Independent Analysis of Decelerations and Resting Periods through CEEMDAN and Spectral-Based Feature Extraction Improves Cardiotocographic Assessment
Abstract
1. Introduction
2. State of the Art
2.1. Analysis in Frequency-Domain
2.2. Nonlinear Features
2.3. Time-Variant Techniques
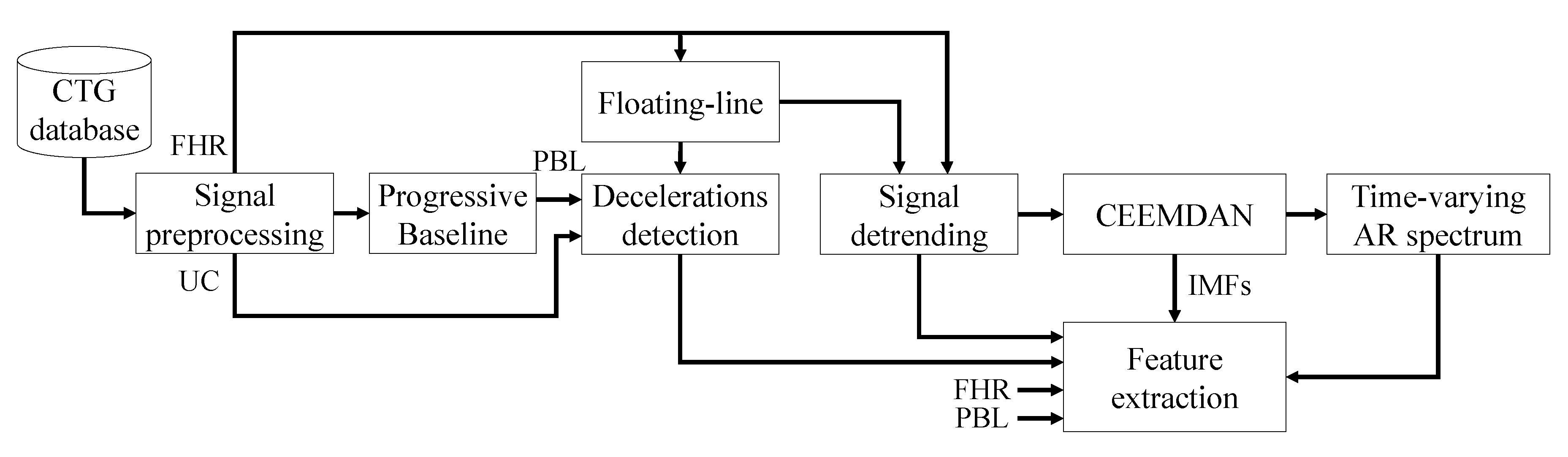
3. Methodology
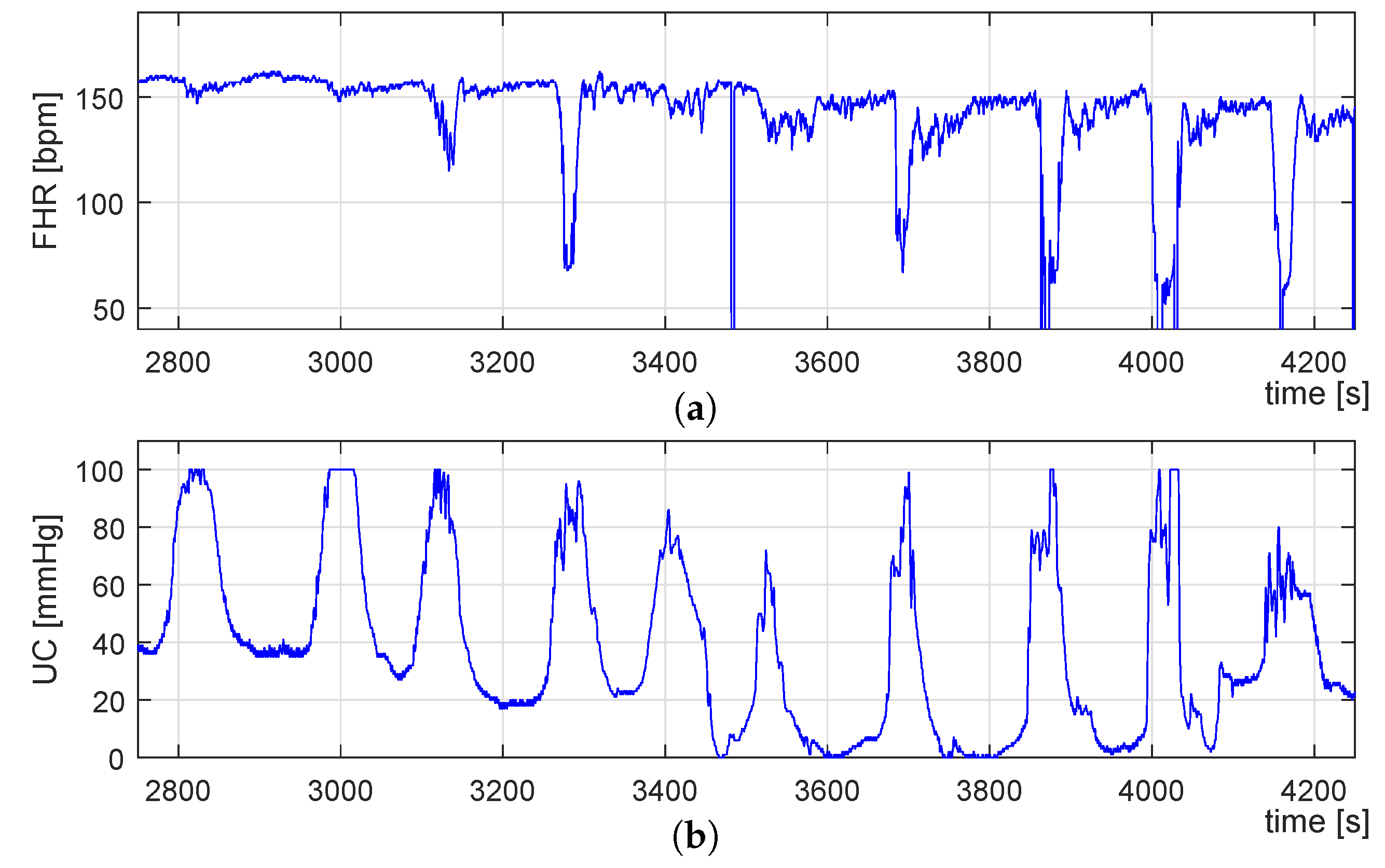
3.1. CTG Recordings Dataset
3.2. CTG Signal Analysis
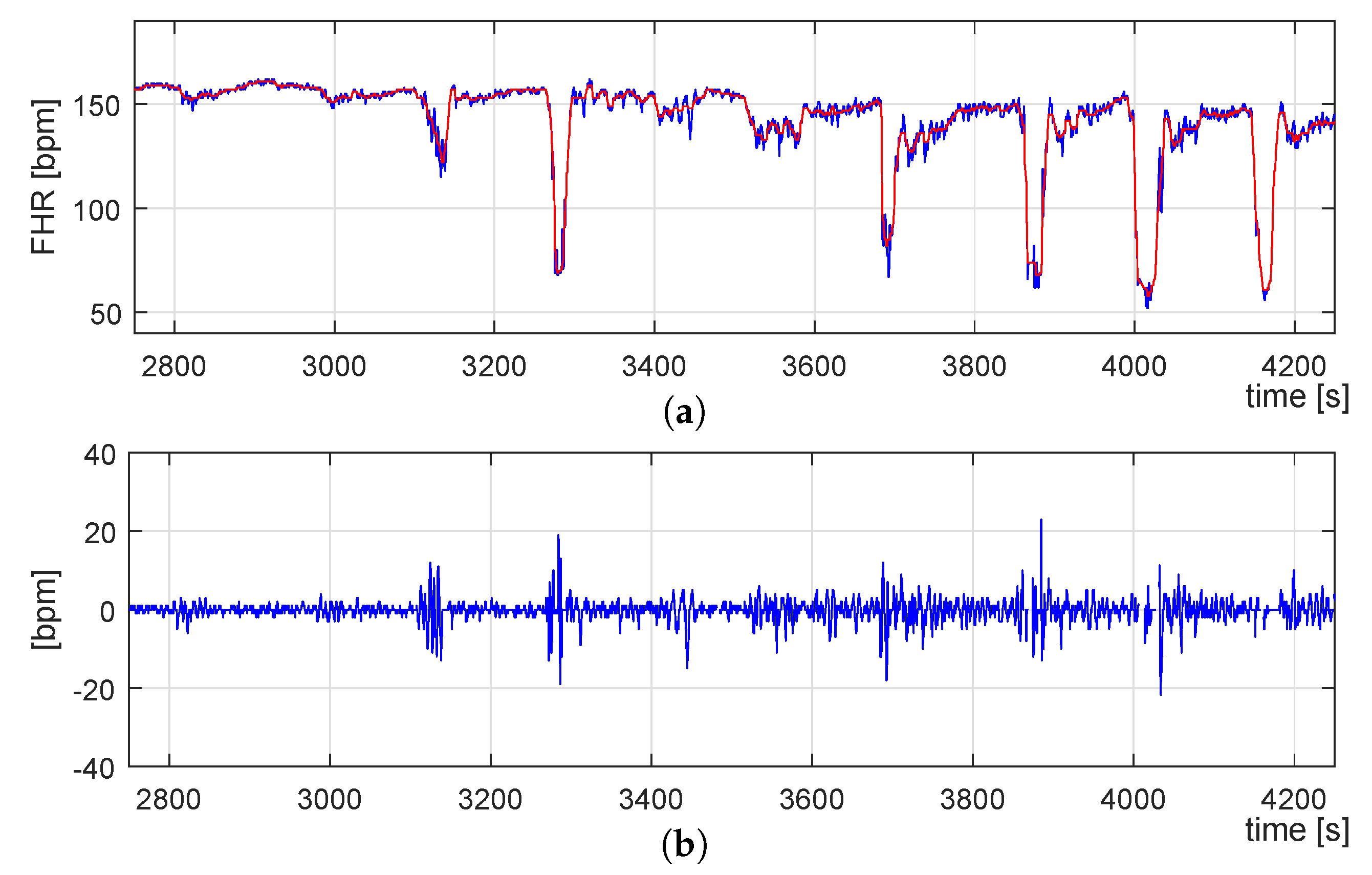
3.2.1. Signal Preprocessing
3.2.2. FHR Signal Detrending
- We randomly selected a set of ten CTG recordings from the CTU-UHB database.
- Each FHR signal was filtered by a median filter using different window lengths in the range of 6 to 12 s in steps of 1 s.
- The extracted traces (seven for each signal) were superimposed on the corresponding raw FHR signal in order to examine which one tracks the FHR decelerations and accelerations better.
- After a visual analysis, we selected the trace computed by a sliding window of 10 s length.
3.2.3. FHR Signal Decomposition and TV-AR Spectrum Computation
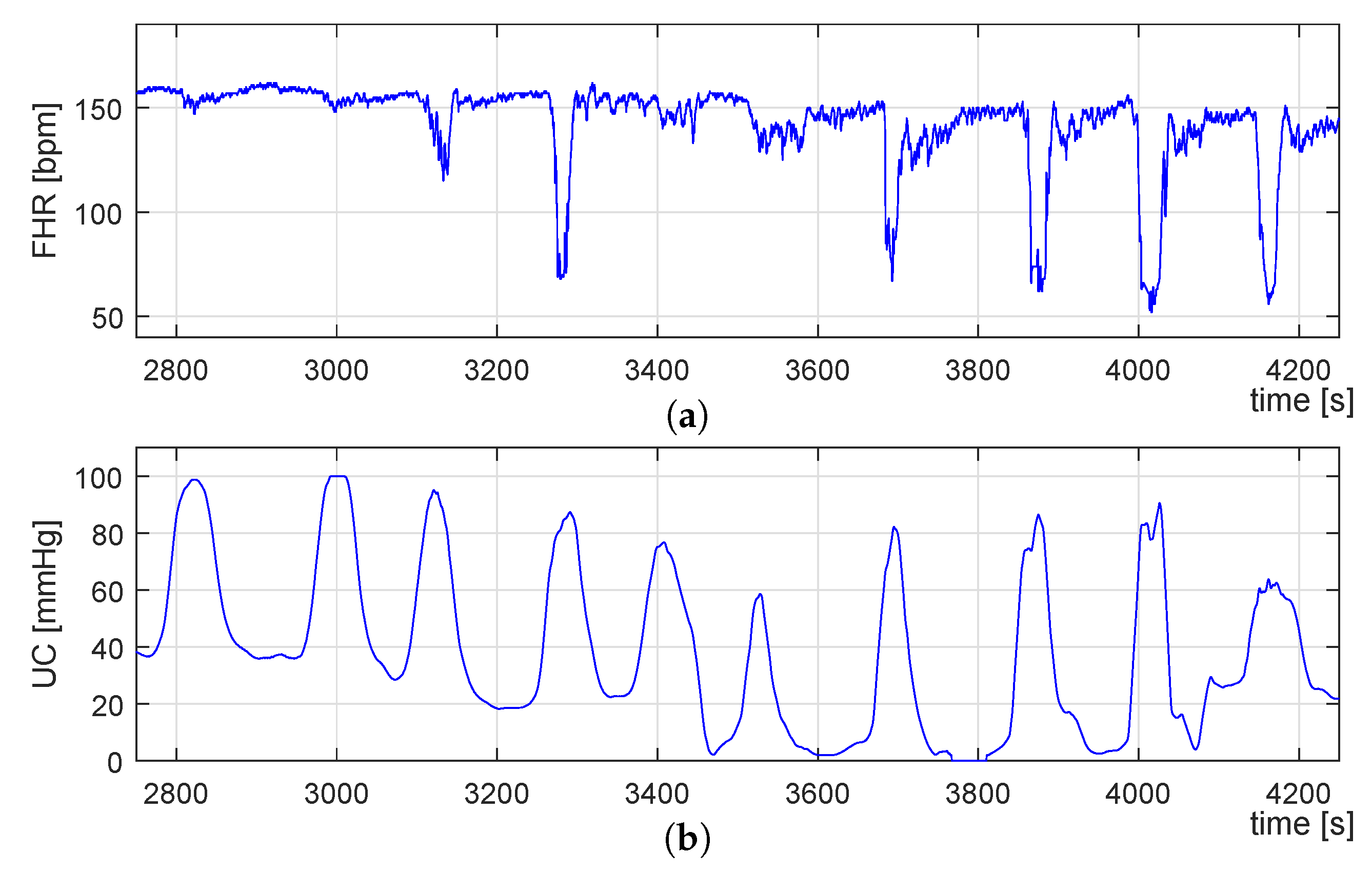
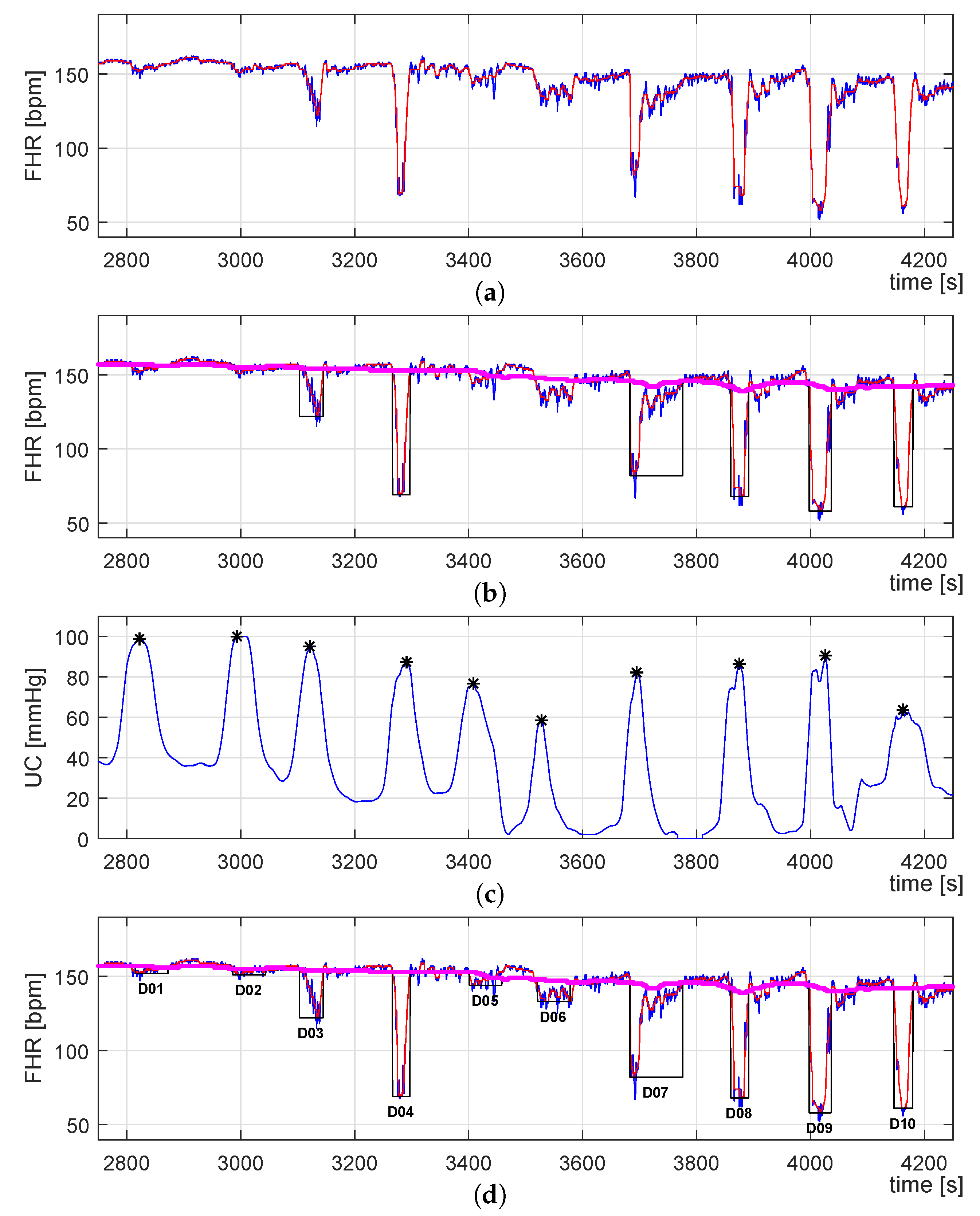
3.3. Identification of UC-Deceleration Episodes
- A virtual baseline (VBL) is estimated by filtering the FHR signal using the same median filter for the floating-line computation but with a different sliding window. Following [65], the sliding window size was set to 400 s length.
- Then, the VBL allows defining a range in amplitude delimit by the low (L) and high (H) traces, which corresponds to the signal data that will be considered for the PBL computation. These traces are described by Equations (8) and (9), where n corresponds to the sample number, and FHR is set to 10 bpm following [66].
- Finally, the PBL is computed by considering only the data described by FHRLH (see Equation (10)) by using the same median filter used for the VBL extraction.
4. Evaluation and Results
4.1. Definition of Proposed Features
4.2. Feature Computation
4.3. Features Evaluation and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ayres-de Campos, D.; Spong, C.Y.; Chandraharan, E.; FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int. J. Gynecol. Obstet. 2015, 131, 13–24. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Practice bulletin no. 116: Management of intrapartum fetal heart rate tracings. Obstet. Gynecol. 2010, 116, 1232. [Google Scholar] [CrossRef] [PubMed]
- Rooth, G.; Huch, A.; Huch, R. FIGO News: Guidelines for the use of fetal monitoring. Int. J. Gynecol. Obstet. 1987, 25, 159–167. [Google Scholar]
- Santo, S.; Ayres-de Campos, D. Human factors affecting the interpretation of fetal heart rate tracings: An update. Curr. Opin. Obstet. Gynecol. 2012, 24, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Haritopoulos, M.; Illanes, A.; Nandi, A.K. Survey on cardiotocography feature extraction algorithms for foetal welfare assessment. In Proceedings of the XIV Mediterranean Conference on Medical and Biological Engineering and Computing 2016, Paphos, Greece, 31 March–2 April 2016; Springer: Cham, Switzerland, 2016; pp. 1193–1198. [Google Scholar]
- Freeman, R.K.; Garite, T.J.; Nageotte, M.P.; Miller, L.A. Fetal Heart Rate Monitoring; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Ugwumadu, A.; Steer, P.; Parer, B.; Carbone, B.; Vayssiere, C.; Maso, G.; Arulkumaran, S. Time to optimise and enforce training in interpretation of intrapartum cardiotocograph. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Nunes, I.; Ayres-de Campos, D. Computer analysis of foetal monitoring signals. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 30, 68–78. [Google Scholar] [CrossRef]
- Lutomski, J.E.; Meaney, S.; Greene, R.A.; Ryan, A.C.; Devane, D. Expert systems for fetal assessment in labour. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Garabedian, C.; De Jonckheere, J.; Butruille, L.; Deruelle, P.; Storme, L.; Houfflin-Debarge, V. Understanding fetal physiology and second line monitoring during labor. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 113–117. [Google Scholar] [CrossRef]
- Ugwumadu, A. Are we (mis) guided by current guidelines on intrapartum fetal heart rate monitoring? Case for a more physiological approach to interpretation. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1063–1070. [Google Scholar] [CrossRef]
- Cesarelli, M.; Romano, M.; Ruffo, M.; Bifulco, P.; Pasquariello, G. Foetal heart rate variability frequency characteristics with respect to uterine contractions. J. Biomed. Sci. Eng. 2010, 3, 1014. [Google Scholar] [CrossRef][Green Version]
- Romano, M.; Bifulco, P.; Cesarelli, M.; Sansone, M.; Bracale, M. Foetal heart rate power spectrum response to uterine contraction. Med. Biol. Eng. Comput. 2006, 44, 188–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hruban, L.; Spilka, J.; Chudáček, V.; Janků, P.; Huptych, M.; Burša, M.; Hudec, A.; Kacerovský, M.; Koucký, M.; Procházka, M.; et al. Agreement on intrapartum cardiotocogram recordings between expert obstetricians. J. Eval. Clin. Pract. 2015, 21, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Quirk, J.G.; Djurić, P.M. Inference about Causality from Cardiotocography Signals Using Gaussian Processes. In Proceedings of the ICASSP 2019-2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Brighton, UK, 12–17 May 2019; pp. 2852–2856. [Google Scholar]
- Sletten, J.; Kiserud, T.; Kessler, J. Effect of uterine contractions on fetal heart rate in pregnancy: A prospective observational study. Acta Obstet. Gynecol. Scand. 2016, 95, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, P.; Illanes, A.; Ortmeier, F. Spectral-Based Analysis of Progressive Dynamical Changes in the Fetal Heart Rate Signal During Labor by Using Empirical Mode Decomposition. In Proceedings of the 2018 Computing in Cardiology (CinC), Maastricht, The Netherlands, 23–26 September 2018; pp. 1–4. [Google Scholar]
- Fuentealba, P.; Illanes, A.; Ortmeier, F. Foetal heart rate assessment by empirical mode decomposition and spectral analysis. Curr. Dir. Biomed. Eng. 2019, 5, 381–383. [Google Scholar] [CrossRef]
- Warmerdam, G.J.J.; Vullings, R.; Van Laar, J.O.E.H.; Bergmans, J.W.M.; Schmitt, L.; Oei, S.G. Detection rate of fetal distress using contraction-dependent fetal heart rate variability analysis. Physiol. Meas. 2018, 39, 025008. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Park, I.Y.; Shin, J.C.; Song, J.; Tafreshi, R.; Lim, J. Specific change in spectral power of fetal heart rate variability related to fetal acidemia during labor: Comparison between preterm and term fetuses. Early Hum. Dev. 2012, 88, 203–207. [Google Scholar] [CrossRef]
- Gonçalves, H.; Rocha, A.P.; Ayres-de Campos, D.; Bernardes, J. Internal versus external intrapartum foetal heart rate monitoring: The effect on linear and nonlinear parameters. Physiol. Meas. 2006, 27, 307. [Google Scholar] [CrossRef]
- Signorini, M.G.; Magenes, G.; Cerutti, S.; Arduini, D. Linear and nonlinear parameters for the analysisof fetal heart rate signal from cardiotocographic recordings. IEEE Trans. Biomed. Eng. 2003, 50, 365–374. [Google Scholar] [CrossRef]
- Siira, S.M.; Ojala, T.H.; Vahlberg, T.J.; Rosén, K.G.; Ekholm, E.M. Do spectral bands of fetal heart rate variability associate with concomitant fetal scalp pH? Early Hum. Dev. 2013, 89, 739–742. [Google Scholar] [CrossRef]
- Van Laar, J.; Peters, C.; Houterman, S.; Wijn, P.F.; Kwee, A.; Oei, S. Normalized spectral power of fetal heart rate variability is associated with fetal scalp blood pH. Early Hum. Dev. 2011, 87, 259–263. [Google Scholar] [CrossRef]
- Van Laar, J.; Peters, C.; Vullings, R.; Houterman, S.; Bergmans, J.; Oei, S. Fetal autonomic response to severe acidaemia during labour. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, J.; Peters, C.; Vullings, R.; Houterman, S.; Oei, S. Power spectrum analysis of fetal heart rate variability at near term and post term gestation during active sleep and quiet sleep. Early Hum. Dev. 2009, 85, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, J.; Porath, M.; Peters, C.; Oei, S. Spectral analysis of fetal heart rate variability for fetal surveillance: Review of the literature. Acta Obstet. Gynecol. Scand. 2008, 87, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Cesarelli, M.; Romano, M.; Bifulco, P.; Fedele, F.; Bracale, M. An algorithm for the recovery of fetal heart rate series from CTG data. Comput. Biol. Med. 2007, 37, 663–669. [Google Scholar] [CrossRef]
- Siira, S.M.; Ojala, T.H.; Vahlberg, T.J.; Jalonen, J.O.; Välimäki, I.A.; Rosén, K.G.; Ekholm, E.M. Marked fetal acidosis and specific changes in power spectrum analysis of fetal heart rate variability recorded during the last hour of labour. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Warrick, P.A.; Hamilton, E.F. Fetal heart-rate variability response to uterine contractions during labour and delivery. In Proceedings of the Computing in Cardiology (CinC), Krakow, Poland, 9–12 September 2012; pp. 417–420. [Google Scholar]
- Cazares, S.; Moulden, M.; Redman, C.W.; Tarassenko, L. Tracking poles with an autoregressive model: A confidence index for the analysis of the intrapartum cardiotocogram. Med. Eng. Phys. 2001, 23, 603–614. [Google Scholar] [CrossRef]
- Illanes, A.; Haritopoulos, M. Fetal heart rate feature extraction from cardiotocographic recordings through autoregressive model’s power spectral-and pole-based analysis. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 5842–5845. [Google Scholar]
- Granero-Belinchon, C.; Roux, S.G.; Abry, P.; Doret, M.; Garnier, N.B. Information Theory to Probe Intrapartum Fetal Heart Rate Dynamics. Entropy 2017, 19, 640. [Google Scholar] [CrossRef]
- Warrick, P.A.; Hamilton, E.F. Mutual information estimates of CTG synchronization. In Proceedings of the 2015 Computing in Cardiology Conference (CinC), Nice, France, 6–9 September 2015; pp. 137–139. [Google Scholar]
- Magenes, G.; Bellazzi, R.; Fanelli, A.; Signorini, M. Multivariate analysis based on linear and non-linear FHR parameters for the identification of IUGR fetuses. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014; pp. 1868–1871. [Google Scholar]
- Costa, M.D.; Schnettler, W.T.; Amorim-Costa, C.; Bernardes, J.; Costa, A.; Goldberger, A.L.; Ayres-de Campos, D. Complexity-loss in fetal heart rate dynamics during labor as a potential biomarker of acidemia. Early Hum. Dev. 2014, 90, 67–71. [Google Scholar] [CrossRef]
- Ferrario, M.; Signorini, M.G.; Magenes, G. Complexity analysis of the fetal heart rate variability: Early identification of severe intrauterine growth-restricted fetuses. Med Biol. Eng. Comput. 2009, 47, 911–919. [Google Scholar] [CrossRef]
- Ferrario, M.; Signorini, M.G.; Magenes, G.; Cerutti, S. Comparison of entropy-based regularity estimators: Application to the fetal heart rate signal for the identification of fetal distress. IEEE Trans. Biomed. Eng. 2006, 53, 119–125. [Google Scholar] [CrossRef]
- Romano, M.; Bracale, M.; Cesarelli, M.; Campanile, M.; Bifulco, P.; De Falco, M.; Sansone, M.; Di Lieto, A. Antepartum cardiotocography: A study of fetal reactivity in frequency domain. Comput. Biol. Med. 2006, 36, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Azemi, G.; Boashash, B. Improved characterization of HRV signals based on instantaneous frequency features estimated from quadratic time–frequency distributions with data-adapted kernels. Biomed. Signal Process. Control 2014, 10, 153–165. [Google Scholar] [CrossRef]
- Fuentealba, P.; Illanes, A.; Ortmeier, F. Foetal heart rate signal spectral analysis by using time-varying autoregressive modelling. Curr. Dir. Biomed. Eng. 2018, 4, 579–582. [Google Scholar] [CrossRef]
- Bursa, M.; Lhotska, L. The Use of Convolutional Neural Networks in Biomedical Data Processing. In Proceedings of the International Conference on Information Technology in Bio-and Medical Informatics, Lyon, France, 28–31 August 2017; Springer: Cham, Switzerland, 2017; pp. 100–119. [Google Scholar]
- Cattani, C.; Doubrovina, O.; Rogosin, S.; Voskresensky, S.L.; Zelianko, E. On the creation of a new diagnostic model for fetal well-being on the base of wavelet analysis of cardiotocograms. J. Med. Syst. 2006, 30, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Georgoulas, G.; Stylios, C.D.; Groumpos, P.P. Predicting the risk of metabolic acidosis for newborns based on fetal heart rate signal classification using support vector machines. IEEE Trans. Biomed. Eng. BME 2006, 53, 875. [Google Scholar] [CrossRef]
- Romano, M.; Iuppariello, L.; Ponsiglione, A.M.; Improta, G.; Bifulco, P.; Cesarelli, M. Frequency and time domain analysis of foetal heart rate variability with traditional indexes: A critical survey. Comput. Math. Methods Med. 2016, 2016, 9585431. [Google Scholar] [CrossRef]
- Colominas, M.A.; Schlotthauer, G.; Torres, M.E. Improved complete ensemble EMD: A suitable tool for biomedical signal processing. Biomed. Signal Process. Control 2014, 14, 19–29. [Google Scholar] [CrossRef]
- Kay, S.M.; Marple, S.L. Spectrum analysis—A modern perspective. Proc. IEEE 1981, 69, 1380–1419. [Google Scholar] [CrossRef]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Krupa, N.; Ali, M.; Zahedi, E.; Ahmed, S.; Hassan, F.M. Antepartum fetal heart rate feature extraction and classification using empirical mode decomposition and support vector machine. Biomed. Eng. Online 2011, 10, 6. [Google Scholar] [CrossRef]
- Krupa, B.; Ali, M.M.; Zahedi, E. The application of empirical mode decomposition for the enhancement of cardiotocograph signals. Physiol. Meas. 2009, 30, 729. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, N.E. A study of the characteristics of white noise using the empirical mode decomposition method. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 2004, 460, 1597–1611. [Google Scholar] [CrossRef]
- Huang, J. Study of Autoregressive (AR) Spectrum Estimation Algorithm for Vibration Signals of Industrial Stream Turbines. Int. J. Control Autom. 2014, 7, 349–362. [Google Scholar] [CrossRef]
- Chudáček, V.; Spilka, J.; Burša, M.; Janku, P.; Hruban, L.; Huptych, M.; Lhotská, L. Open access intrapartum CTG database. BMC Pregnancy Childbirth 2014, 14, 16. [Google Scholar] [CrossRef]
- Kumar, N.; Suman, A.; Sawant, K. Relationship between immediate postpartum umbilical cord blood pH and fetal distress. Int. J. Contemp. Pediatr. 2016, 3, 113–119. [Google Scholar] [CrossRef]
- Spilka, J.; Georgoulas, G.; Karvelis, P.; Oikonomou, V.P.; Chudáček, V.; Stylios, C.; Lhotská, L.; Janku, P. Automatic evaluation of FHR recordings from CTU-UHB CTG database. In Information Technology in Bio-and Medical Informatics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 47–61. [Google Scholar]
- Fuentealba, P.; Illanes, A.; Ortmeier, F. Progressive fetal distress estimation by characterization of fetal heart rate decelerations response based on signal variability in cardiotocographic recordings. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; pp. 1–4. [Google Scholar]
- Al-Angari, H.M.; Kimura, Y.; Hadjileontiadis, L.J.; Khandoker, A.H. A hybrid EMD-kurtosis method for estimating fetal heart rate from continuous Doppler signals. Front. Physiol. 2017, 8, 641. [Google Scholar] [CrossRef]
- Romano, M.; Faiella, G.; Clemente, F.; Iuppariello, L.; Bifulco, P.; Cesarelli, M. Analysis of foetal heart rate variability components by means of empirical mode decomposition. In Proceedings of the XIV Mediterranean Conference on Medical and Biological Engineering and Computing 2016, Paphos, Greece, 31 March–2 April 2016; Springer: Cham, Switzerland, 2016; pp. 71–74. [Google Scholar]
- Lu, Y.; Li, X.; Wei, S.; Liu, X. Fetal heart rate baseline estimation with analysis of fetal movement signal. Bio Med Mater. Eng. 2014, 24, 3763–3769. [Google Scholar] [CrossRef]
- Ortiz, M.; Bojorges, E.; Aguilar, S.; Echeverria, J.; Gonzalez-Camarena, R.; Carrasco, S.; Gaitan, M.; Martinez, A. Analysis of High Frequency Fetal Heart Rate Variability Using the Empirical Mode Decomposition. Comput. Cardiol. 2005, 32, 675–678. [Google Scholar]
- Fuentealba, P.; Illanes, A.; Ortmeier, F. Cardiotocograph Data Classification Improvement by Using Empirical Mode Decomposition. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5646–5649. [Google Scholar]
- Wu, Z.; Huang, N.E. Ensemble empirical mode decomposition: A noise-assisted data analysis method. Adv. Adapt. Data Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Torres, M.E.; Colominas, M.A.; Schlotthauer, G.; Flandrin, P. A complete ensemble empirical mode decomposition with adaptive noise. In Proceedings of the 2011 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Prague, Czech Republic, 22–27 May 2011; pp. 4144–4147. [Google Scholar]
- Manolakis, D.G.; Ingle, V.K.; Kogon, S.M. Statistical and Adaptive Signal Processing: Spectral Estimation, Signal Modeling, Adaptive Filtering, and Array Processing; McGraw-Hill: Boston, MA, USA, 2000. [Google Scholar]
- Fuentealba, P.; Illanes, A.; Ortmeier, F. Analysis of the foetal heart rate in cardiotocographic recordings through a progressive characterization of decelerations. Curr. Dir. Biomed. Eng. 2017, 3, 423–427. [Google Scholar] [CrossRef]
- Agostinelli, A.; Braccili, E.; Marchegiani, E.; Rosati, R.; Sbrollini, A.; Burattini, L.; Morettini, M.; Di Nardo, F.; Fioretti, S.; Burattini, L. Statistical baseline assessment in cardiotocography. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 3166–3169. [Google Scholar]
- Gibbons, J.D.; Chakraborti, S. Nonparametric statistical inference. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 977–979. [Google Scholar]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S. Approximate entropy (ApEn) as a complexity measure. Chaos Interdiscip. J. Nonlinear Sci. 1995, 5, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M.; Viscarello, R.R. Approximate entropy: A regularity measure for fetal heart rate analysis. Obstet. Gynecol. 1992, 79, 249–255. [Google Scholar] [PubMed]
- Fergus, P.; Selvaraj, M.; Chalmers, C. Machine learning ensemble modelling to classify caesarean section and vaginal delivery types using Cardiotocography traces. Comput. Biol. Med. 2018, 93, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Spilka, J.; Chudáček, V.; Kouckỳ, M.; Lhotská, L.; Huptych, M.; Janku, P.; Georgoulas, G.; Stylios, C. Using nonlinear features for fetal heart rate classification. Biomed. Signal Process. Control 2012, 7, 350–357. [Google Scholar] [CrossRef]
- Spilka, J.; Chudácek, V.; Kouckỳ, M.; Lhotská, L. Assessment of non-linear features for intrapartal fetal heart rate classification. In Proceedings of the 2009 9th International Conference on Information Technology and Applications in Biomedicine, Larnaca, Cyprus, 4–7 November 2009; pp. 1–4. [Google Scholar]
- Spilka, J.; Georgoulas, G.; Karvelis, P.; Chudáček, V.; Stylios, C.D.; Lhotská, L. Discriminating normal from “abnormal” pregnancy cases using an automated fhr evaluation method. In Proceedings of the Hellenic Conference on Artificial Intelligence, Ioannina, Greece, 15–17 May 2014; Springer: Cham, Switzerland, 2014; pp. 521–531. [Google Scholar]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- He, H.; Bai, Y.; Garcia, E.A.; Li, S. ADASYN: Adaptive synthetic sampling approach for imbalanced learning. In Proceedings of the 2008 IEEE International Joint Conference on Neural Networks, IJCNN 2008, Hong Kong, China, 1–8 June 2008; pp. 1322–1328. [Google Scholar]
- Warmerdam, G.J.; Vullings, R.; Van Laar, J.O.; Bergmans, J.; Schmitt, L.; Oei, S. Selective heart rate variability analysis to account for uterine activity during labor and improve classification of fetal distress. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2950–2953. [Google Scholar]


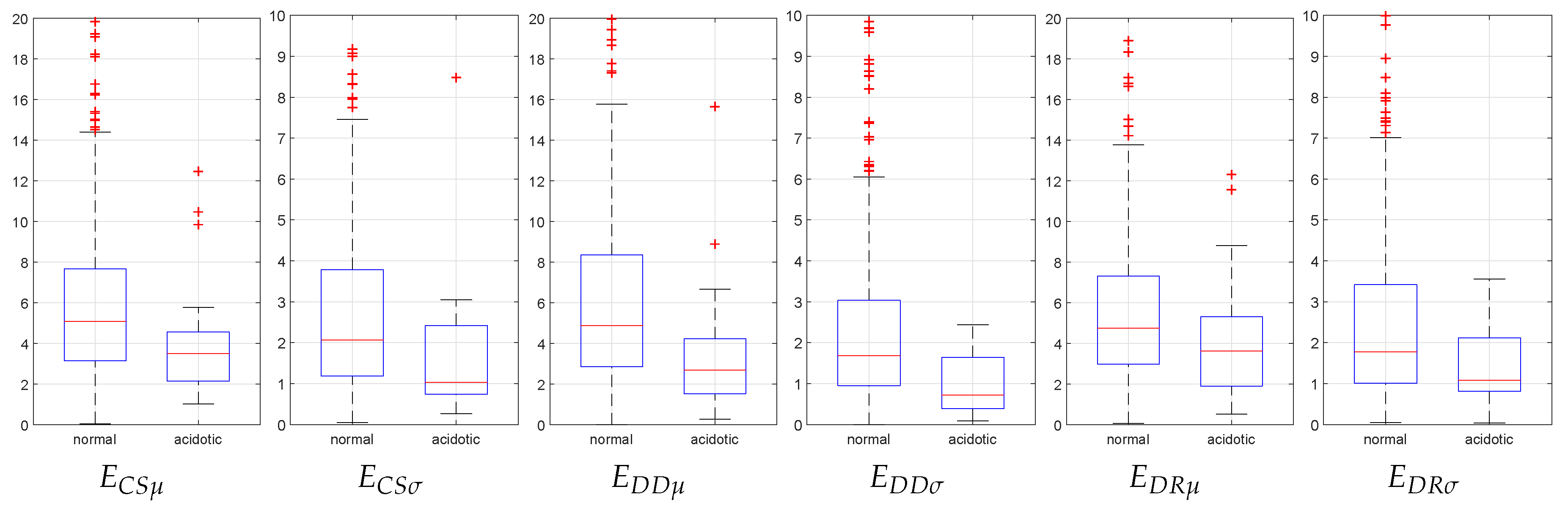
| Feature | Normal Cases | Acidotic Cases | Significance (p-Value) |
|---|---|---|---|
| 0.0213 | |||
| 0.0079 | |||
| 0.0016 | |||
| 0.0015 | |||
| 0.0708 | |||
| 0.0277 |
| Feature Set | Significant Features | Type | |
|---|---|---|---|
| IMF1-E | , M, RMS | modal-spectral | |
| IMF1- | , M, RMS | ||
| IMF2- | ApEn, SampEn | ||
| IMF4- | ApEn, SampEn | ||
| IMF6-E | , , mad, RMS | ||
| IMF6- | , , mad, RMS | ||
| IMF8-E | , , mad, RMS | ||
| IMF8- | , , mad, RMS | ||
| IMF10-E | ApEn, SampEn | ||
| IMF10- | ApEn, SampEn | ||
| FHR signal | , mad, ApEn, SampEn | conventional | |
| PBL | , mad | ||
| IMF6 | ApEn | ||
| IMF7 | ApEn | ||
| Feature Set | Significant Features | Type | |
|---|---|---|---|
| IMF1-E | , , mad, RMS | modal-spectral | |
| IMF4-E | |||
| IMF4- | , RMS | ||
| IMF5- | M | ||
| IMF6-E | , M, , mad, RMS | ||
| IMF6- | , M, , mad, RMS | ||
| IMF6- | M | ||
| FHR signal | , M, RMS | conventional | |
| PBL | |||
| Feature Set | Significant Features | Type | |
|---|---|---|---|
| IMF1-E | , M, RMS | modal-spectral | |
| IMF1- | , M, RMS | ||
| IMF2-E | , mad | ||
| IMF2- | ApEn | ||
| IMF6-E | , ApEn, SampEn, RMS | ||
| IMF8-E | ApEn, SampEn | ||
| IMF10- | SampEn | ||
| IMF7- | , , mad | ||
| FHR signal | , mad, ApEn, SampEn | conventional | |
| PBL | , mad | ||
| detrended FHR | ApEn, SampEn | ||
| FHR Segment | Optimal Feature Sets | QI (%) |
|---|---|---|
| CS | CS4, CS10, CS11, CS12 | 81.7 |
| DD | DD1, DD3, DD8 | 66.8 |
| DR | DR8, DR9, DR10 | 76.3 |
| DD_DR | DR8, DR9, DR10 | 76.3 |
| CS_DD | CS4, CS11, CS12, DD4, DD8 | 82.5 |
| CS_DR | CS4, CS11, CS12, DR5 | 83.2← |
| CS_DD_DR | CS4, CS11, CS12, DR5 | 83.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentealba, P.; Illanes, A.; Ortmeier, F. Independent Analysis of Decelerations and Resting Periods through CEEMDAN and Spectral-Based Feature Extraction Improves Cardiotocographic Assessment. Appl. Sci. 2019, 9, 5421. https://doi.org/10.3390/app9245421
Fuentealba P, Illanes A, Ortmeier F. Independent Analysis of Decelerations and Resting Periods through CEEMDAN and Spectral-Based Feature Extraction Improves Cardiotocographic Assessment. Applied Sciences. 2019; 9(24):5421. https://doi.org/10.3390/app9245421
Chicago/Turabian StyleFuentealba, Patricio, Alfredo Illanes, and Frank Ortmeier. 2019. "Independent Analysis of Decelerations and Resting Periods through CEEMDAN and Spectral-Based Feature Extraction Improves Cardiotocographic Assessment" Applied Sciences 9, no. 24: 5421. https://doi.org/10.3390/app9245421
APA StyleFuentealba, P., Illanes, A., & Ortmeier, F. (2019). Independent Analysis of Decelerations and Resting Periods through CEEMDAN and Spectral-Based Feature Extraction Improves Cardiotocographic Assessment. Applied Sciences, 9(24), 5421. https://doi.org/10.3390/app9245421





