removal of cell wall polysaccharide in pneumococcal capsular polysaccharides by selective degradation via deamination. Carbohydr. Polym. 2019, 218, 199–207. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, X.; Liu, N.; Gao, Y.; Wang, L.; Xu, G.; Li, X.; Yang, Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, W.; Gong, H.; Yang, Y.; Zhang, A.; Liu, H.; Cao, J.; Guo, F.; Cui, K. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chem. 2019, 300, 125194. [Google Scholar] [CrossRef]
- Zhan, P.; Tang, K.; Chen, X.; Yu, L. Complete genome sequence of Maribacter sp. T28, a polysaccharide-degrading marine flavobacteria. J. Biotechnol. 2017, 259, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT 2018, 95, 223–229. [Google Scholar] [CrossRef]
- Shao, L.-L.; Xu, J.; Shi, M.-J.; Wang, X.-L.; Li, Y.-T.; Kong, L.-M.; Hider, R.C.; Zhou, T. Preparation, antioxidant and antimicrobial evaluation of hydroxamated degraded polysaccharides from Enteromorpha prolifera. Food Chem. 2017, 237, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Lodge, J.S.; Trabold, T.A. Characteristics of food processing wastes and their use in sustainable alcohol production. Renew. Sustain. Energy Rev. 2018, 81, 510–523. [Google Scholar] [CrossRef]
- Afdhol, M.K.; Lubis, H.Z.; Siregar, C.P. Bioethanol production from tea waste as a basic ingredient in renewable energy sources. J. Earth Energy Eng. 2019, 8, 21. [Google Scholar] [CrossRef]
- Puspawati, S.; Wagiman; Ainuri, M.; Nugraha, D.A.; Haslianti. The production of bioethanol fermentation substrate from Eucheuma cottonii seaweed through hydrolysis by cellulose enzyme. Agric. Agric. Sci. Procedia 2015, 3, 200–205. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.; Wen, L.; Zhu, H.; Jiang, Y.; John, A.; Yu, L.; Yang, B. Effect of morin on the degradation of water-soluble polysaccharides in banana during softening. Food Chem. 2019, 287, 346–353. [Google Scholar] [CrossRef]
- Phwan, C.K.; Ong, H.C.; Chen, W.H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energy Convers. Manag. 2018, 173, 81–94. [Google Scholar] [CrossRef]
- Conde-Mejía, C.; Jiménez-Gutiérrez, A.; Gómez-Castro, F.I. Purification of bioethanol from a fermentation process: Alternatives for dehydration. In Computer Aided Chemical Engineering; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 38, pp. 373–378. ISBN 9780444634283. [Google Scholar]
- Fitria; Ruan, H.; Fransen, S.C.; Carter, A.H.; Tao, H.; Yang, B. Selecting winter wheat straw for cellulosic ethanol production in the Pacific Northwest, U.S.A. Biomass Bioenergy 2019, 123, 59–69. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, S.; Wang, M.; Cao, R.; Zhang, R.; Zhan, R.; Wang, K. A novel glycoside hydrolase family 42 enzyme with bifunctional β-galactosidase and α-L-arabinopyranosidase activities and its synergistic effects with cognate glycoside hydrolases in plant polysaccharides degradation. Int. J. Biol. Macromol. 2019, 140, 129–139. [Google Scholar] [CrossRef]
- Alfonsín, V.; Maceiras, R.; Gutiérrez, C. Bioethanol production from industrial algae waste. Waste Manag. 2019, 87, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ren, Y.; Jiang, F.; Liang, J.; Du, S. Pretreatment of quinoa straw with 1-butyl-3-methylimidazolium chloride and physiochemical characterization of biomass. Renew. Energy 2020, 146, 1364–1371. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, N.; Fu, X.; Wang, L.; Yang, Y.; Ren, Y.; Liu, J.; Wang, L. Structural characteristics, biological, rheological and thermal properties of the polysaccharide and the degraded polysaccharide from raspberry fruits. Int. J. Biol. Macromol. 2019, 132, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sayed, W.; Cabrol, A.; Abdallah, R.; Taha, S.; Amrane, A.; Djelal, H. Enhancement of ethanol production from synthetic medium model of hydrolysate of macroalgae. Renew. Energy 2018, 124, 3–10. [Google Scholar] [CrossRef]
- Bar, R. Effect of interphase mixing on a water–organic solvent two-liquid phase microbial system: Ethanol fermentation. J. Chem. Technol. Biotechnol. 1988, 43, 49–62. [Google Scholar] [CrossRef]
- Park, J.; Shiroma, R.; Al-Haq, M.I.; Zhang, Y.; Ike, M.; Arai-Sanoh, Y.; Ida, A.; Kondo, M.; Tokuyasu, K. A novel lime pretreatment for subsequent bioethanol production from rice straw—Calcium capturing by carbonation (CaCCO) process. Bioresour. Technol. 2010, 101, 6805–6811. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jin, Y.S.; Cha, Y.L.; Seo, J.H. Bioethanol production from cellulosic hydrolysates by engineered industrial Saccharomyces cerevisiae. Bioresour. Technol. 2017, 228, 355–361. [Google Scholar] [CrossRef]
- Guerrero, A.B.; Ballesteros, I.; Ballesteros, M. The potential of agricultural banana waste for bioethanol production. Fuel 2018, 213, 176–185. [Google Scholar] [CrossRef]
- Jin, X.; Song, J.; Liu, G.-Q. Bioethanol production from rice straw through an enzymatic route mediated by enzymes developed in-house from Aspergillus fumigatus. Energy 2019, 116395. [Google Scholar] [CrossRef]
- Derman, E.; Abdulla, R.; Marbawi, H.; Sabullah, M.K. Oil palm empty fruit bunches as a promising feedstock for bioethanol production in Malaysia. Renew. Energy 2018, 129, 285–298. [Google Scholar] [CrossRef]
- Carrillo-Nieves, D.; Rostro Alanís, M.J.; de la Cruz Quiroz, R.; Ruiz, H.A.; Iqbal, H.M.N.; Parra-Saldívar, R. Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 63–74. [Google Scholar] [CrossRef]
- Ussiri, D.A.; Lal, R. Miscanthus agronomy and bioenergy feedstock potential on minesoils. Biofuels 2014, 5, 741–770. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Yang, H.; Shi, Z.; Xu, G.; Qin, Y.; Deng, J.; Yang, J. Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Bioresour. Technol. 2019, 274, 261–266. [Google Scholar] [CrossRef]
- Wang, K.S.; Wu, C.H.; Creek, J.L.; Shuler, P.J.; Tang, Y. Evaluation of effects of selected wax inhibitors on wax appearance and disappearance temperatures. Pet. Sci. Technol. 2003, 21, 359–368. [Google Scholar] [CrossRef]
- Hidayat, F.; Abdurrahman, M. A prospective method to increase oil recovery in waxy-shallow reservoir. IOP Conf. Ser. Mater. Sci. Eng. 2018, 306, 012040. [Google Scholar] [CrossRef]
- Abdurrahman, M.; Ferizal, F.H.; Husna, U.Z.; Pangaribuan, L. Possibility of wax control techniques in Indonesian oil fields. AIP Conf. Proc. 2018, 1941. [Google Scholar]
- Fernandiaz, R.; Amri, I.; Utama, P.S. Modeling of oil flows in Langgak field pipeline. J. Earth Energy Eng. 2019, 8, 12–20. [Google Scholar] [CrossRef]
- Ellison, B.T.; Gallagher, C.T.; Lorimer, S.E. The physical chemistry of wax, hydrates, and asphaltene. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 1–4 May 2000. [Google Scholar]
- Alves, B.F.; Pereira, P.H.R.; Nunes, R.D.C.P.; Lucas, E.F. Influence of solvent solubility parameter on the performance of EVA copolymers as pour point modifiers of waxy model-systems. Fuel 2019, 258, 116196. [Google Scholar] [CrossRef]
- Ridzuan, N.; Adam, F.; Yaacob, Z. Evaluation of the inhibitor selection on wax deposition for Malaysian crude oil. Pet. Sci. Technol. 2016, 34, 366–371. [Google Scholar] [CrossRef]
- Towler, B.F.; Jaripatke, O.; Mokhatab, S. Experimental investigations of the mitigation of paraffin wax deposition in crude oil using chemical additives. Pet. Sci. Technol. 2011, 29, 468–483. [Google Scholar] [CrossRef]
- Xu, G.; Xue, Y.; Zhao, Z.; Lian, X.; Lin, H.; Han, S. Influence of poly(methacrylate-co-maleic anhydride) pour point depressant with various pendants on low-temperature flowability of diesel fuel. Fuel 2018, 216, 898–907. [Google Scholar] [CrossRef]
- Zaky, M.T.; Mohamed, N.H.; Farag, A.S. Separation of some paraffin wax grades using solvent extraction technique. Fuel Process. Technol. 2011, 92, 2024–2029. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; de Lourdes Mosqueira, M.; Zapata-Rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; de la Cruz Clavel-López, J.; Aburto, J. Transportation of heavy and extra-heavy crude oil by pipeline: A review. J. Pet. Sci. Eng. 2011, 75, 274–282. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, W. Pour point depressant (PPD) and flow improver additives (FIA) of crude oil and its study method progress. Adv. Mater. Res. 2012, 524–527, 1844–1847. [Google Scholar] [CrossRef]
- Chanda, D.; Sarmah, A.; Borthakur, A.; Rao, K.V.; Subrahmanyam, B.; Das, H.C. Combined effect of asphaltenes and flow improvers on the rheological behaviour of Indian waxy crude oil. Fuel 1998, 77, 1163–1167. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Rønningsen, H.P. Influence of wax inhibitors on wax appearance temperature, pour point, and viscosity of waxy crude oils. Energy Fuels 2003, 17, 321–328. [Google Scholar] [CrossRef]
- Deka, B.; Sharma, R.; Mandal, A.; Mahto, V. Synthesis and evaluation of oleic acid based polymeric additive as pour point depressant to improve flow properties of Indian waxy crude oil. J. Pet. Sci. Eng. 2018, 170, 105–111. [Google Scholar] [CrossRef]
- Castro, L.V.; Vazquez, F. Copolymers as flow improvers for Mexican crude oils. Energy Fuels 2008, 22, 4006–4011. [Google Scholar] [CrossRef]
- Soliman, E.A.; Elkatory, M.R.; Hashem, A.I.; Ibrahim, H.S. Synthesis and performance of maleic anhydride copolymers with alkyl linoleate or tetra-esters as pour point depressants for waxy crude oil. Fuel 2018, 211, 535–547. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, Y.; Zhang, H.; Liu, Y. Investigation on gelation nucleation kinetics of waxy crude oil emulsions by their thermal behavior. J. Pet. Sci. Eng. 2019, 181, 106230. [Google Scholar] [CrossRef]
- Huang, H.; Wang, W.; Peng, Z.; Li, K.; Gan, D.; Zhang, S.; Ding, Y.; Wu, H.; Gong, J. The effect of cooling processes on the yield stress of waxy model oil with nanocomposite pour point depressant. J. Pet. Sci. Eng. 2019, 175, 828–837. [Google Scholar] [CrossRef]
- Huang, H.; Wang, W.; Peng, Z.; Ding, Y.; Li, K.; Li, Q.; Gong, J. The influence of nanocomposite pour point depressant on the crystallization of waxy oil. Fuel 2018, 221, 257–268. [Google Scholar] [CrossRef]
- Sharma, R.; Mahto, V.; Vuthaluru, H. Synthesis of PMMA/modified graphene oxide nanocomposite pour point depressant and its effect on the flow properties of Indian waxy crude oil. Fuel 2019, 235, 1245–1259. [Google Scholar] [CrossRef]
- Xie, M.; Chen, F.; Liu, J.; Yang, T.; Yin, S.; Lin, H.; Xue, Y.; Han, S. Synthesis and evaluation of benzyl methacrylate-methacrylate copolymers as pour point depressant in diesel fuel. Fuel 2019, 255, 115880. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Zhang, X.; Yang, F.; Sun, G.; Zhao, Y. Performance improvement of the ethylene-vinyl acetate copolymer (EVA) pour point depressant by small dosage of the amino-functionalized polymethylsilsesquioxane (PAMSQ) microsphere. Fuel 2018, 220, 167–176. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, X.; Ma, J.; Zhang, B. Investigation into a pour point depressant for shengli crude oil. Ind. Eng. Chem. Res. 2012, 51, 11605–11612. [Google Scholar] [CrossRef]
- Monger-McClure, T.G.; Tackett, J.E.; Merrill, L.S. Comparisons of cloud point measurement and paraffin prediction methods. SPE Prod. Facil. 1999, 14, 4–16. [Google Scholar] [CrossRef]
- Hammami, A.; Ratulowski, J.; Coutinho, J.A.P. Cloud points: Can we measure or model them? Pet. Sci. Technol. 2003, 21, 345–358. [Google Scholar] [CrossRef]
- Towler, B.F.; Rebbapragada, S. Mitigation of paraffin wax deposition in cretaceous crude oils of Wyoming. J. Pet. Sci. Eng. 2004, 45, 11–19. [Google Scholar] [CrossRef]
| Pretreatment Method | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Chemical | Acids | High glucose yield | High cost of acid, must be recovered | [78] |
| Short reaction and residence time | Solvents need to be recycled, costly, causes corrosion to reactors, leads to formation of some inhibitors | [43,79] | ||
| Lignin and hemicellulose are removed | [79] | |||
| Base combined with oxidizing agents | Proceeds at ambient temperature | Generation of degradation products | [78] | |
| Formation of toxic inhibitors | ||||
| Reactor corrosion problems | ||||
| Organic solvents | Causes lignin and hemicellulose hydrolysis, useful for lignin recovery | High cost | [41] | |
| Solvents need to be drained and recycled | [43] | |||
| Ozonolysis | High delignification efficiency | Large amounts of ozone needed | [78] | |
| High glucose yield | Process economically unviable | |||
| Mild environmental conditions (temperature and pressure) | [41] | |||
| No formation of toxic inhibitors | [78] | |||
| Ionic liquids | Low generation of degradation products | High commercial price of solvents | [80] | |
| Low formation of toxic inhibitors | Solvents need to be drained and recycled | [43] | ||
| Reduce cellulose crystallinity | Lack of studies of the impact of enzymes on the next stage | [41] | ||
| Physical | Microwave irradiation | Faster heat transfer | Low penetration in bulk products | |
| Shorter reaction times | [78] | |||
| Low generation of degradation products | ||||
| Milling | Reduces cellulose crystallinity | High power and energy consumption | [43] | |
| Cost effective especially for the herbaceous biomass and agricultural residues | [79] | |||
| Mechanical comminution | Reduces cellulose crystallinity | High power and energy consumption | [41] | |
| Reduces of degree of polymerization | [78] | |||
| Extrusion | Low generation of degradation products | Temperature regulation difficulties, limited cooling capacities | ||
| Low formation of toxic inhibitors | Limited residence time | |||
| Physicochemical | Liquid hot water | No catalyst required | High energy consumption | [41,81] |
| Low-cost reactor construction | Not developed on commercial scale | [78] | ||
| Low corrosion potential | ||||
| Low generation of degradation products | ||||
| Steam explosion | Causes lignin transformation and hemicellulose solubilization | Generation of toxic compounds | [80] | |
| Cost effective | Partial hemicellulose degradation | [78] | ||
| Higher yield of glucose and hemicellulose in a two-step method | [43] | |||
| CO2 explosion | Increase accessible surface area | Does not affect lignin and hemicelluloses | [43] | |
| Cost effective | Very high pressure requirements | [33,41] | ||
| Does not generate of toxic compounds | ||||
| Ammonia fiber explosion | Increases accessible surface area | Not efficient for raw materials with high lignin content | [79] | |
| Low formation of inhibitors | High cost of large amount of ammonia | [78] | ||
| High cost of ammonia | ||||
| Soaking aqueous ammonia | Performed at lower temperature | [78] | ||
| Glycan and xylene remain in the solid | ||||
| Low formation of inhibitors | ||||
| Biological | Degrades lignin and hemicellulose | Low rate of hydrolysis | [41] | |
| Low energy consumption | [43] | |||
| Low-capital cost | ||||
| No chemicals requirement | [78] | |||
| Mild environmental conditions | [79] | |||
| Characteristic | Slack Waxes | ||
|---|---|---|---|
| Light | Middle | Heavy | |
| Congealing point, °C | 48 | 59 | 62.5 |
| Kinematic viscosity, 98.9 °C | 3.04 | 4.30 | 6.00 |
| Refractive index, 98.9 °C | 1.4224 | 1.4270 | 1.4402 |
| Density, 70 °C | 0.7920 | 0.8035 | 0.8107 |
| Mean molecular weight | 384 | 446 | 477 |
| Oil content, wt.% | 5.32 | 6.23 | 23.05 |
| Cone penetration, 25 °C | 17 | 13 | 28 |
| Needle penetration, °C | 43 | 40 | 59 |
| Sulfur content, wt.% | 0.09 | 0.10 | 0.22 |
| Color (ASTM D-1500) | 1.0 | 1.5 | 3.0 |
| Carbon Distribution Analysis | |||
| % CA | 5.93 | 8.41 | 10.46 |
| % CN | 12.85 | 14.20 | 29.44 |
| % CR | 18.78 | 22.61 | 39.90 |
| % CP | 81.22 | 77.39 | 60.10 |
| Molecular Type Composition | |||
| Total saturates, wt.% | 97.63 | 96.97 | 86.18 |
| n-paraffin content, wt.% | 74.71 | 62.89 | 35.07 |
| Iso- and cyclo-paraffin content, wt.% | 22.92 | 34.08 | 51.11 |
| Total aromatics, wt.% | 2.37 | 3.03 | 13.82 |
| Mono-aromatics, wt.% | 2.37 | 3.03 | 11.52 |
| di-aromatics, wt.% | - | 2.30 | |
| % CA—percentage of aromatic carbon per average molecule | |||
| % CN—percentage of naphthenic carbon per average molecule = % CR−% CA | |||
| % CR—% CN + % CA | |||
| % CP—percentage of paraffinic carbon per average molecule = 100% CR | |||
| Inhibitor | Chemical Name | ∆WAT (°C) | PIElight (%) | PIEheavy (%) | PIEltotal (%) |
|---|---|---|---|---|---|
| A | Poly alkyl enamine | −2.7 | 71 | 13 | 52 |
| B | Proprietary | −3.3 | 87 | 75 | 83 |
| C | Polyolefin amide alkene amine | −0.1 | 18 | −9 | 9 |
| D | Polyolefin amide alkene amine | −0.2 | 33 | 3 | 23 |
| E | Poly acrylate | 0.0 | 42 | 28 | 37 |
| F | Poly alkyl acrylate | −3.4 | 72 | 18 | 54 |
| G | Olefin amide copolymer | −1.7 | 55 | −3 | 36 |
| H | Proprietary | −4.2 | 73 | 29 | 58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afdhol, M.K.; Abdurrahman, M.; Hidayat, F.; Chong, F.K.; Mohd Zaid, H.F. Review of Solvents Based on Biomass for Mitigation of Wax Paraffin in Indonesian Oilfield. Appl. Sci. 2019, 9, 5499. https://doi.org/10.3390/app9245499
Afdhol MK, Abdurrahman M, Hidayat F, Chong FK, Mohd Zaid HF. Review of Solvents Based on Biomass for Mitigation of Wax Paraffin in Indonesian Oilfield. Applied Sciences. 2019; 9(24):5499. https://doi.org/10.3390/app9245499
Chicago/Turabian StyleAfdhol, M. K., M. Abdurrahman, F. Hidayat, F. K. Chong, and H. F. Mohd Zaid. 2019. "Review of Solvents Based on Biomass for Mitigation of Wax Paraffin in Indonesian Oilfield" Applied Sciences 9, no. 24: 5499. https://doi.org/10.3390/app9245499
APA StyleAfdhol, M. K., Abdurrahman, M., Hidayat, F., Chong, F. K., & Mohd Zaid, H. F. (2019). Review of Solvents Based on Biomass for Mitigation of Wax Paraffin in Indonesian Oilfield. Applied Sciences, 9(24), 5499. https://doi.org/10.3390/app9245499





