Development of Detection System with Low Predictive Errors for Determining Vitamin C Content of Indian Jujube
Abstract
1. Introduction
2. Materials and Methods
2.1. Indian Jujube Samples
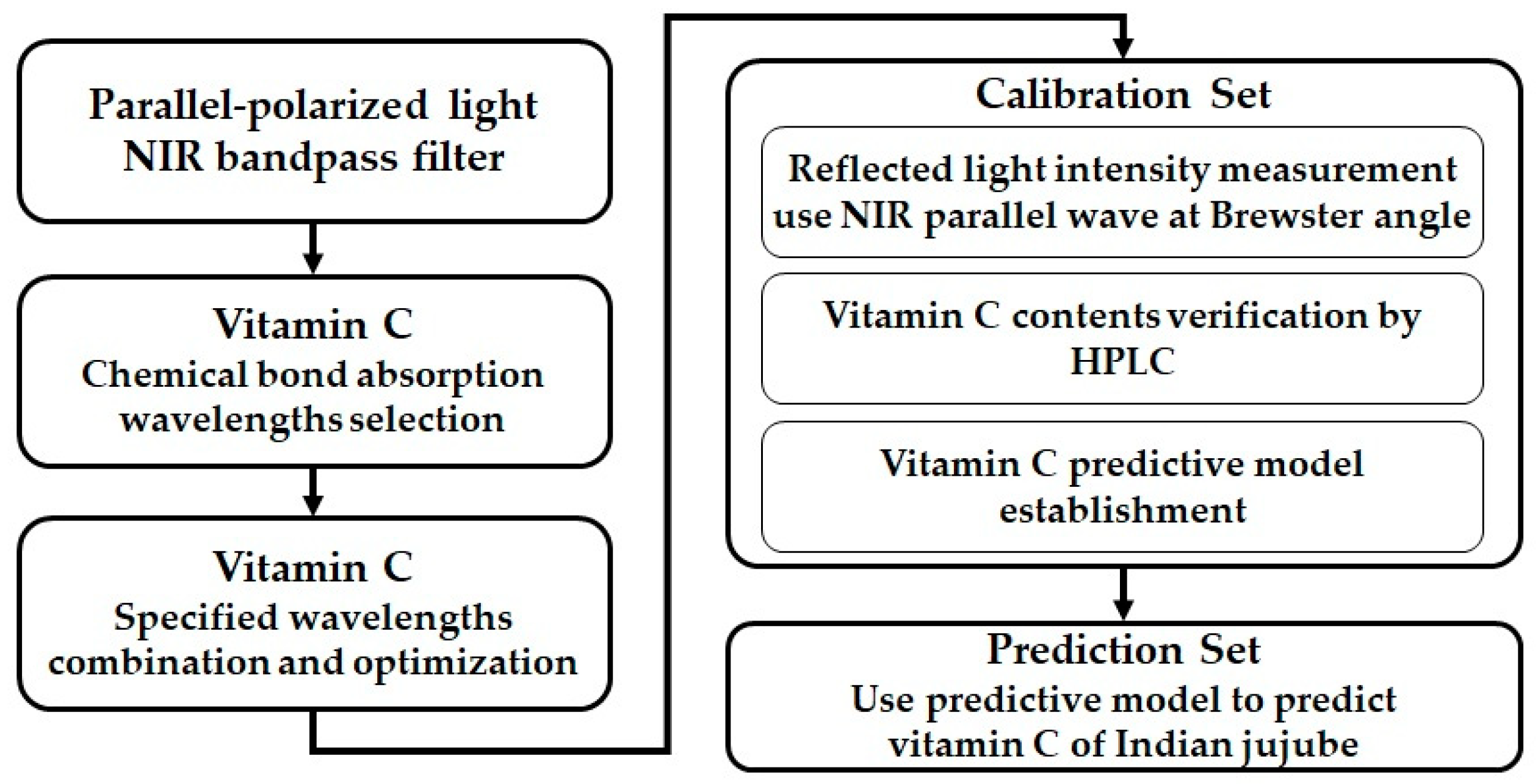
2.2. Experimental Procedures
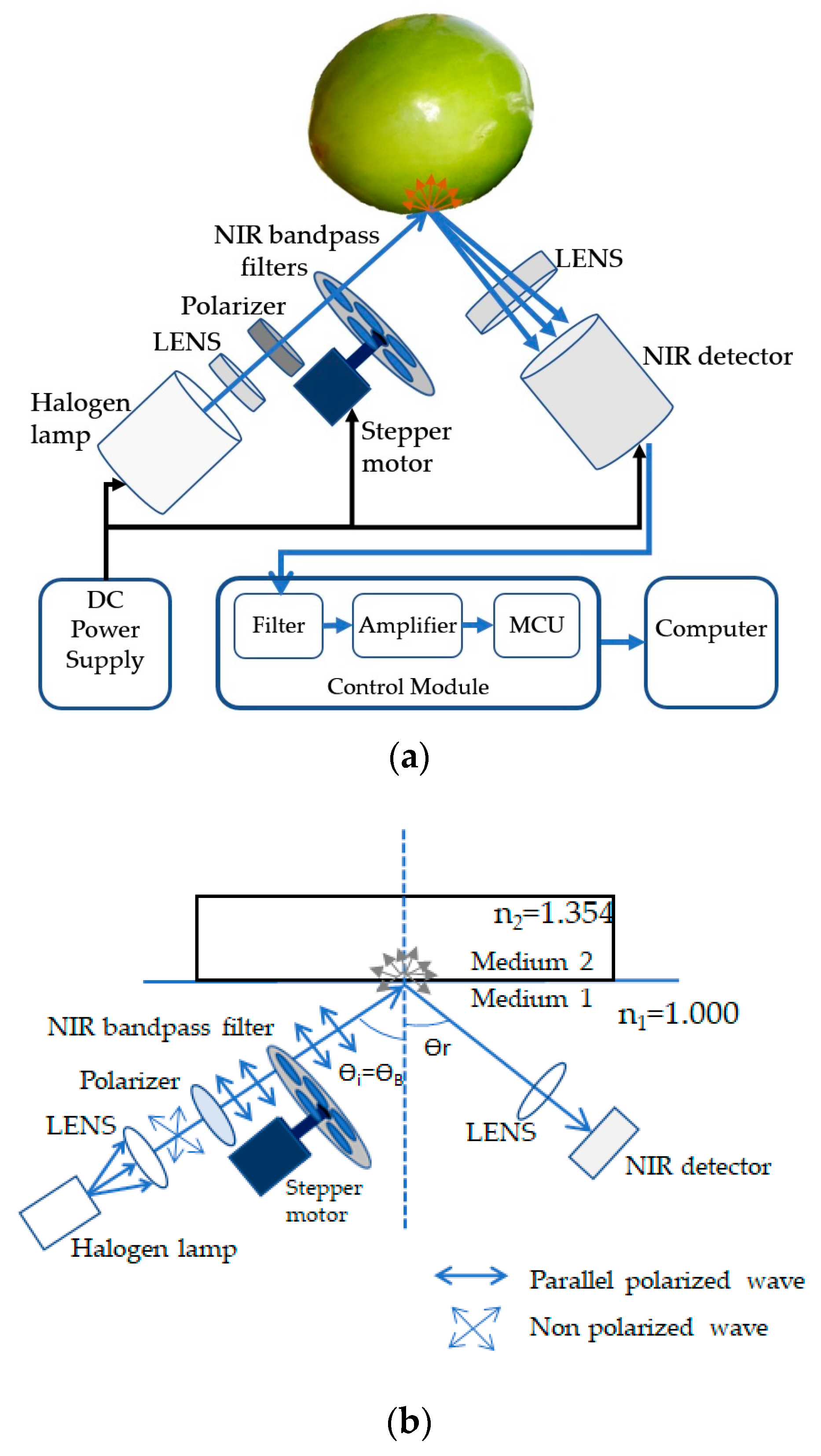
2.3. Structure of the Nondestructive Detection System
2.3.1. Light Source, LENS and Polarizer
2.3.2. NIR Bandpass Filter
2.3.3. Photodetector
2.3.4. Signal Processing Module
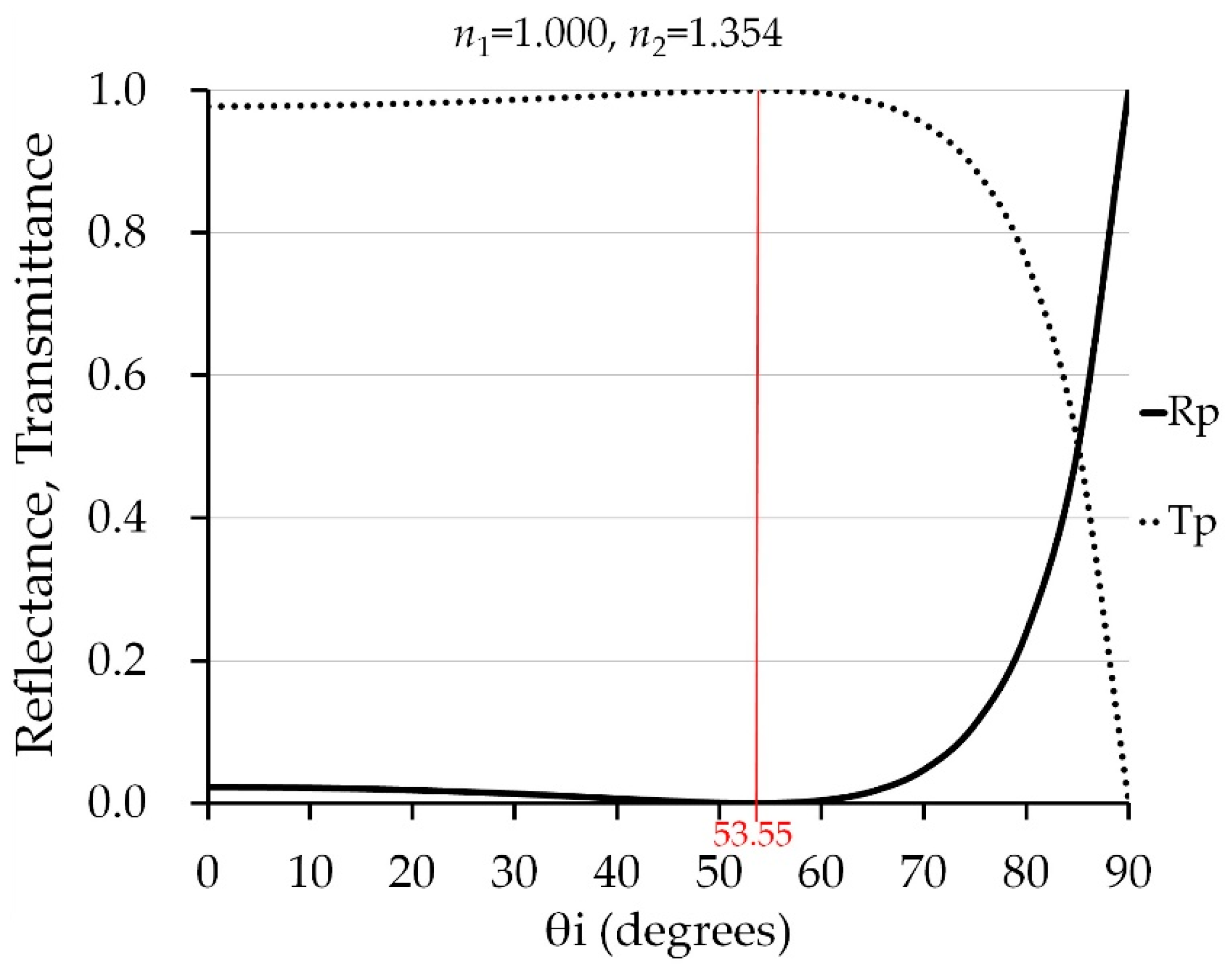
2.4. Angle of Incidence and Polarization for Measuring Vitamin C Content of Indian Jujube
2.5. Calibration of Detection System and Construction of a Predictive Algorithm Model
2.6. Predictions by the Detection System and Verification of the Predictive Algorithm Model
3. Results
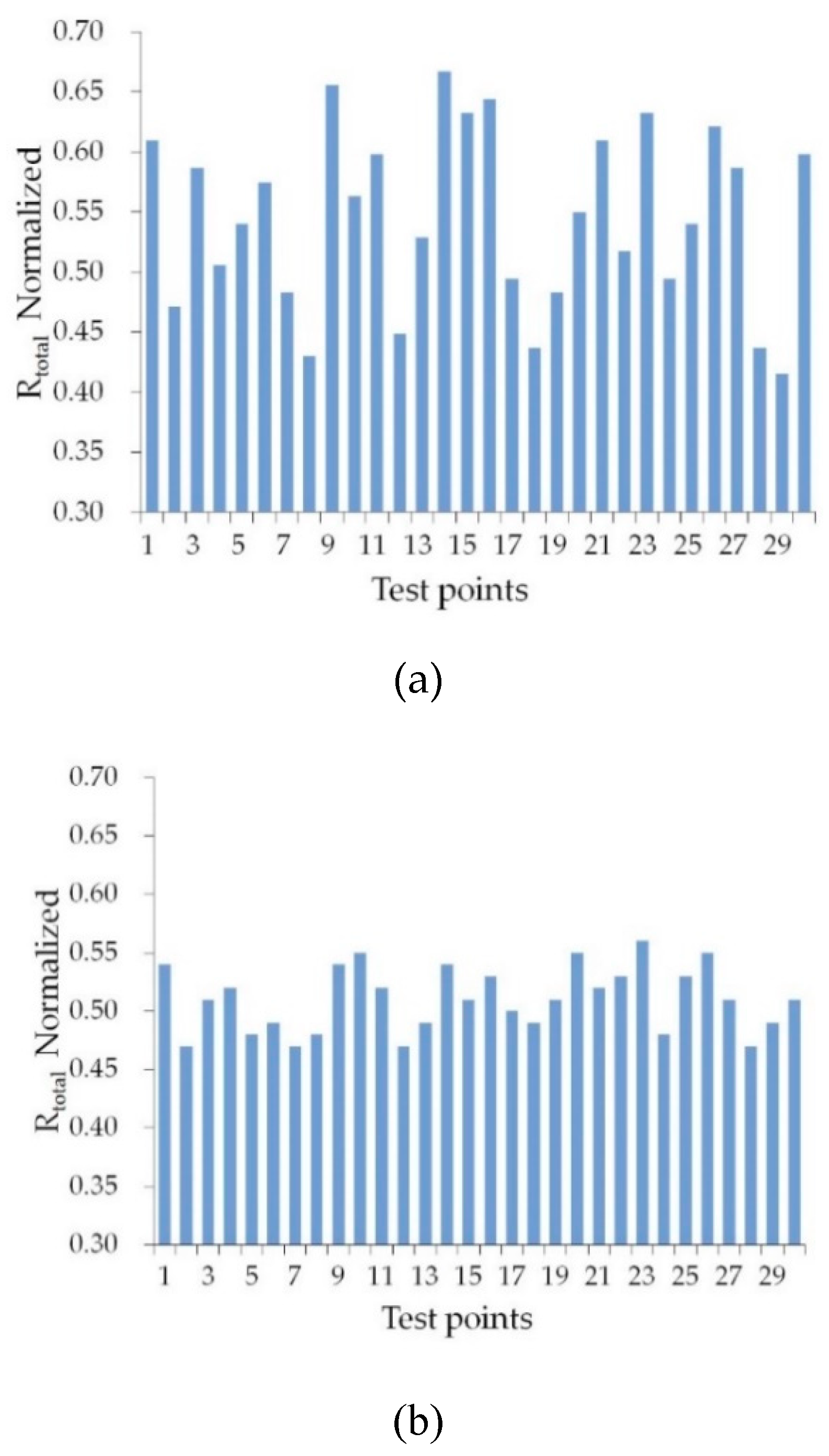
3.1. Distribution of Rtotal Values for p-Waves Incident at θB
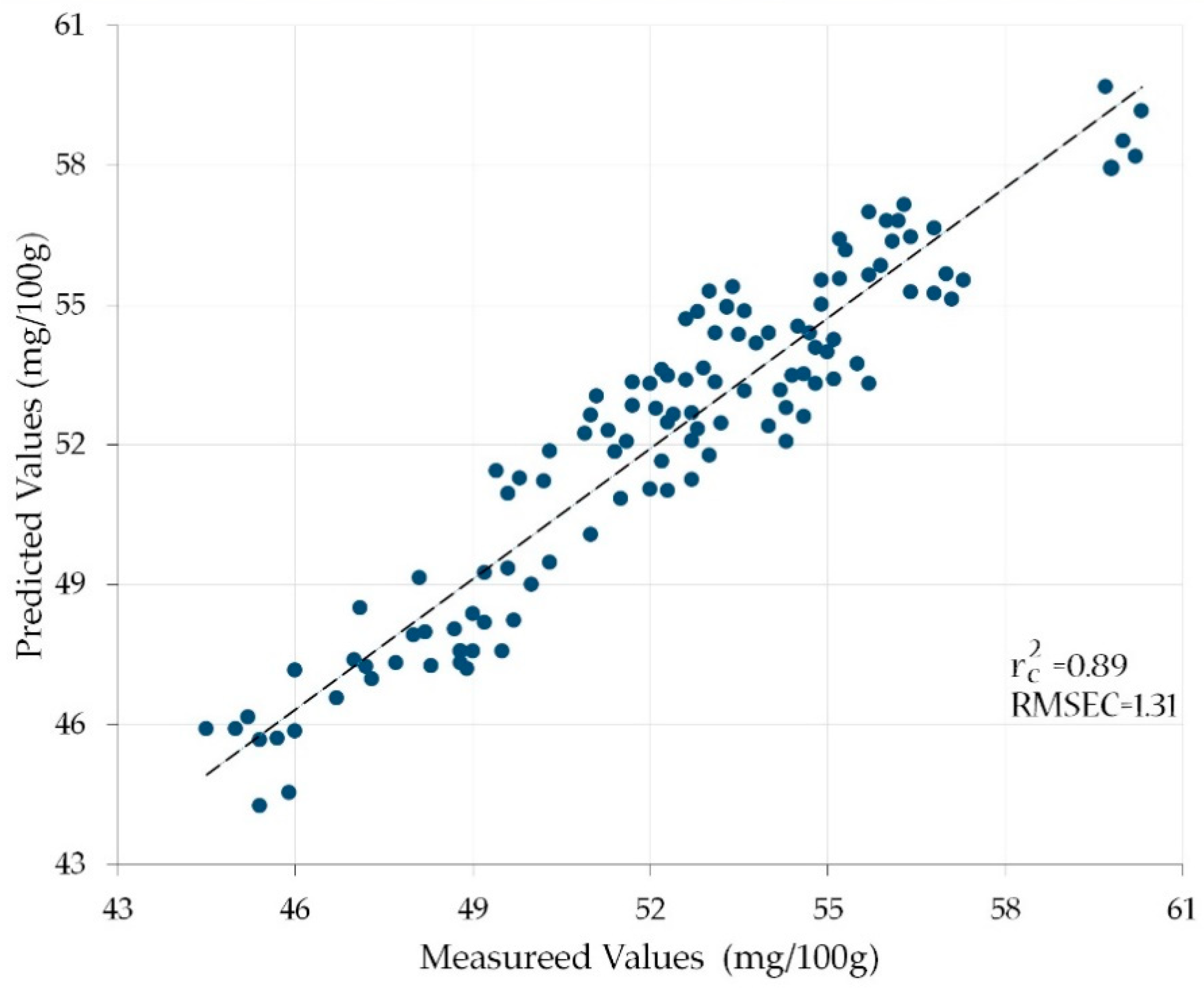
3.2. Correlation between the Vitamin C Contents of the Calibration Set and the Reflectances of the Characteristic Wavelength Combinations
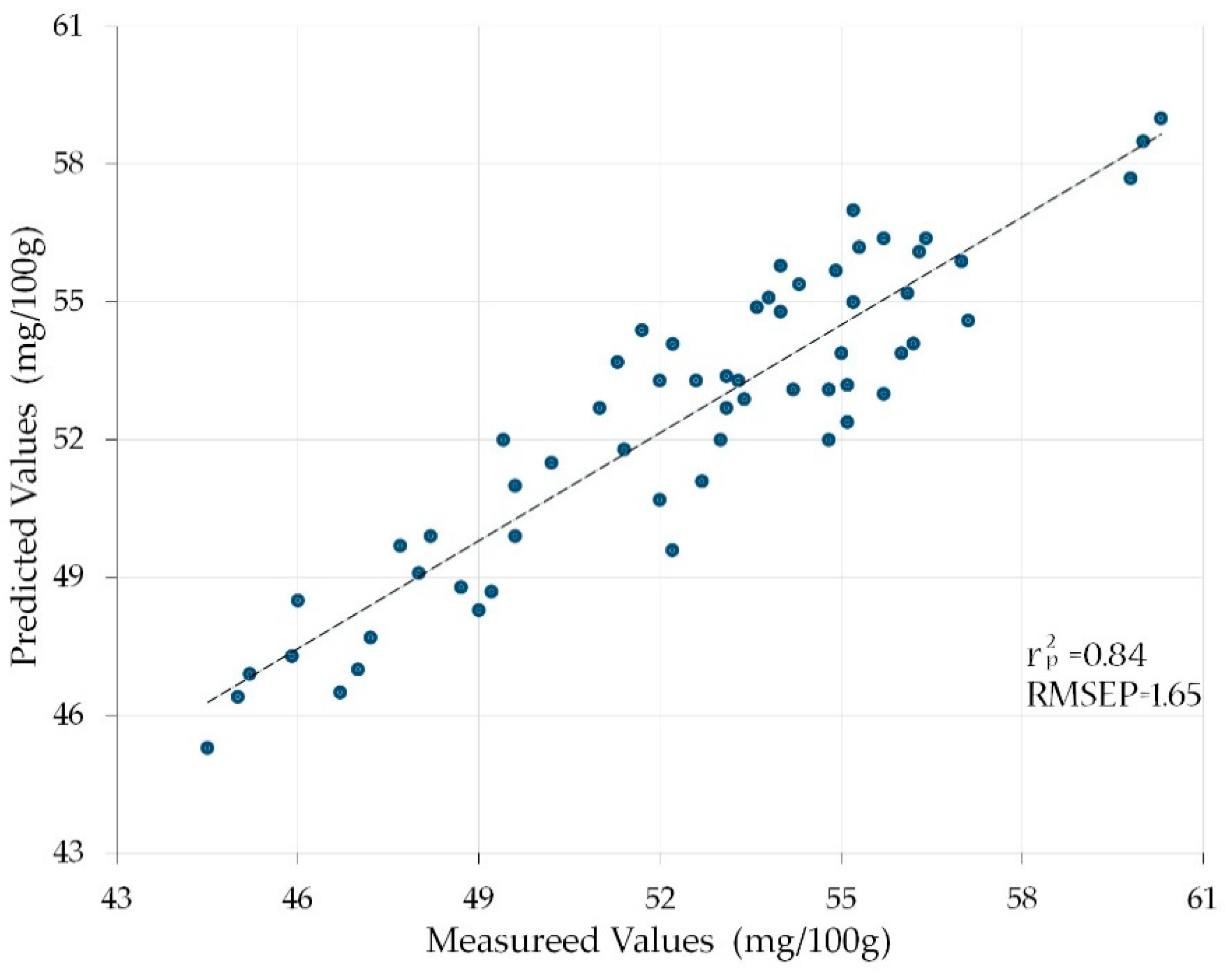
3.3. Analyzing the Predicted Vitamin C Contents of the Prediction Set
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gorton, H.C.; Javis, K. The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections. J. Manip. Physiol. Ther. 1999, 22, 530–533. [Google Scholar] [CrossRef]
- Mattila, P.H.; Hellström, J.; McDougall, G.; Dobson, G.; Pihlava, J.M.; Tiirikka, T.; Stewart, D.; Karjalainen, R. Polyphenol and vitamin C contents in European commercial blackcurrant juice products. Food Chem. 2011, 127, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Nicolaı, B.M.; Beullens, K.; Bobelyn, E. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar]
- McGlone, V.A.; Kawano, S. Firmness, dry-matter and soluble-solids assessment of postharvest Kiwifruit by NIR spectroscopy. Postharvest Biol. Technol. 1998, 13, 131–141. [Google Scholar] [CrossRef]
- Alander, J.T.; Bochko, V.; Martinkauppi, B.; Saranwong, S.; Mantere, T. A review of optical nondestructive visual and near-infrared methods for food quality and safety. Int. J. Spectrosc. 2013, 2013, 341402. [Google Scholar] [CrossRef]
- Fu, X.; Ying, Y.; Lu, H.; Yu, H.; Liu, Y. Evaluation of vitamin C content in kiwifruit by diffuse reflectance FT-NIR spectroscopy. Proc. SPIE Opt. Sens. Sens. Syst. Nat. Resour. Food Saf. Qual. 2005, 5996, 1–10. [Google Scholar] [CrossRef]
- Xia, J.; Li, P.; Li, X.; Wang, W.; Ding, X. Effect of different pretreatment method of nondestructive measure vitamin C content of umbilical orange with near-infrared spectroscopy. Trans. CSAM 2007, 38, 107–111. [Google Scholar]
- Xia, J.; Li, X.; Li, P.; Wang, W.; Ding, X. Approach to nondestructive measurement of vitamin c content of orange with near-infrared spectroscopy treated by wavelet transform. Trans. CSAE 2007, 23, 170–174. [Google Scholar]
- Peiris, K.H.S.; Dull, G.G.; Leffler, R.G. Near-infrared spectrometric method for nondestructive determination of soluble solids content of peaches. J. Am. Soc. Hortic. Sci. 1998, 123, 898–905. [Google Scholar] [CrossRef]
- Elmasry, G.; Wang, N.; Elsayed, A. Hyperspectral imaging for nondestructive determination of some quality attributes for strawberry. J. Food Eng. 2007, 81, 98–107. [Google Scholar] [CrossRef]
- Jaiswal, P.; Jha, S.N.; Bharadwaj, R. Non-destructive prediction of quality of intact banana using spectroscopy. Sci. Hortic. 2012, 135, 14–22. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J. Rapid determination of vitamin C by NIR, MIR and FT-Raman techniques. J. Pharm. Pharmacol. 2002, 54, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.B.; Huang, L.S.; Chen, X.L.; Liao, L. Rapid quantification of vitamin C in navel oranges by near-infrared diffuse reflectance spectroscopy. Appl. Mech. Mater. 2014, 602, 1534–1537. [Google Scholar] [CrossRef]
- Qing, Z.S.; Ji, B.P.; Zude, M. Wavelength selection for predicting physicochemical properties of apple fruit based on near-infrared spectroscopy. J. Food Qual. 2007, 30, 511–526. [Google Scholar] [CrossRef]
- Zude, M.; Herold, B.; Roger, J.M. Non-destructive tests on the prediction of apple fruit flesh firmness and soluble solids content on tree and in shelf life. J. Food Eng. 2006, 77, 254–260. [Google Scholar] [CrossRef]
- Sutherland, G.B.B.M.; Jones, A.V. Use of polarized radiation in infra-red analysis. Nature 1947, 160, 567–568. [Google Scholar] [CrossRef]
- Tsepulin, V.G.; Perchik, A.V.; Tolstoguzov, V.L. Thin film thickness measurement error reduction by wavelength selection in spectrophotometry. J. Phys. Conf. Ser. 2015, 584, 012011. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Terry, L.A.; Landahl, S.; Cronje, P.J.; Nieuwoudt, H.H.; Hanssense, A.; SaeyseBart, W.; Nicolaï, B.M. Evaluation of fourier transform-NIR spectroscopy for integrated external and internal quality assessment of Valencia oranges. J. Food Compos. Anal. 2013, 31, 144–154. [Google Scholar] [CrossRef]
- Mack, C.A. Field Guide to Optical Lithography; SPIE Press: Bellingham, WA, USA, 2006. [Google Scholar]
- Daood, H.G.; Biacs, P.A.; Dakar, M.A.; Hajdu, F. Ion-pair chromatography and photodiode-array detection of vitamin C and organic acids. J. Chromatogr. Sci. 1994, 32, 481–487. [Google Scholar] [CrossRef]
- Nishiyama, I.; Yamashita, Y.; Yamanaka, M.; Shimohashi, A.; Fukuda, T.; Oota, T. Varietal difference in vitamin C content in the fruit of kiwifruit and other Actinidia species. J. Agric. Food Chem. 2004, 52, 5472–5475. [Google Scholar] [CrossRef]
- De Whalley, H.C.S. ICUMSA Methods of Sugar Analysis; Elsevier Publishing Company: Amsterdam, The Netherlands, 1964. [Google Scholar]
- Lu, R. Nondestructive measurement of firmness and soluble solids content for apple fruit using hyperspectral scattering images. Sens. Instrum. Food Qual. Saf. 2007, 1, 19–27. [Google Scholar] [CrossRef]
- López, M.G.; García-González, A.S.; Franco-Robles, E. Carbohydrate analysis by NIRS-Chemometrics. Dev. Near Infrared Spectrosc. 2017, 10, 67208. [Google Scholar]
- Aenugu, H.P.R.; Kumar, D.S.; Srisudharson, N.P.; Ghosh, S.; Banji, D. Near infra-red spectroscopy—An overview. Int. J. ChemTech Res. 2011, 3, 825–836. [Google Scholar]
- Fox, G.P.; O’Donnell, N.H.; Stewart, P.N.; Gleadow, R.M. Estimating hydrogen cyanide in forage sorghum (Sorghum bicolor) by near-infrared spectroscopy. J. Agric. Food Chem. 2012, 60, 6183–6187. [Google Scholar] [CrossRef]
- Rajan, G.; Bhattacharjee, H.; Bedi, S.; Reo, J. Primer on NIR spectroscopy as a PAT tool in the tablet manufacture process. Am. Pharm. Rev. 2010, 13, 128–135. [Google Scholar]
- Bento, A.C.; Dias, D.T.; Olenka, L.; Medina, A.N.; Baesso, M.L. On the application of the photoacoustic methods for the determination of thermo-optical properties of polymers. Braz. J. Phys. 2002, 32, 483–494. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Paliotta, M.; Amoriello, T.; Carbone, K. FT-NIR spectroscopy and multivariate classification strategies for the postharvest quality of green-fleshed kiwifruit varieties. Sci. Hortic. 2019, 257, 108622. [Google Scholar] [CrossRef]
- Rady, A.; Sugiharto, S.; Adedeji, A.A. Evaluation of carrot quality using visible-near infrared spectroscopy and multivariate analysis. J. Food Res. 2018, 7, 80–93. [Google Scholar] [CrossRef]
- Sun, X.; Chen, X.; Liu, Y.; Gao, B. Vis-NIR measurement of vitamin C in mandarin by PLS regression and wavelength selection. Adv. Biomed. Photonics Imaging 2008, 114–119. [Google Scholar] [CrossRef]
- Pissard, A.; Fernández Pierna, J.A.; Baeten, V.; Sinnaeve, G.; Lognay, G.; Mouteau, A.; Dupont, P.; Rondia, A.; Lateur, M. Non-destructive measurement of vitamin C, total polyphenol and sugar content in apples using near-infrared spectroscopy. J. Sci. Food Agric. 2013, 93, 238–244. [Google Scholar] [CrossRef]
- Volery, P.; Nzabonimpa, R.; Bessas, N. Quantitative determination of vitamin C at sub-percent level in infant cereals by NIR spectroscopy. In Near Infrared Spectroscopy: Proceedings of the 11th International Conference; NIR Publications: Chichester, UK, 2004. [Google Scholar]
| Calibration Set (120 Samples) | Prediction Set (60 Samples) | |||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | S.D. | Min | Max | Mean | S.D. | |
| Weight (g) | 120 | 167 | 145 | 5.8 | 116 | 155 | 140 | 6.5 |
| Vitamin C (mg/100 g) | 45 | 60 | 53 | 2.5 | 45 | 60 | 52 | 2.3 |
| Item | Wavelength (nm) | Part No. | Full Width at Half Maximum | Manufacturer |
|---|---|---|---|---|
| 1 | 1050 | FB1050-10 | ±10 nm | Thorlabs |
| 2 | 1100 | FB1100-10 | ±10 nm | Thorlabs |
| 3 | 1150 | FB1150-10 | ±10 nm | Thorlabs |
| 4 | 1200 | FB1200-10 | ±10 nm | Thorlabs |
| 5 | 1250 | FB1250-10 | ±10 nm | Thorlabs |
| 6 | 1300 | FB1300-12 | ±12 nm | Thorlabs |
| 7 | 1350 | FB1350-12 | ±12 nm | Thorlabs |
| 8 | 1400 | FB1400-12 | ±12 nm | Thorlabs |
| 9 | 1450 | FB1450-12 | ±12 nm | Thorlabs |
| 10 | 1500 | FB1500-12 | ±12 nm | Thorlabs |
| 11 | 1550 | FB1550-12 | ±12 nm | Thorlabs |
| 12 | 1600 | FB1600-12 | ±12 nm | Thorlabs |
| 13 | 1650 | FB1650-12 | ±12 nm | Thorlabs |
| Wavelength (nm) | Nonpolarized Wave | Parallel-Polarized Wave | ||
|---|---|---|---|---|
| Coefficient of Calibration | Root-Mean-Square Error of Calibration (RMSEC) (mg/100 g) | Coefficient of Calibration | Root-Mean-Square Error of Calibration (RMSEC) (mg/100 g) | |
| 1050 | 0.65 | 3.07 | 0.70 | 2.10 |
| 1100 | 0.52 | 3.28 | 0.58 | 2.49 |
| 1150 | 0.64 | 3.14 | 0.68 | 2.17 |
| 1200 | 0.79 | 3.02 | 0.85 | 1.49 |
| 1250 | 0.55 | 3.37 | 0.61 | 2.39 |
| 1300 | 0.54 | 3.14 | 0.59 | 2.46 |
| 1350 | 0.57 | 3.09 | 0.64 | 2.30 |
| 1400 | 0.80 | 3.07 | 086 | 1.43 |
| 1450 | 0.78 | 3.12 | 0.83 | 1.58 |
| 1500 | 0.74 | 3.15 | 0.78 | 1.80 |
| 1550 | 0.77 | 3.06 | 0.81 | 1.67 |
| 1600 | 0.73 | 3.09 | 0.76 | 1.88 |
| 1650 | 0.55 | 3.28 | 0.60 | 2.43 |
| Wavelength Combinations (nm) | Coefficient of Calibration | Root-Mean-Square Error of Calibration (RMSEC) (mg/100 g) | ||
|---|---|---|---|---|
| A | N = 3 | 1200, 1400, 1450 | 0.86 | 1.44 |
| B | N = 4 | 1200, 1400, 1450, 1500 | 0.87 | 1.39 |
| C | N = 4 | 1200, 1400, 1450, 1550 | 0.86 | 1.42 |
| D | N = 5 | 1200, 1400, 1450, 1500, 1550 | 0.89 | 1.31 |
| E | N = 5 | 1200, 1400, 1450, 1500, 1600 | 0.88 | 1.34 |
| F | N = 5 | 1200, 1400, 1450, 1550, 1600 | 0.88 | 1.32 |
| G | N = 6 | 1150, 1200, 1400, 1450, 1500, 1550 | 0.90 | 1.33 |
| H | N = 6 | 1050, 1200, 1400, 1450, 1500, 1550 | 0.91 | 1.32 |
| I | N = 6 | 1200, 1400, 1450, 1500, 1550, 1600 | 0.92 | 1.30 |
| Wavelengths Combination | Prediction Set | |||
|---|---|---|---|---|
| Coefficient of Prediction | Root-Mean-Square Error of Prediction (RMSEP) (mg/100 g) | |||
| D | N = 5 | 1200, 1400, 1450, 1500, 1550 nm | 0.84 | 1.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, S.-H.; Hsu, H.-C.; Han, P. Development of Detection System with Low Predictive Errors for Determining Vitamin C Content of Indian Jujube. Appl. Sci. 2019, 9, 5317. https://doi.org/10.3390/app9245317
Hua S-H, Hsu H-C, Han P. Development of Detection System with Low Predictive Errors for Determining Vitamin C Content of Indian Jujube. Applied Sciences. 2019; 9(24):5317. https://doi.org/10.3390/app9245317
Chicago/Turabian StyleHua, Shih-Hao, Hsun-Ching Hsu, and Pin Han. 2019. "Development of Detection System with Low Predictive Errors for Determining Vitamin C Content of Indian Jujube" Applied Sciences 9, no. 24: 5317. https://doi.org/10.3390/app9245317
APA StyleHua, S.-H., Hsu, H.-C., & Han, P. (2019). Development of Detection System with Low Predictive Errors for Determining Vitamin C Content of Indian Jujube. Applied Sciences, 9(24), 5317. https://doi.org/10.3390/app9245317




