Pore Structure Degradation of Different Cement Mortars Exposed to Sulphuric Acid
Abstract
1. Introduction
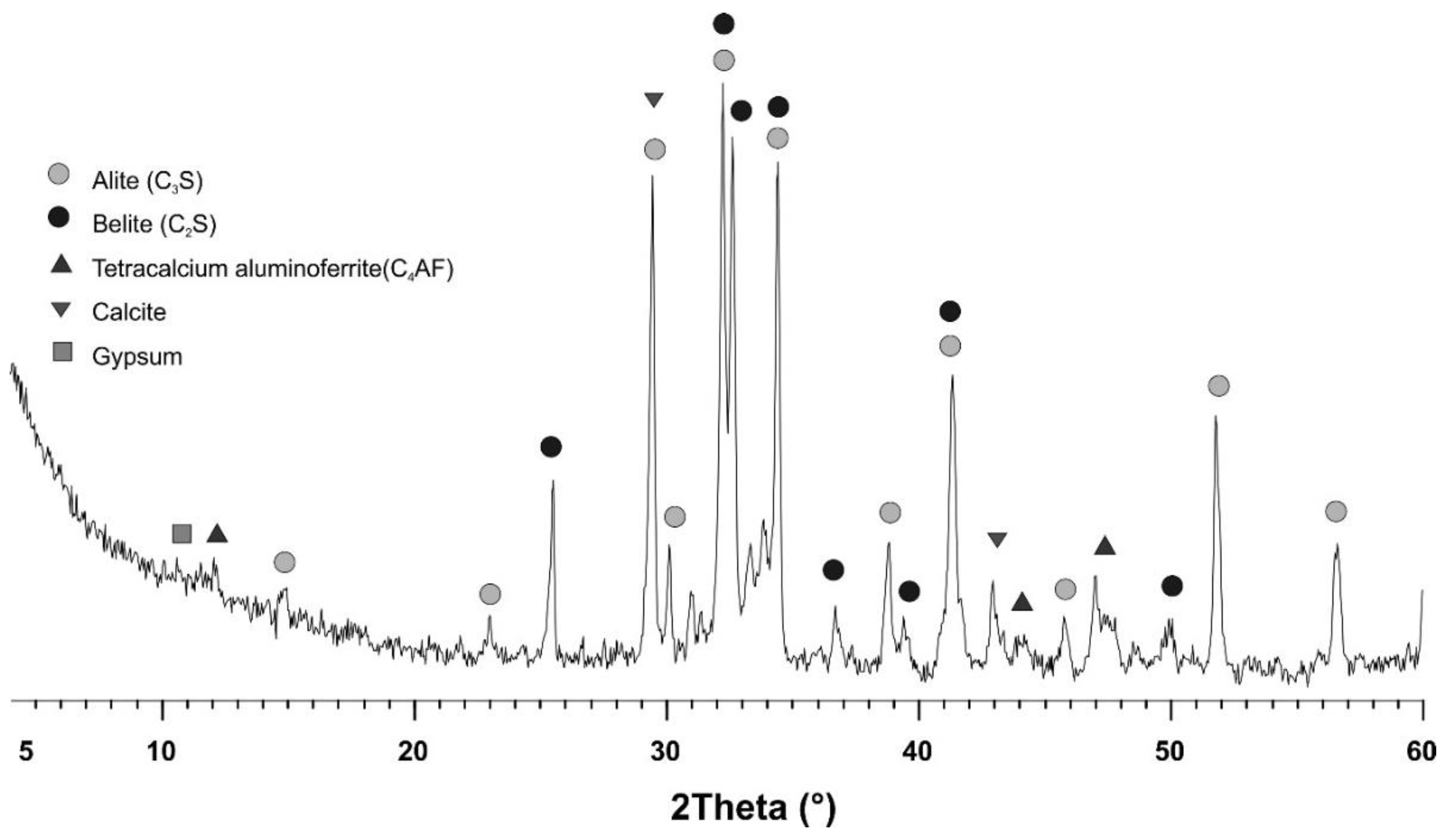
2. Materials
3. Methods
3.1. Manufacturing of Specimens, Curing Process, and Sulphuric Acid Exposure
3.2. Mercury Intrusion Porosimetry
3.3. Compressive Strength
3.4. Mass Loss
3.5. Open Porosity
- ρo is the open porosity(%)
- ms is the mass of the saturated sample (g)
- md is the mass of the dry sample (g)
- mh is the mass of the sample submerged in water (g)
4. Results and Discussion
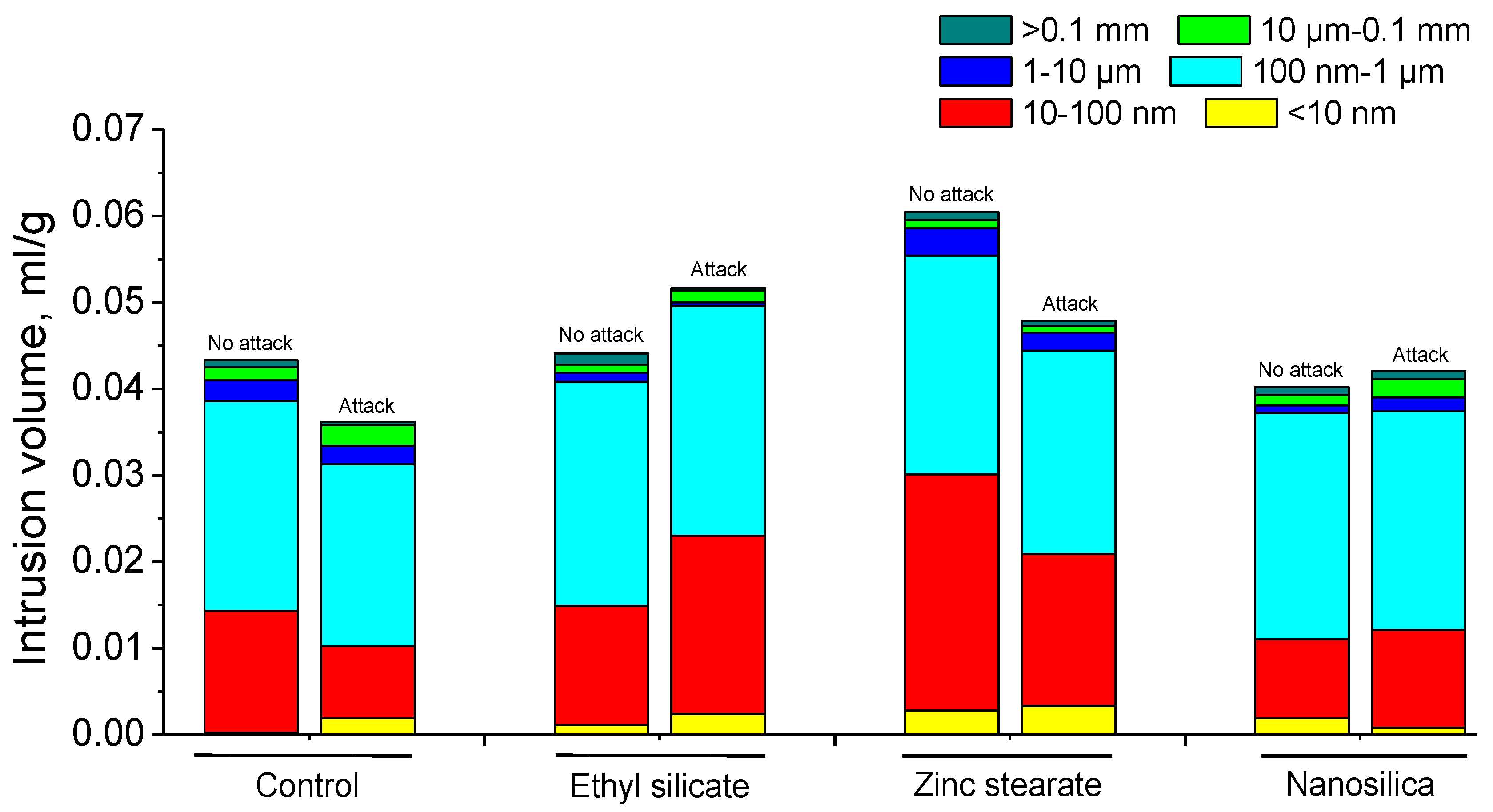
4.1. Mercury Intrusion Porosimetry
4.2. Compressive Strength
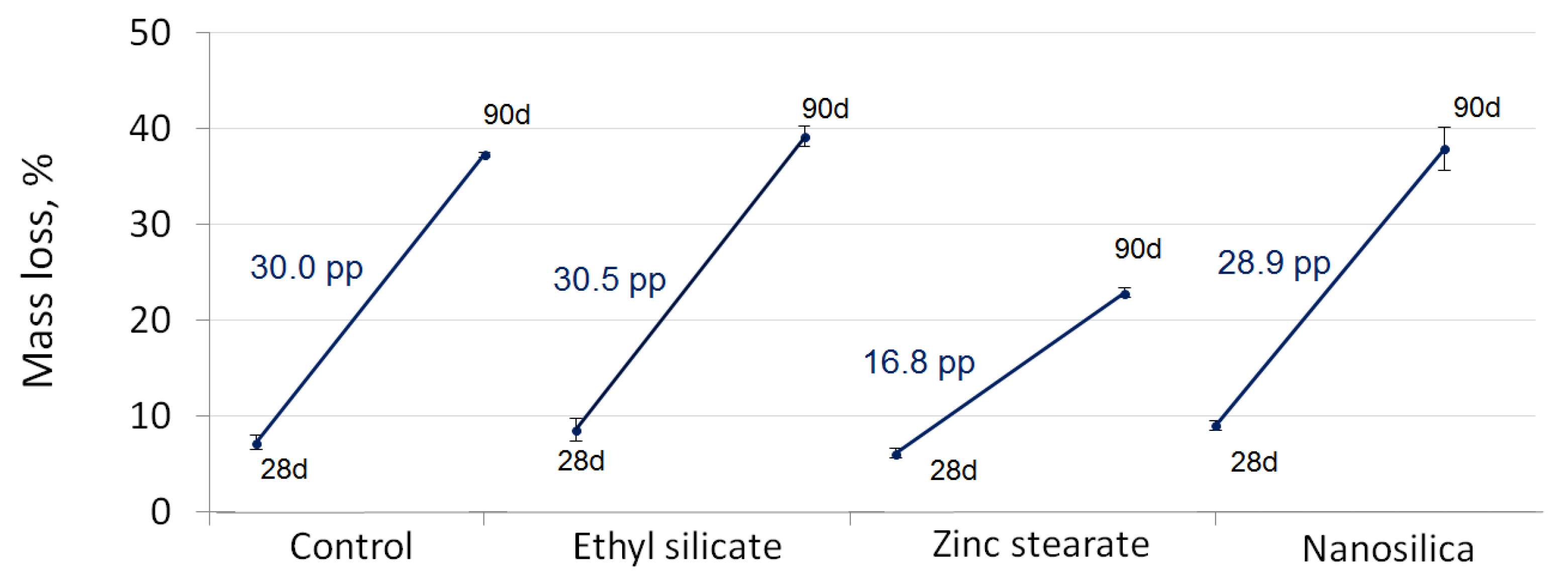
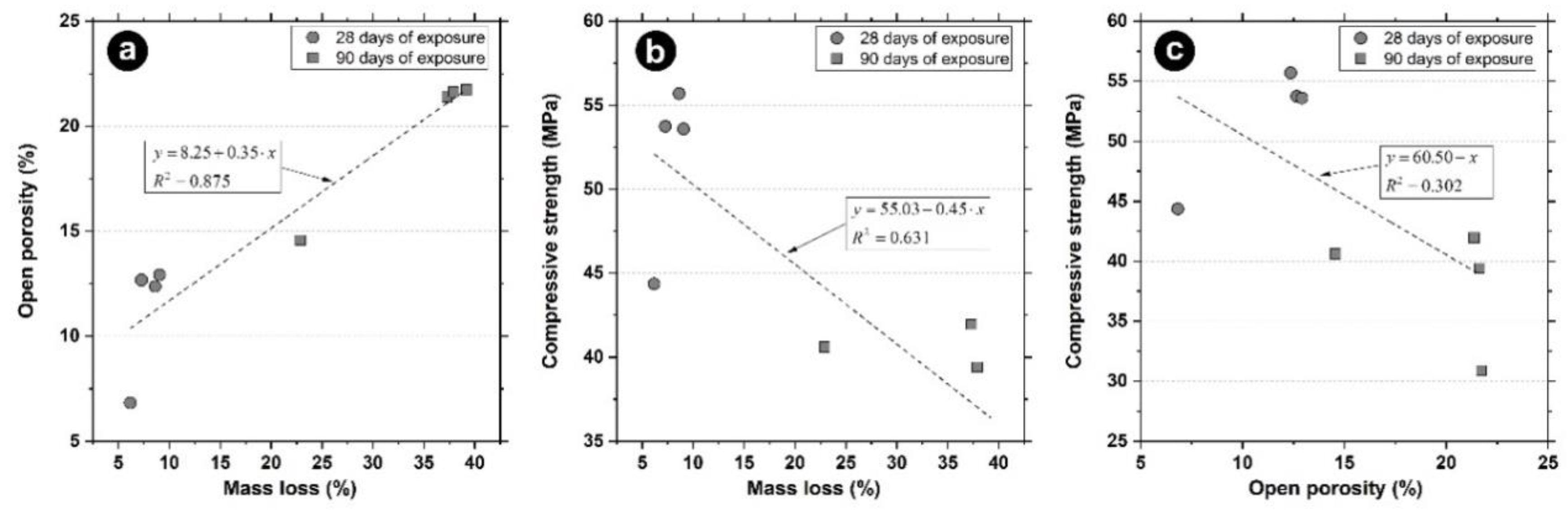
4.3. Mass Loss Caused by Sulphuric Acid Exposure
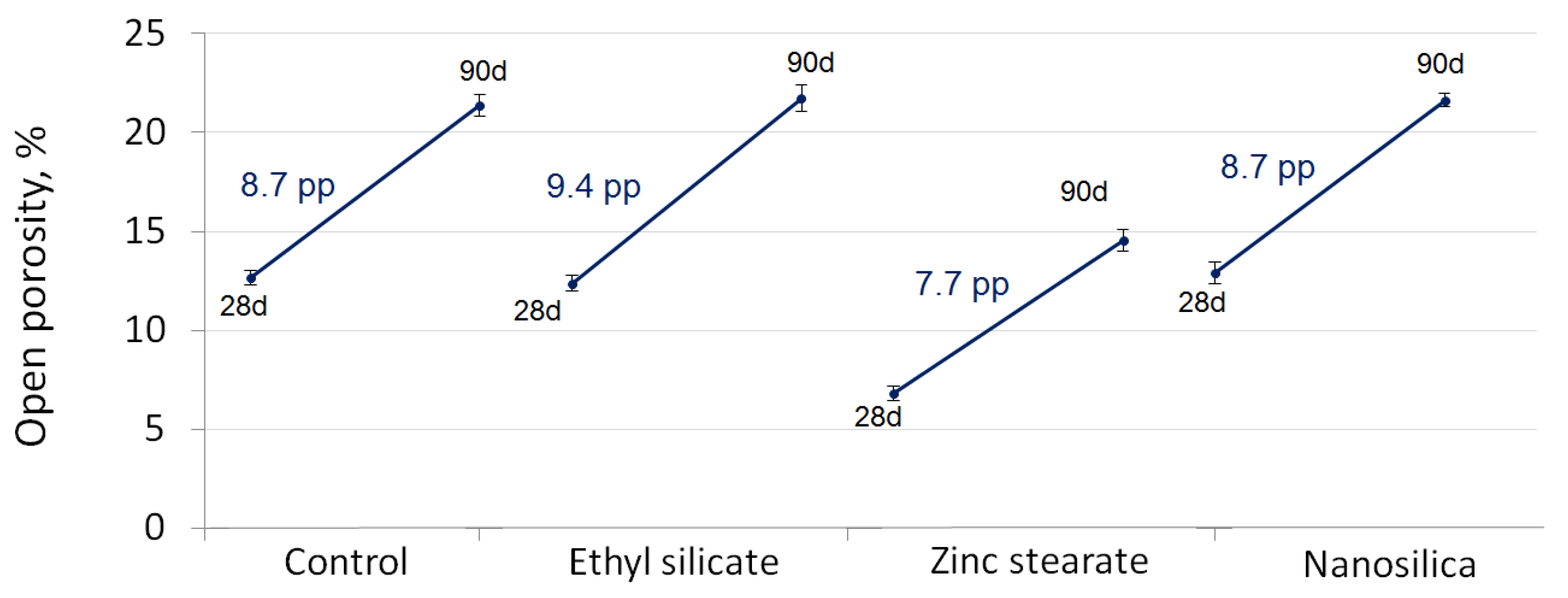
4.4. Open Porosity
5. Conclusions
- There was a small variation in total porosity for all the mortars; they also were of similar magnitude.
- After the sulphuric acid attack, the control mortars and mortars with zinc stearate showed a less-refined microstructure with a predominance of pores of higher sizes. On the contrary, pore structure was more refined in mortars treated with ethyl silicate coating exposed to the attack. Mortars with nanosilica exhibited limited changes in pore size distribution after the acid exposure, compared to the optimal condition.
- The mercury retained after the end of the porosimetry test was of a similar order of magnitude in all the mortars studied. The control mortar and mortars with ethyl silicate exhibited an increase in retained Hg, probably due to the formation of microcracks, resulting in a higher tortuosity of the microstructure. However, mortars with ethyl silicate coating and nanosilica showed a reduction in retained Hg, probably due to silting of the pores.
- The reduction in compressive strength observed in all the mortars was due to the appearance of expansive compounds that cause microcracking, weakening the structure of the mortars.
- The mortar less affected in terms of open porosity and mass loss after the acid exposure was the one with zinc stearate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quinn, M.-L. Early smelter sites: A neglected chapter in the history and geography of acid rain in the United States. Atmos. Environ. 1989, 23, 1281–1292. [Google Scholar] [CrossRef]
- Lanzón, M.; García-Ruiz, P.A. Deterioration and damage evaluation of rendering mortars exposed to sulphuric acid. Mater. Struct. 2010, 43, 417–427. [Google Scholar] [CrossRef]
- García-Vera, V.; Tenza-Abril, A.; Saval, J.; Lanzón, M. Influence of crystalline admixtures on the short-term behaviour of mortars exposed to sulphuric acid. Materials 2018, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Skalny, J.; Marchand, J.; Odler, I. Sulfate Attack on Concrete; Spon Press: London, UK; New York, NY, USA, 2002; ISBN 0-419-24550-2. [Google Scholar]
- Hadigheh, S.A.; Gravina, R.J.; Smith, S.T. Effect of acid attack on FRP-to-concrete bonded interfaces. Constr. Build. Mater. 2017, 152, 285–303. [Google Scholar] [CrossRef]
- García-Vera, V.E.; Lanzón, M. Physical-chemical study, characterisation and use of image analysis to assess the durability of earthen plasters exposed to rain water and acid rain. Constr. Build. Mater. 2018, 187, 708–717. [Google Scholar] [CrossRef]
- Jalal, M.; Pouladkhan, A.; Harandi, O.F.; Jafari, D. Comparative study on effects of Class F fly ash, nano silica and silica fume on properties of high performance self compacting concrete. Constr. Build. Mater. 2015, 94, 90–104. [Google Scholar] [CrossRef]
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M. Applications of using nano material in concrete: A review. Constr. Build. Mater. 2017, 133, 91–97. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Mahdikhani, M.; Bamshad, O.; Fallah Shirvani, M. Mechanical properties and durability of concrete specimens containing nano silica in sulfuric acid rain condition. Constr. Build. Mater. 2018, 167, 929–935. [Google Scholar] [CrossRef]
- Khanzadi, M.; Tadayon, M.; Sepehri, H.; Sepehri, M.; Student, M.S. Influence of nano-silica particles on mechanical properties and permeability of concrete. In Proceedings of the Second International Conference on Sustainable Construcion Materials and Technologies, Ancona, Italy, 28–30 June 2010; Zachar, J., Claisse, P., Naik, T.R., Ganjian, E., Eds.; Universita Ploitecnica delle Marche: Ancona, Italy, 2010. [Google Scholar]
- Fallah, S.; Nematzadeh, M. Mechanical properties and durability of high-strength concrete containing macro-polymeric and polypropylene fibers with nano-silica and silica fume. Constr. Build. Mater. 2017, 132, 170–187. [Google Scholar] [CrossRef]
- Lanzón, M.; García-Ruiz, P.A. Effectiveness and durability evaluation of rendering mortars made with metallic soaps and powdered silicone. Constr. Build. Mater. 2008, 22, 2308–2315. [Google Scholar] [CrossRef]
- Lanzón, M.; Martínez, E.; Mestre, M.; Madrid, J.A. Use of zinc stearate to produce highly-hydrophobic adobe materials with extended durability to water and acid-rain. Constr. Build. Mater. 2017, 139, 114–122. [Google Scholar] [CrossRef]
- Hashem, F.S. Sulfate attack on hardened Portland cement pastes with different porosities in presence of water-repelling admixtures. Adv. Cem. Res. 2009, 21, 75–82. [Google Scholar] [CrossRef]
- Cogan, H.; Setterstrom, C. Ethyl Silicates. Ind. Eng. Chem. 1947, 39, 1364–1368. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Pigino, B.; Leemann, A.; Franzoni, E.; Lura, P. Ethyl silicate for surface treatment of concrete-Part II: Characteristics and performance. Cem. Concr. Compos. 2012, 34, 313–321. [Google Scholar] [CrossRef]
- Sandrolini, F.; Franzoni, E.; Pigino, B. Ethyl silicate for surface treatment of concrete–Part I: Pozzolanic effect of ethyl silicate. Cem. Concr. Compos. 2012, 34, 306–312. [Google Scholar] [CrossRef]
- García-Vera, V.E.; Tenza-Abril, A.J.; Lanzón, M. The effectiveness of ethyl silicate as consolidating and protective coating to extend the durability of earthen plasters. Constr. Build. Mater. 2020, 236, 117445. [Google Scholar] [CrossRef]
- AENOR UNE-EN 197-1:2011. Cement-Part 1: Composition, Specifications and Conformity Criteria for Common Cements; AENOR: Madrid, Spain, 2011. [Google Scholar]
- AENOR UNE-EN 933-1:2012. Tests for Geometrical Properties of Aggregates-Part 1: Determination of Particle Size Distribution—Sieveing Method; AENOR: Madrid, Spain, 2012. [Google Scholar]
- AENOR UNE-EN 196-1:2005. Methods of Testing Cement-Part 1: Determination of Strength; AENOR: Madrid, Spain, 2005. [Google Scholar]
- Soroushian, P.; Chowdhury, H.; Ghebrab, T. Evaluation of water-repelling additives for use in concrete-based sanitary sewer infrastructure. J. Infrastruct. Syst. 2009, 15, 106–110. [Google Scholar] [CrossRef]
- García-Vera, V.E.; Tenza-Abril, A.J.; Lanzón, M.; Saval, J.M. Exposing sustainable mortars with nanosilica, zinc stearate, and ethyl silicate coating to sulfuric acid attack. Sustainability 2018, 10, 3769. [Google Scholar] [CrossRef]
- Ortega, J.M.; Esteban, M.D.; Williams, M.; Sánchez, I.; Climent, M.A. Short-term performance of sustainable silica fume mortars exposed to sulfate attack. Sustainability 2018, 10, 2517. [Google Scholar] [CrossRef]
- Ortega, J.; Esteban, M.; Rodríguez, R.; Pastor, J.; Ibanco, F.; Sánchez, I.; Climent, M. Influence of silica fume addition in the long-term performance of sustainable cement grouts for micropiles exposed to a sulphate aggressive medium. Materials 2017, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and acid resistance of ambient-cured geopolymer mortars containing nano-silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef]
- Bonakdar, A.; Mobasher, B. Multi-parameter study of external sulfate attack in blended cement materials. Constr. Build. Mater. 2010, 24, 61–70. [Google Scholar] [CrossRef]
- AENOR UNE-EN 1936:2007. Natural Stone Test Methods-Determination of Real Density and Apparent Density, and of Total and Open Porosity; AENOR: Madrid, Spain, 2007. [Google Scholar]
- Tixier, R.; Mobasher, B. Modeling of damage in cement-based materials subjected to external sulfate attack. I: Formulation. J. Mater. Civ. Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Yang, C.; Zhou, L.; Yin, J. Accelerated sulfate attack on mortars using electrical pulse. Constr. Build. Mater. 2015, 95, 875–881. [Google Scholar] [CrossRef]
- Ma, H.; Li, Z. Microstructures and mechanical properties of polymer modified mortars under distinct mechanisms. Constr. Build. Mater. 2013, 47, 579–587. [Google Scholar] [CrossRef]
- Ortega, J.; Esteban, M.; Rodríguez, R.; Pastor, J.; Ibanco, F.; Sánchez, I.; Climent, M. Long-term behaviour of fly ash and slag cement grouts for micropiles exposed to a sulphate aggressive medium. Materials 2017, 10, 598. [Google Scholar] [CrossRef]
- Diamond, S. Mercury porosimetry. An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Diamond, S. Aspects of concrete porosity revisited. Cem. Concr. Res. 1999, 29, 1181–1188. [Google Scholar] [CrossRef]
- Cabeza, M.; Merino, P.; Miranda, A.; Nóvoa, X.R.; Sanchez, I. Impedance spectroscopy study of hardened Portland cement paste. Cem. Concr. Res. 2002, 32, 881–891. [Google Scholar] [CrossRef]
- Williams, M.; Ortega, M.; Sánchez, I.; Cabeza, M.; Climent, M.A. Non-destructive study of the microstructural effects of sodium and magnesium sulphate attack on mortars containing silica fume using impedance spectroscopy. Appl. Sci. 2017, 7, 648. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look. Part 1: Summary of experimental results. Cem. Concr. Res. 2002, 32, 915–921. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Impedance spectroscopy study of the effect of environmental conditions in the microstructure development of OPC and slag cement mortars. Arch. Civ. Mech. Eng. 2015, 15, 569–583. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look-Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Jiménez Montoya, P.; García Meseguer, A.; Morán Cabré, F. Hormigón Armado, 14th ed.; Gustavo Gili: Barcelona, Spain, 2000; ISBN 9788425218255. [Google Scholar]
- Falchi, L.; Zendri, E.; Muller, U.; Fontana, P. The influence of water-repellent admixtures on the behaviour and the effectiveness of Portland limestone cement mortars. Cem. Concr. Compos. 2015, 59, 107–118. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W.; Shen, C. An investigation of the microstructure and durability of a fluidized bed fly ash-metakaolin geopolymer after heat and acid exposure. Mater. Des. 2015, 74, 125–137. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A.; Dong, P.; Bassim, N. Acid attack on geopolymer cement mortar based on waste-glass powder and calcium aluminate cement at mild concentration. Constr. Build. Mater. 2018, 193, 363–372. [Google Scholar] [CrossRef]
- Kwasny, J.; Aiken, T.A.; Soutsos, M.N.; McIntosh, J.A.; Cleland, D.J. Sulfate and acid resistance of lithomarge-based geopolymer mortars. Constr. Build. Mater. 2018, 166, 537–553. [Google Scholar] [CrossRef]

| Element | Percentage (%) |
|---|---|
| CaO | 55.36 |
| SiO2 | 14.89 |
| SO3 | 4.62 |
| Al2O3 | 3.36 |
| MgO | 3.29 |
| Fe2O3 | 3.06 |
| K2O | 1.06 |
| TiO2 | 0.25 |
| Na2O | 0.23 |
| P2O5 | 0.14 |
| SrO | 0.12 |
| Cl | 0.11 |
| MnO | 0.04 |
| Other elements | <0.30 |
| Mortar Type | Exposure Medium | Total Porosity (%) |
|---|---|---|
| Control | No attack | 10.1 |
| Attack | 8.6 | |
| Ethyl silicate | No attack | 10.0 |
| Attack | 11.7 | |
| Zinc stearate | No attack | 13.5 |
| Attack | 11.1 | |
| Nanosilica | No attack | 9.4 |
| Attack | 9.9 |
| Control | Ethyl Silicate | Zinc Stearate | Nanosilica | |||||
|---|---|---|---|---|---|---|---|---|
| No Attack | Attack | No Attack | Attack | No Attack | Attack | No Attack | Attack | |
| <10 nm | 0.0002 | 0.0019 | 0.0011 | 0.0024 | 0.0028 | 0.0033 | 0.0019 | 0.0008 |
| 10–100 nm | 0.0141 | 0.0083 | 0.0138 | 0.0206 | 0.0273 | 0.0176 | 0.0091 | 0.0113 |
| 100 nm–1 µm | 0.0243 | 0.0211 | 0.0259 | 0.0266 | 0.0253 | 0.0235 | 0.0262 | 0.0253 |
| 10–100 µm | 0.0024 | 0.0021 | 0.0011 | 0.0004 | 0.0032 | 0.0021 | 0.0009 | 0.0016 |
| 10 µm–0.1 mm | 0.0015 | 0.0024 | 0.0009 | 0.0014 | 0.0009 | 0.0008 | 0.0012 | 0.0021 |
| >0.1 mm | 0.0008 | 0.0004 | 0.0013 | 0.0003 | 0.0010 | 0.0006 | 0.0009 | 0.0010 |
| Mortar Type | Exposure Medium | Hg Retained (%) |
|---|---|---|
| Control | No attack | 47.8 |
| Attack | 53.9 | |
| Ethyl silicate | No attack | 62.1 |
| Attack | 47.8 | |
| Zinc stearate | No attack | 55.7 |
| Attack | 57.4 | |
| Nanosilica | No attack | 57.5 |
| Attack | 53.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, J.M.; García-Vera, V.E.; Solak, A.M.; Tenza-Abril, A.J. Pore Structure Degradation of Different Cement Mortars Exposed to Sulphuric Acid. Appl. Sci. 2019, 9, 5297. https://doi.org/10.3390/app9245297
Ortega JM, García-Vera VE, Solak AM, Tenza-Abril AJ. Pore Structure Degradation of Different Cement Mortars Exposed to Sulphuric Acid. Applied Sciences. 2019; 9(24):5297. https://doi.org/10.3390/app9245297
Chicago/Turabian StyleOrtega, José Marcos, Victoria E. García-Vera, Afonso Miguel Solak, and Antonio José Tenza-Abril. 2019. "Pore Structure Degradation of Different Cement Mortars Exposed to Sulphuric Acid" Applied Sciences 9, no. 24: 5297. https://doi.org/10.3390/app9245297
APA StyleOrtega, J. M., García-Vera, V. E., Solak, A. M., & Tenza-Abril, A. J. (2019). Pore Structure Degradation of Different Cement Mortars Exposed to Sulphuric Acid. Applied Sciences, 9(24), 5297. https://doi.org/10.3390/app9245297








