1. Introduction
Most oral diseases are of infectious origin. Periodontal infections, dental caries, jawbone osteomyelitis and osteonecrosis, and mucosal fungal infections like thrush are all caused by microorganisms forming persistent and hard-to-eradicate structures referred to as biofilms [
1]. One of the key features of biofilm organisms is their ability to colonize soft and hard tissues [
2,
3], and to spread throughout the host, changing the status of the infection from localized to a more generalized one in susceptible hosts. Therefore, untreated, aggressive oral infections can lead to life-threatening infection and inflammation in virtually any body organ, including the brain, heart, and skeleton, to name a few [
4,
5,
6]. Another key feature of biofilm infections is the high tolerance to antibiotics and antiseptics [
7]. Microorganisms, such as
Enterococcus faecalis,
Staphylococcus aureus,
Aggregatibacter actinomycetemcomitans,
Pseudomonas aeruginosa,
Streptococcus mutans, and
Candida albicans, are not only well-recognized biofilm-formers and important pathogens in oral cavity infections [
8,
9,
10,
11,
12,
13], but they are also associated with high levels of intrinsic and acquired resistance to antimicrobials [
14,
15]. Due to antibiotic inefficiency in the context of persistent biofilm infections, the remaining therapeutic option is the local application of antiseptic agents, which display strong, unspecific mechanisms of antimicrobial and antibiofilm action [
16]. The antiseptics broadly used against bacterial biofilms are, among others, povidone-iodine, polihexanide, and chlorhexidine [
17,
18,
19]. However, the latter one, due to a quickly growing bulk of evidence indicating increasing microbial cross-resistance following application [
20,
21,
22], is being substituted with other antiseptic substances.
In turn, povidone-iodine (PVP-I) displays strong antimicrobial and anti-biofilm activity and it is widely used in oral mouthwashes. However, it has a poor taste and flavor, and can interfere with the regeneration of epidermal cells and granulation tissues [
23]; moreover, if it is used in abundance, it may penetrate through wounds to blood and accumulate in the thyroid gland. Therefore, other antiseptics are presently applied as an alternative for PVP-iodine; polihexanidine is one of the most promising among them [
24].
Regarding the specificity of the oral cavity environment, eradication of biofilm-forming microbes is a challenge, not only because of biofilm persistence and species-specific resistance to antibiotics, but tongue movements and the ongoing production of saliva decreases the active concentrations of liquid antimicrobials and sprays, while elasticity and mucosity of the oral cavity membranes leads to loss of antimicrobial hydrogels from the infection site. Therefore, despite the high in vitro antibiofilm efficacy of various forms of antiseptics, oral infections remain a hard-to-solve health issue. The aforementioned factors indicate the importance and necessity of using an appropriate carrier containing antimicrobial substances for the delivery to sites of infection in the oral cavity. Such a carrier should be able to provide a high local concentration of an antimicrobial agent at the infection site, display high elasticity, resistance to rending, and the ability to adhere to mucosa and teeth [
25].
Bacterial cellulose (BC) is a polymer that can address these challenges. This multifunctional biomaterial is synthesized by a plethora of so-called acetic acid bacteria, of which
Komagataeibacter xylinus is recognized as a model cellulose-producer. BC displays a high elasticity, the ability to absorb and release high volumes of antimicrobial compounds, a lack of cytotoxicity, and a resistance to shear stresses [
26]. The process of BC fabrication consists of relatively cost-effective stages of bacterial cell removal by means of alkalization and subsequent rinsing in order to stabilize pH, in addition to washing off bacterial and medium leftovers or debris [
27]. Our team has been studying BC properties and optimizing BC fabrication. We were able to increase the yield of BC by 500% compared to any previously published methods, making it more amenable to rapid and larger scale production [
28]. The physicochemical properties of BC depend on the culture conditions, such as the carbon and nitrogen sources in the culture medium composition, oxygen level, temperature, pH, and the type of cultures. This gives the opportunity to relatively easily modify BC carrier nanostructures without using expensive or time-consuming chemical processes. Thus, we previously designed BC carriers with various physicochemical properties that enabled the current study. Our bacterial cellulose (BC) pellicles display diverse properties, including a surface area in the range from 7.21 to 11.04 m
2/g, a pore volume of 3.11–3.96 cm
3/g, pore diameter 0.011–0.109 nm, water holding capacity 32–64%, water release capacity 10,600–33,400%, swelling ratio 132–389%, polymerization degree 2260–4780, and total crystallinity index 1.22–1.96 [
29]. Overall, BC has already found use in infected wound healing applications [
30] and osteo-regenerative procedures, to name a few [
31]. The high biocompability of this polymer (native or coupled with such antimicrobials as silver, povidone-iodine, or octenidine) for wound healing was convincingly demonstrated in a vast review by Portela et al. where many in vitro, animal model and in vivo studies are presented [
30]. However, BC has not been tested in combination with antimicrobial substances against oral biofilms as in the current study. Thus, the aim of this research was to evaluate the applicability of novel BC dressings chemisorbed with potent antiseptics for eradicating in vitro biofilms formed by pathogens that are etiological factors for various infections within the oral cavity.
2. Materials and Methods
2.1. Microbial Strains Used
For experimental purposes, the following strains from the American Tissue and Cell Culture Collection (ATCC) were applied: S. aureus 6538; P. aeruginosa 15442, E. faecalis 29212, Str. mutans 25175, A. actinomycetemcomitans 29522, C. albicans 10231 and K. xylinus 23769.
2.2. Antiseptics Used
The following antiseptics were used in this research:
- (a)
Prontosan, later referred to as “P” (Aesculap Chifa, Warsaw, Poland), which contained 0.1% undecylenamidopropyl betaine, 0.1% polyhexamethylene biguanide (polihexanide), and purified water.
- (b)
ProntOral, later referred to as “PO” (Aesculap Chifa), which contained water, PEG-40 (poly(ethylene) glycol 40), cured castor oil, aroma, sodium cyclamate, undecylenamidopropyl betaine, and polyhexamethylene biguanide (polihexanide).
- (c)
Braunol, latter referred to as “B” (Aesculap Chifa), where 100 g of “B” contains 7.5 g of iodine-povidone with 10% of active iodine, purified water, sodium phosphate dibasic, sodium iodine, sodium hydroxide, laureate ether, and macrogol.
2.3. Manufacture of Hydroxyapatite Discs
Discs used as a surface for the biofilm formation were obtained from commercially available hydroxyapatite (HA) powder that was 9.6 µm in diameter (MT3300, LowWet; Tomita Pharmaceutical, Kumamoto City, Japan) with an impurity level below the point of detection. The powder was pressed without a binder. Sintering was performed at 900 °C. The resultant discs were compressed using the universal testing system for static tensile, compression, and bending tests (Instron model 3384, Instron, Norwood, MA, USA). The height of these discs was 3 mm, while the diameter was 12 mm. The quality of the manufactured HA discs was checked using confocal microscopy and microcomputed tomography (microCT) using a LEXT OLS4000 microscope (Olympus, Center Valley, PA, USA) and a Metrotom 1500 microtomograph (Carl Zeiss, Oberkochen, Germany).
2.4. Artificial Saliva Composition
Artificial saliva (AS) used in this research was composed of: 2.5 g/L mucin, 0.25 g/L sodium chloride, 0.2 g/L potassium chloride, 0.2 g/L calcium chloride, 2.0 g/L yeast extract, 5.0 g/L protease peptone, 1.25 mL/L 40% urea, and 3.0 g/L of sucrose. AS was adjusted to a pH of 6 [
32].
2.5. Estimation of the Minimal Biofilm Eradication Concentration of Oral Antiseptics Against Bacteria
A total of 100 μL of S. aureus, P. aeruginosa, E. faecalis, or C. albicans suspension (105 cfu/mL) in tryptic soy broth (TSB) medium was introduced to each well and incubated at 37 °C in 5% CO2 for 24 h without shaking. For Str. mutans and A. actinomycetemcomitans, conditions for culturing were analogous with the exception that TSB supplemented with 3% sucrose was applied. Next, 100 μL of medium was removed, leaving biofilm-forming organisms attached to the bottom of a 96-well plate. Subsequently, geometric dilutions of oral antiseptics (OAs) in medium were applied to the wells and left for another 24 h at 37 °C in 5% CO2. The culture with no added antiseptic served as a positive control for microorganism growth, while the well containing the sterile medium only served as a sterility control for the experiment. After incubation, 5 μL of triphenyl tetrazolium chloride (TTC, Sigma Aldrich, Taufkirchern, Germany) was added to each well and incubated for 5 h at 37 °C. A change of colorless TTC to red formazan confirmed the presence of metabolically active microorganisms. The antiseptic concentration in the first colorless well, neighboring the red well, was taken as the minimal biofilm eradication concentration (MBEC) value. Because thick cell walls of C. albicans may impede the penetration of TTC and E. faecalis may not always metabolize TTC due to its specific metabolism pathways, an additional evaluation of cell viability for these species was performed; for example, the biofilms in plate wells were agitated using an automated pipette, removed from 96-well plates, and spotted on a stable Sabouraud or Mueller-Hinton (M-H) agar plate (Biomaxima, Warsaw, Poland) for C. albicans and E. faecalis, respectively and incubated for 48 h. The presence of living colonies on agar indicated the lack of MBEC, while the absence of colonies in the place where spotting was performed confirmed that the particular concentration of oral antiseptic can be considered the MBEC. For the purpose of clarity, results are presented as the percentage of dilution of a specific oral antiseptic product, wherein the original oral antiseptic product was considered to be 100%. These experiments were performed using six repeats.
2.6. Preparation of Prototypical Bacterial Cellulose Dressings
The K. xylinus strain was first cultivated in stationary conditions for 7 days at 28 °C in a dedicated Herstin-Schramm (H-S) medium. The 7-day cultures were shaken to remove the bacteria from the cellulose and used to inoculate the fresh H-S medium in a stationary 24-well culture plate at 28 °C to obtain disc-shape BC membranes with an 18 mm diameter. After 7 days, the BC membranes were taken from the medium and purified by the application of 0.1 M NaOH at 80 °C for 90 min and then washed with double-distilled water until the pH stabilized.
2.7. Saturation of Bacterial Cellulose Dressings with Oral Antiseptics
The obtained prototypical BC dressings, as described in
Section 2.6 immediately above, were placed in the wells of fresh 24-well culture plates containing 2 mL of each oral antiseptic. Each plate was left for 24 h at 4 °C. The control setting for this experiment was comprised of BC dressings containing no antimicrobial substance, e.g., dressings saturated with 0.9% NaCl in a manner analogical to the BC dressings saturated with antiseptics. In the later parts of this manuscript, BC dressings saturated with Prontosan were dubbed P-BC, dressings saturated with ProntoOral were dubbed PO-BC, BC dressings saturated with Braunol were dubbed B-BC, while control BC dressings saturated with saline were referred to as the N-BC. If oral-antiseptic-containing BC dressings are discussed collectively, they will be referred to as the OA-BC dressings.
2.8. Estimation of Oral Antiseptics’ Volume Trapped within a Bacterial Cellulose (BC) Carrier
To estimate the amount of OA trapped within a BC carrier, we first calculated the weight of water trapped within wet cellulose discs. To do so, we weighted wet BC dressings. Next, we placed BC dressings in the dryer at 25 °C for 72 h. Each day, the BC was weighted until the weight stopped dropping. The difference in weight between wet and dried cellulose was used to estimate the weight of water trapped within the BC. These experiments were performed in six repeats. Next, we introduced wet BC carriers into 24-well plate and immersed each carrier with 2 mL of a specific OA. The 24-well plate was sealed with adhesive tape to prevent liquids from evaporating and left for 24 h at 4 °C. After incubation, the OA-BC dressings were placed into O-ring holders and the whole setting was introduced to the 50 mL tube and subjected to centrifugation for 20 min at 12,000 rpm. The volume of obtained filtrates was measured to estimate the volume of specific OA trapped within the BC carrier. The value of the absorbances of filtrates at 230 nm were recorded and compared with the absorbances of working solutions of P and PO. The intense brown color of the PVP-I required dilution of the antiseptic 10× before spectrometric measurement could be done. These data are presented as a percentage of the absorbance of the OA-filtrate released from BC divided by the value of the absorbance of OA’s working solution. These experiments were performed using six replicates.
2.9. The Oas’ Release from the BC Dressings
To assess the release of the antimicrobials tested using BC disks, a spectrometric method using the MultiScan Go (Thermo Fisher, Warsaw, Poland) spectrometer was used. BC discs were incubated for 300 min at 37 °C in closed beakers containing 5 mL of clean, sterile water. At times of 5, 15, 30, 45, 60, 90, 120, 180, and 300 min, 200 μL of the resulting solution was transferred to wells on a transparent UV microplate (Becton Dickinson and Company, Franklin Lakes, NY, USA). Absorbance was determined spectroscopically at a 230 nm wavelength for each of the tested OA. Antiseptic working concentrations were used, with the exception of PVP-I, whose intense brown color forced dilution of the antiseptic 10× for measurement. After analysis, 200 μL of the test solutions were returned to beakers containing antiseptics. The experiments were performed in triplicates.
2.10. Estimation of the Antimicrobial Activity of BC Dressings Saturated with Oral Antiseptics Using a Modified Disc Diffusion Method
The BC dressings impregnated with oral antiseptics were placed onto the surface of the M-H agar medium (BioMaxima, Poland) seeded with the suspension of S. aureus, P. aeruginosa, E. faecalis, and C. albicans at a density of 0.5 McFarland. For Str. Mutans and A. actinomycetemcomitans, M-H agar medium supplemented with 3% sucrose was applied. Next, the cultures were carried out at 37 °C in 5% CO2 for 24 h. The average diameters of the inhibition zone (in mm) were calculated for each tested sample. The tests were performed in triplicates. The results were presented as growth inhibition zone (inhibition zone (mm) minus BC diameter (mm)). These experiments were performed using six repeats.
2.11. Confirmation of Ability of Tested Strains to Form Biofilm on Hydroxyapatite (HA) Disc Surface by Means of Scanning Electron Microscopy
A total of 2 mL of TSB suspension containing 1.5 × 108 CFU/mL of tested strains was introduced to wells of 24-well plates containing HA discs and incubated for 24 h at 37 °C in 5% CO2. For Str. mutans and A. actinomycetemcomitans, TSB suspension was enriched with 3% sucrose. Subsequently, biofilm-containing HA discs were gently rinsed with 0.9% NaCl to remove non-adherent cells. Next, the biofilm-containing HA discs were fixed via immersion in 2% glutarate for 4 h at 4 °C. After incubation, the samples were rinsed three times (for 2 min) with water to remove the fixative. Dehydration in increasing concentrations of ethanol (1× 25%, 50%, 70%, 80%, and 90%, and 2× 10% and 100%) was performed for 10 min (for 10–90% concentrations of ethanol) and for 15 min for the 100% ethanol concentration. The ethanol was then rinsed off and the samples were dried at 37 °C. Then the biofilm-containing agar plugs were sputtered using a Au:Pd mixture and examined using a scanning electron microscope (ZEISS EVO MA, Oberkochen, Germany) .
2.12. Evaluation of the Activity of Antiseptic-Saturated BC Dressings in Conditions Imitating an Oral Cavity Environment
The HA discs were soaked with 2 mL of AS for 1 h. Subsequently, the abundance of AS was removed and 2 mL of TSB suspension containing 1.5 × 108 CFU/mL of tested strains was introduced to wells of 24-well plates containing HA discs and incubated for 24 h at 37 °C in 5% CO2. For Str. mutans and A. actinomycetemcomitans, a TSB suspension enriched with 3% sucrose was applied. Subsequently, the biofilm-containing HA discs were gently rinsed with saline to remove non-adherent cells. At the same time, 1 mL of 2% tryptic soy agar (TSA) was poured into wells of a 24-well plate. The plate was left in a sterile chamber until the agar consolidated. Next, the agar plugs were cut out using a customized cork-borer with a 1.8 mm diameter. Afterwards, biofilm-containing HA discs were introduced to the bottom of wells and immersed in 400 µL of AS containing 3% sucrose. Subsequently, OA-BC dressings were placed on the top of this setting and left for 30 min, 1 h, and 24 h at 37 °C in 5% CO2.
The schematic of the experimental setting is presented in
Figure 1.
After incubation, the OA-BC dressings and AS were very gently removed and the HA discs were subjected to biofilm survival analysis. For this purpose, biofilm-containing HA discs were transferred to the fresh wells of a 24-well plate. Next, 2 mL of 0.1% TTC was added. The plates were incubated at 37 °C for 1.5 h. Next, the medium was removed and 2 mL of 96% ethanol was introduced to the wells to extract crystals of red formazan, which were the product of the TTC transformation in the presence of living microorganisms. Subsequently, the plates were incubated for 30 min at room temperature in a shaker (300 rpm/min). After this time, 200 μL of formazan-containing solution was transferred to the wells of a 96-well plate. The absorbance was measured at 490 nm using a MultiScan Go spectrophotometer. Then, absorbances were measured for formazan in samples with biofilms treated with OA-BC and were compared to the analogical values obtained for biofilms treated with N-BC. The survival of biofilms treated with N-BC was considered to be 100% and the survival of biofilms treated with OA-BC was calculated using: (value of the absorbance from sample of biofilm treated with OA-BC)/(value of the absorbance from sample of biofilm treated with N-BC) × 100%.
Because of issues related to the TTC assay (mentioned already in
Section 2.5) that may occur when
C. albicans and
E. faecalis are subjected to analysis, the following quantitative culturing procedure to assess biofilm survival was performed. Biofilm-containing HA discs were transferred to separate test tubes containing 1 mL of 0.1% saponine solution and subjected to vortex-mixing for 1 min to detach the biofilm cells from the HA surface. Serial dilutions of the obtained suspension in saline solution were performed and then cultured onto Sabouraud or M-H agar plates (for
C. albicans and
E. faecalis, respectively). The plates were incubated at 37 °C for 24 h. After incubation, the cfu number of
C. albicans or
E. faecelis was counted. The formula used to determine the biofilm survival was as follows: (value of cfu obtained from biofilm treated with OA-BC)/(cfu obtained from biofilm treated with N-BC) × 100%. These experiments were done using six repeats.
2.13. Presentation of the OA-BC Dressing Applicability Using an Oral Cavity Model
A model of the oral cavity was provided for experimental purposes by Septodont (Warsaw, Poland). The OA-BC dressings were placed in exemplary sites of the oral cavity model to demonstrate the cohesiveness and adhesiveness of the BC material and then photographed.
2.14. Mechanical Strength and Elasticity of BC Dressings
The BC dressings’ tensile strength test was performed using an MTS Synergie 100® machine (MTS System Corp, Eden Praire MN, USA). The tests were carried out at a speed of 10 mm/min at room temperature. Based on the recorded values of force (F) and displacement (Δl), stress–strain graphs were prepared; based on these graphs, mechanical parameters, such as tensile strength, were determined. The results were presented as the average tensile strength values obtained from six samples.
2.15. Statistical Analysis
Calculations were performed using the GraphPad Prism version 7 software (GraphPad Co., San Diego, CA, USA). The normality distribution was calculated by means of the D’Agostino–Pearson omnibus test. Because all values were non-normally distributed, the Kruskal–Wallis test with post-hoc Dunnett analysis were applied. The results of statistical analyses were considered significant if they produced p-values < 0.0001.
3. Results
All used OAs displayed a high antibiofilm activity, e.g., low values of MBEC, in the standard setting of 96-well culturing plates. Results are presented in
Table 1.
It should be noted that oral antiseptics containing polihexanide (PO and P) were more active against all microbial strains analyzed (with the exception of C. albicans) than oral antiseptic containing povidone-iodine (B) in this setting. Also, the PO and P MBEC values obtained regarding a specific strain were the same or differed with maximally one level of dilution.
Using antiseptics proven in standard test settings (e.g., microdilution assays in 96-well plates), we chemisorbed these compounds to BC and tested the combination product in another standard microbiological assay (disc-diffusion methodology) to assess the antimicrobial activity. The only difference was that instead of the antibiotic-saturated discs, BC membranes with chemisorbed antiseptics were used. The average weight of wet BC dressings was 276 ± 60 mg, while their dry weight was 2.4 ± 0.4 mg. The average weight of water trapped within native BC dressings was estimated for 273 µL. The average volume of the filtrate obtained after centrifugation of OA-BC was 196.5 ± 22.7, 213.16 ± 45.45, and 240 ± 40 µL for B, P, and PO, respectively. The estimated concentration of particular OAs in filtrates compared with working solutions was 70 ± 11%, 68 ± 9%, and 64 ± 11% for B, P, and PO, respectively. Knowing the concentration of the active substance in working solutions of particular OAs, the amount of drug incorporated in B-BC could thus be estimated for ≈10.5 mg of povidone-iodine complex and ≈1 mg of active iodine (according to the manufacturer, PVP-I complex contains 10% of active iodine element; please refer to
Section 2.2). For P and PO-BC discs, analogical values were estimated for 0.14 mg and 0.15 mg, respectively.
The appearance of BC membranes used for this experimental setting, together with their micro-structure, is presented in
Figure 2, while specific AOs released from BC dressings is presented in
Figure 3 and
Figure 4.
Antimicrobial activity and the repeatability of results is shown in
Figure 5 with
S. aureus as an exemplar. The summarized results of the antimicrobial activity of BC antiseptics against all strains analyzed are presented in
Table 2.
The B-antiseptic released from BC displayed significantly higher antimicrobial activity against all analyzed microorganisms in comparison to PO and P (Kruskal-Wallis test with post-hoc Dunnett’s analysis,
p < 0.0001,
Table 2). Release of PO and P from BC also inhibited microbial growth, however there were no statistically significant differences between inhibition zone sizes formed in result of activity of these two antiseptics (Kruskal-Wallis test with post-hoc Dunnett’s analysis,
p > 0.0001,
Table 2).
P. aeruginosa was most resistant to all applied antiseptics among tested species, while
A. actinomycetemcomitans was the most sensitive. Very high
C. albicans growth inhibition zones were observed in the setting of antiseptic B (
Table 2).
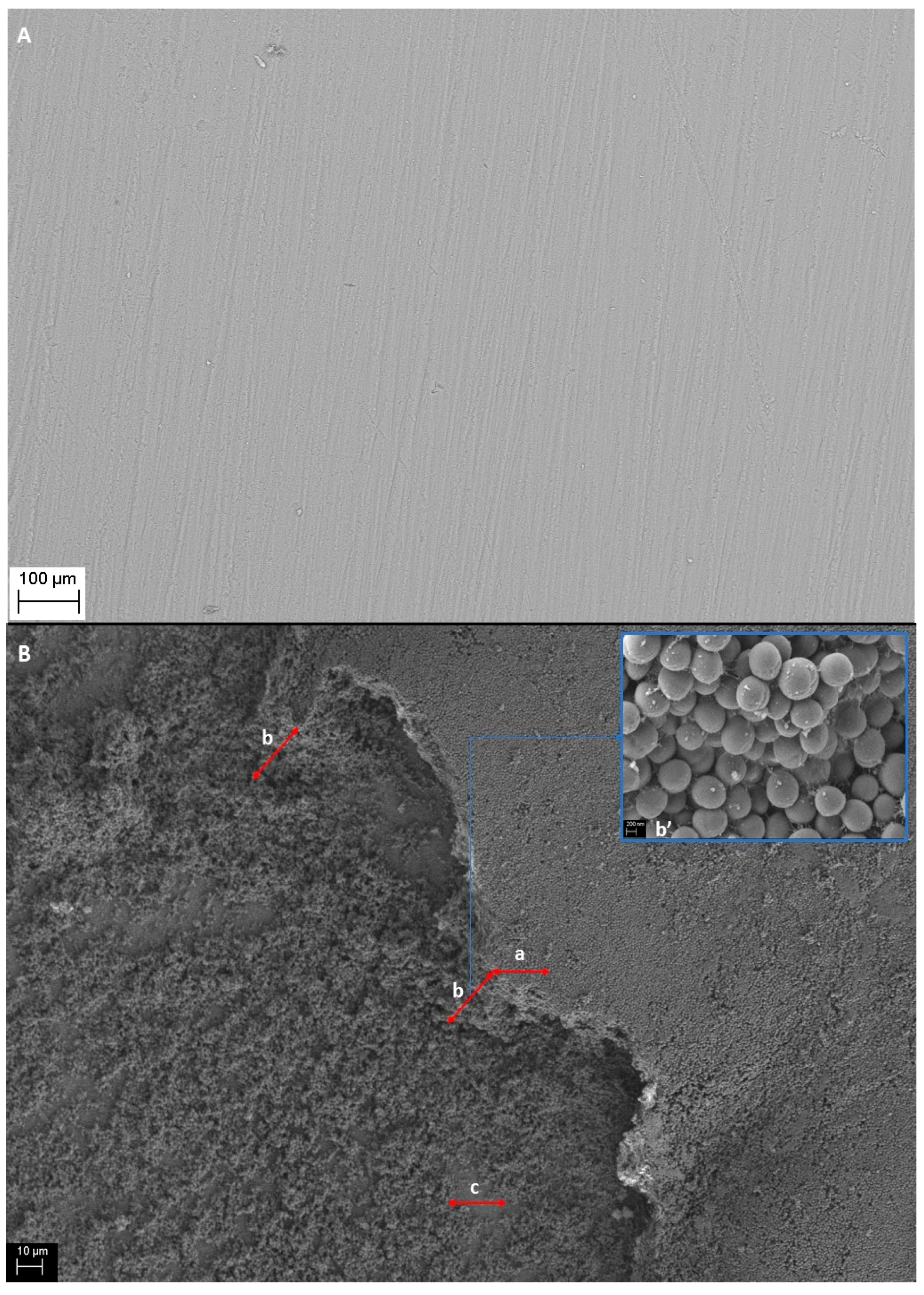
These analyses, with results presented above, were designed to investigate whether antiseptic agents were released from BC carriers to demonstrate antimicrobial activity. Next, another experiment was performed, namely the culturing biofilms of tested pathogens on discs made of HA, which is the main inorganic building component of bone and teeth. The diameter of the customized HA discs was 12 mm; its surface is presented in
Figure 6A. The ability of microorganisms to form multilayer biofilms on the substrate was presented in earlier work of ours [
33,
34,
35]; herein, we show it in
Figure 6B with
S. aureus as an exemplar.
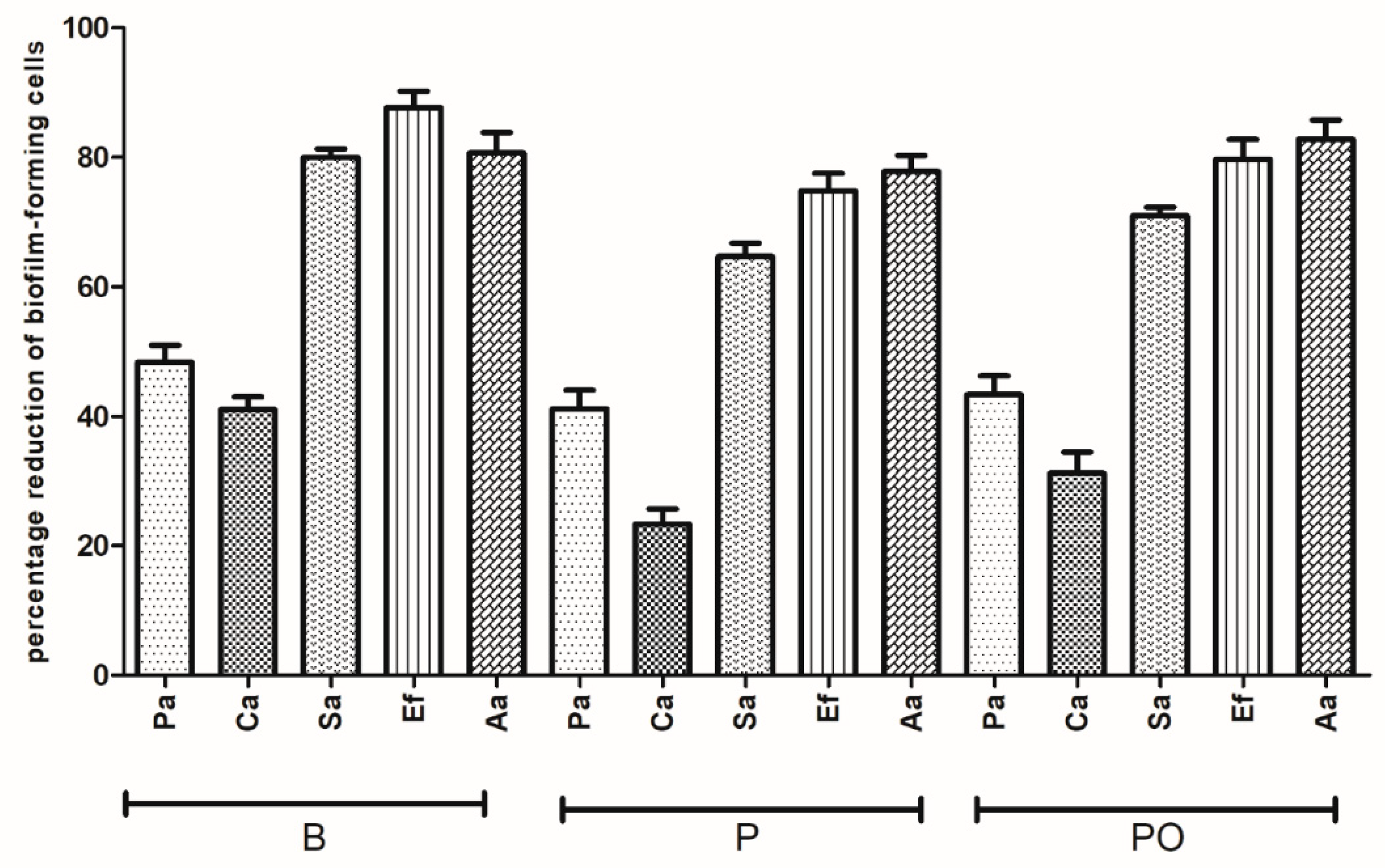
Next, the activity of antiseptic BC carriers was analyzed in in vitro settings imitating the environment of the oral cavity, as presented in
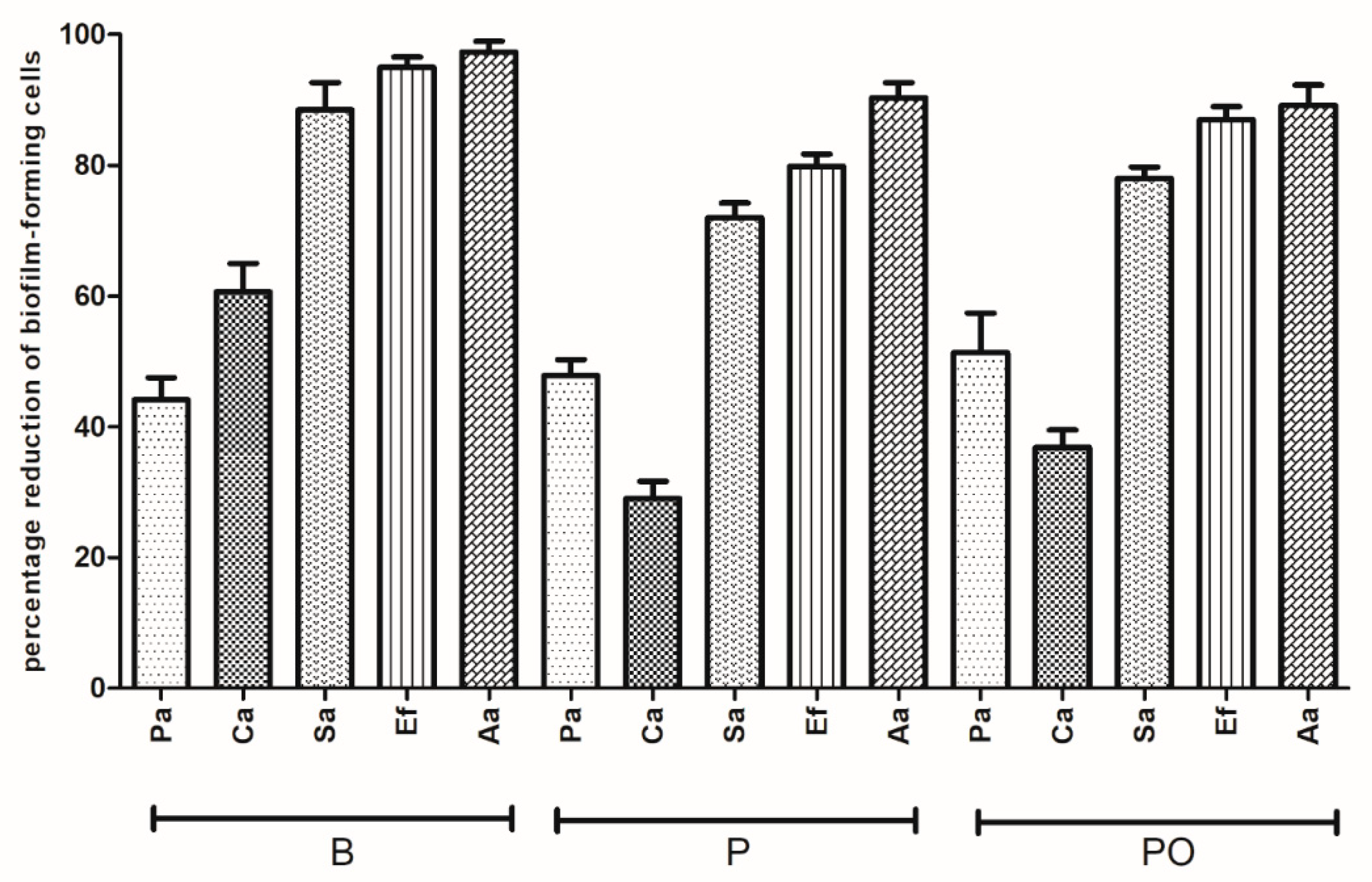
Section 2.12. Over the contact time of 24 h, all antiseptics were able to completely eradicate the biofilm-forming cells from the HA surface. Results of the antiseptic efficiency for shorter contact times (e.g., 1 h and 30 min) are presented in
Figure 7 and
Figure 8, respectively.
As shown in
Figure 7 and
Figure 8, the resistance levels of all pathogens against B, PO, and P were grouped into two clearly distinct sets. One of them clustered
S. aureus,
E. faecalis, and
A. actinomycetemcomitans together, while another one clustered
C. albicans and
P. aeruginosa together. In the case of the first group, antiseptics displayed a high (>70%) antibiofilm efficacy, while in the case of the latter group, the analogical cut-off point was established at 60% and its lowest values were at 23% (
C. albicans biofilm versus P with a 30 min contact time,
Figure 8). Differences observed between the level of eradication of
S. aureus,
E. faecalis, and
A. actinomycetemcomitans versus
C. albicans and
P. aeruginosa biofilms were statistically significant for both contact times and antiseptics applied (Kruskal-Wallis test with post-hoc Dunnett’s analysis,
p < 0.0001), while no significant differences in antibiofilm activity were observed within each of two aforementioned clusters of oral pathogens (Kruskal-Wallis test with post-hoc Dunnett’s analysis,
p > 0.0001).
Regarding the comparison between the antibiofilm activities of specific antiseptics, there were no statistically significant differences between P versus PO and PO versus B against the tested pathogens (Kruskal-Wallis test with post-hoc Dunnett’s analysis, p > 0.0001). In the case of B versus P, differences were also insignificant with the exception of C. albicans biofilms, where B displayed a significantly higher activity in both contact times (Kruskal-Wallis test with post-hoc Dunnett’s analysis, p < 0.0001)
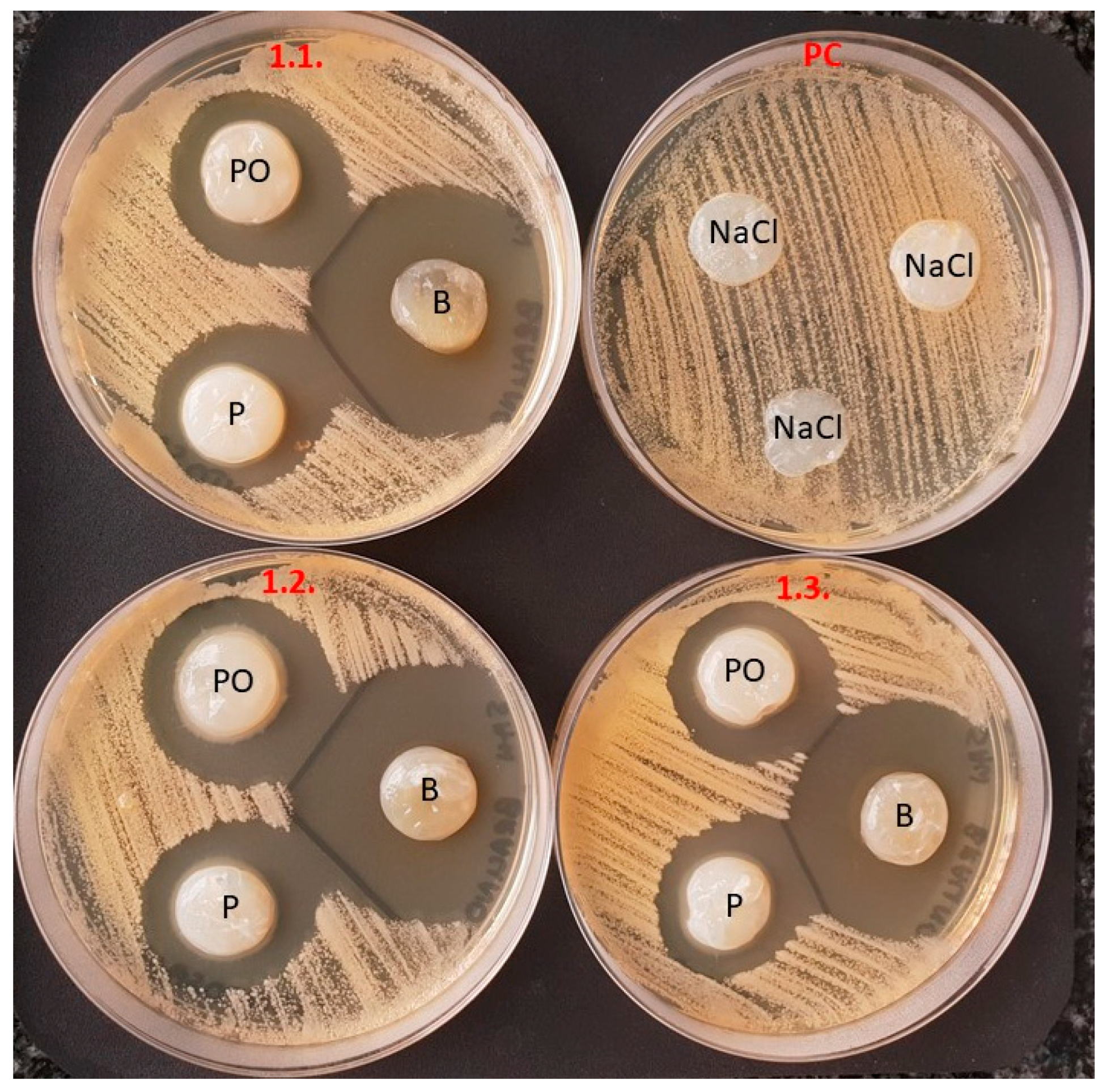
Next, as a proof-of-concept, we evaluated the potential applicability and adhesiveness of BC carriers to various specific surfaces of the oral cavity. To do so in in vitro conditions, we used oral cavity typodont models and raw bovine loin tissue. Results are presented in
Figure 9 and
Figure 10, respectively.
BC antiseptic dressings were easily modeled and stuck firmly to various surfaces of the oral cavity model and bovine tissue. Moreover, as can be seen in
Figure 9 and
Figure 10, BC dressings that were saturated with colorless antiseptic (e.g., P, PO-BC) displayed a high transparency.
Results presented in
Figure 9 and
Figure 10 may raise questions concerning the mechanical strength and elasticity of BC dressings regarding their potential applicability within the oral cavity. The mechanical strength and elasticity modulus of BC dressings used in experiments presented in this manuscript was 2.00 ± 0.86 and 26.18 ± 9.25 MPa, respectively.
4. Discussion
In this study, we evaluated the in vitro ability of prototypical BC dressings, chemisorbed with antiseptics, to eradicate biofilms formed by oral pathogens. For this purpose, we used a micro-dilution methodology as a prerequisite condition to perform further experiments in which we chemisorbed antiseptics to BC carriers. As shown in
Table 1, all antiseptics displayed high antibiofilm activity against
S. aureus,
E. faecalis,
P. aeruginosa,
C. albicans,
Str. mutans, and
A. actinomycetemcomitans. It should be noted that the microdilution technique is one of the most basic experimental settings in microbiology and it does not reflect the in vivo conditions of the oral cavity or any other sites of the human body. In this technique, biofilms grown on a polystyrene surface were cultured in buffered microbiological medium. When antiseptics were introduced, they faced neither physiological impediments related with oral cavity topology nor obstacles such as saliva and the many proteins and enzymes in saliva. These facts may explain the very high antibiofilm efficacy of P, PO, and B antiseptics obtained in this particular experimental setting. Although results presented in
Table 1 are in line with our previous findings, as well as other investigations in this context [
36,
37,
38,
39], it is important to note that antiseptics used to treat in vivo infections may display lower actual activity in comparison to the activity assessed by means of in vitro microdilution methods.
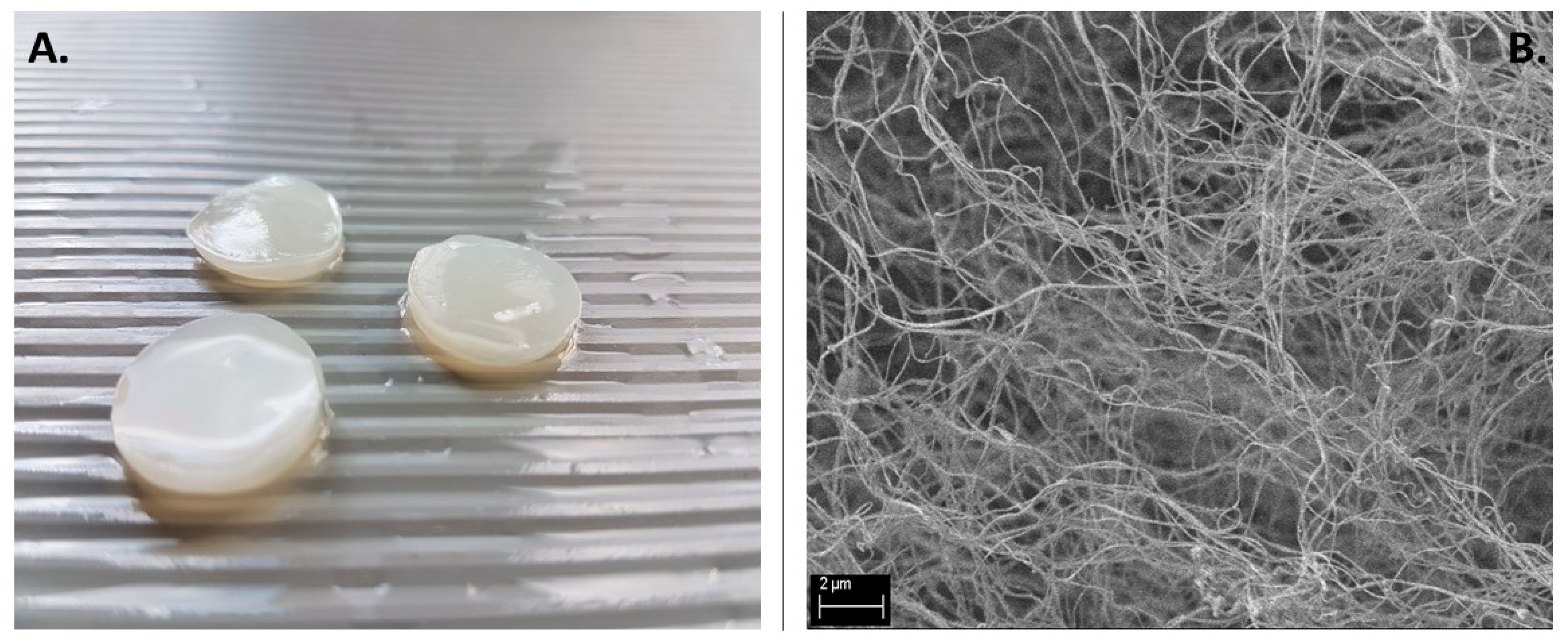
Nevertheless, these studies and results allowed us to perform our next stage of the investigation, namely studying the antimicrobial efficacy of antiseptics released from BC dressings. Dressings (
Figure 2A), owing to their porous composition (
Figure 2B) and chemical composition, were able to absorb and release high volumes of antiseptic liquids [
40]. The results presented in
Figure 3 and
Table 2 confirmed the ability of BC dressings to elute antimicrobials. Antiseptics were released from this material in an effective and reproducible manner (
Figure 3 and
Figure 4), with the maximal release observed within 15 or 30 min for polihexanidine and povidone-iodine, respectively. However, the specificity of wet BC carriers allowed us to only roughly estimate the amount of drugs incorporated into the carrier, though it should be noted that similar results were obtained by Alkhtabib et al. [
41] who analyzed the controlled release of octenidine antiseptic, belonging to the bispyridine family, from BC. The results presented in
Figure 5 confirmed the ability of BC dressings to elute antimicrobials. Also, B-BC dressings, after incubation, almost completely lost their dark brown color (compare B-BC dressing color from
Figure 5 with the B-BC dressing color from
Figure 9 and
Figure 10). This may be explained by the close-to-complete release of povidone-iodine from the BC carrier. B-BC dressings were also the most effective regarding the eradication of microorganisms when a modified disc-diffusion methodology was applied (
Table 2). This very high antimicrobial activity of povidone-iodine in comparison to other antiseptics (including polihexanide) was also confirmed by other research teams [
34,
35]. Antiseptics containing povidone-iodine are commonly used (in a range of concentrations) for treatment procedures in the oral cavity. However, in some cases, the application of this antiseptic substance may be associated with side effects [
42], which motivates our goal to couple BC dressings with other antiseptics. Furthermore, the efficacy of PO and P products, released from BC can be considered high (
Figure 5,
Table 2). Moreover, results obtained for these two products (containing the same active substance, e.g., polihexanide) differed by ≈1–2 mm in diameter for the inhibition growth zones against particular pathogens, showing a comparable spread of active substance throughout the agar surface. When the oral cavity imitating models were used (
Figure 1), all antiseptics were able to eradicate multi-layer biofilms (
Figure 6) preformed on HA surfaces over 24 h of contact time. These results were comprehensive, with data presented by in
Table 1, where we showed that the MBECs reached by all antiseptics within this particular time frame were of very low values. Because antiseptics are usually not applied for such long contact times, we performed analysis of their activity for 1 h and 30 min (
Figure 7 and
Figure 8, respectively). Results presented show that
P. aeruginosa and
C. albicans displayed a higher resistance to all antiseptics released from BC than
S. aureus,
E. faecalis, and
A. actinomycetemcomitans.
P. aeruginosa was also the most resistant to antiseptics for applications using the microdilution and disc-diffusion methodology (
Table 1 and
Table 2, respectively). The high tolerance of this Gram-negative bacteria against various antimicrobials is well-recognized [
43]. In contrast to results presented in
Table 1 and
Table 2,
C. albicans was moderately sensitive to B-antiseptic in the oral cavity model for 30 min and 1 h of contact time (
Figure 7 and
Figure 8, respectively). We assumed that povidone-iodine released from BC (please refer to
Figure 1 for description of this setting) could be partially inhibited by components of the artificial saliva and could display a lower activity against biofilms preformed on HA surfaces. This hypothesis could explain discrepancies in the results obtained by means of disc-diffusion versus oral-cavity-simulating methodologies. It should be noted that the high reactivity of povidone-iodine with various inhibitors (including blood, pus, fat, protein-containing solutions, or even glove powder) was already presented by Zamora et al. [
44].
Altogether, results presented in
Figure 7 and
Figure 8 show that for short contact times of 30 min and 1 h, the applicability of antiseptic BC dressings was higher when the biofilms to be eradicated are formed by
S. aureus,
E. faecalis, and
A. actinomycetemcomitans than by
P. aeruginosa and
C. albicans. Nevertheless, all antiseptics released from the BC carriers displayed a high activity against oral biofilms formed in vitro in our experimental model. We are aware that the application of other models of biofilm testing, such as the Amsterdam Active Adhesion Model [
45], could provide valuable data and additionally elucidate phenomena concerning the interaction between the BC dressing, drug release, and biofilm eradication. However, the methodology and results presented in our manuscript also may be translated to specific conditions of a pathologically altered oral cavity, where the flow of saliva to the infection site is disturbed. Therefore, to some extent, these results can be used to estimate the efficacy of antiseptics released from a BC carrier in the eradication of pathogenic oral biofilms. It should be noted that our in vitro study is of a preliminary character. However, only in vivo results may be considered conclusive for the matter discussed above, and the data we provide here is a necessary investigative step, which justifies and rationalizes the undertaking of further animal model and clinical studies.
Finally, we performed a proof-of-concept analysis and applied antiseptic BC dressings on oral cavity typodont models and bovine tissue to investigate the dressing behavior on topologically-challenging surfaces of the oral cavity. The dressings stuck firmly to particular surfaces resembling teeth (
Figure 9) and gingival tissue (
Figure 10).
One can hypothesize that BC dressings may display sufficient mechanical properties to be applied in an oral cavity. However, the elasticity modulus value of BC was lower than the analogous parameter presented by other teams for collagen fibrils (100–360 MPa) [
46,
47], and it was higher than the elasticity of chitosan (7.06 MPa [
48]), which is one of the biopolymers frequently used as a carrier in oral mucosal delivery systems. Moreover, BC dressings saturated with colorless PO and P antiseptics displayed a high transparency, which may be of benefit for clinical applications for various treatment procedures performed within the oral cavity. We also suggest that in a patient’s oral cavity, BC dressings may efficiently absorb blood, exudate, and sequestrate microorganisms as we have shown in previous experiments [
49]. In another work of ours, we confirmed the high biocompatibility of a BC dressing toward a fibroblast cell line [
50]. These results are in line with data provided by Wiegand et al. [
51], who demonstrated the high biocompatibility of BC chemisorbed with povidone-iodine and octenidine toward a keratinocyte cell line. Despite these promising in vitro results, further experiments on animal models and subsequent pilot clinical studies are required.
The number of infective complications within the oral cavity resulting in various pathologic manifestations is vast; therefore, a vast number of drug forms is needed to treat these diseases. These forms may be classified into pills, mucoadhesive films, mouth rinses, aerosols, and hydrogels. Hydrogels offer excellent potential as oral therapeutic systems due to their inherent biocompatibility, diversity, tunable properties, and the possibility of controlled drug delivery [
52]. BC displays all the beneficial features of hydrogels of natural origin: nontoxicity, biocompatibility, and physiochemical suitability [
30]. As we have shown in our earlier work, BC does not induce macrophages to release free radicals [
50]. The main potential disadvantage of a BC carrier may be related with certain level of differences in porosity and thickness, typical for natural biomaterials, which have an impact on water-related properties [
53]. Therefore, to perform the analyses presented in this manuscript, we used BC obtained during a single cultivation period.
The results presented in this study indicate the potential of antiseptic BC dressings for eradicating pathogenic biofilms formed in the oral cavity. Our design and optimization of this unique biomaterial may allow for clinical applications of antibiotic-eluting BC carriers toward biofilm-mediated infectious diseases like periodontitis, osteomyelitis, and osteonecrosis, which we will investigate in future translational studies.