Solvothermal Synthesis of Size-Controlled Monodispersed Superparamagnetic Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanoparticles
2.3. Characterization
3. Results and Discussion
3.1. Structure and Morphology Analyses
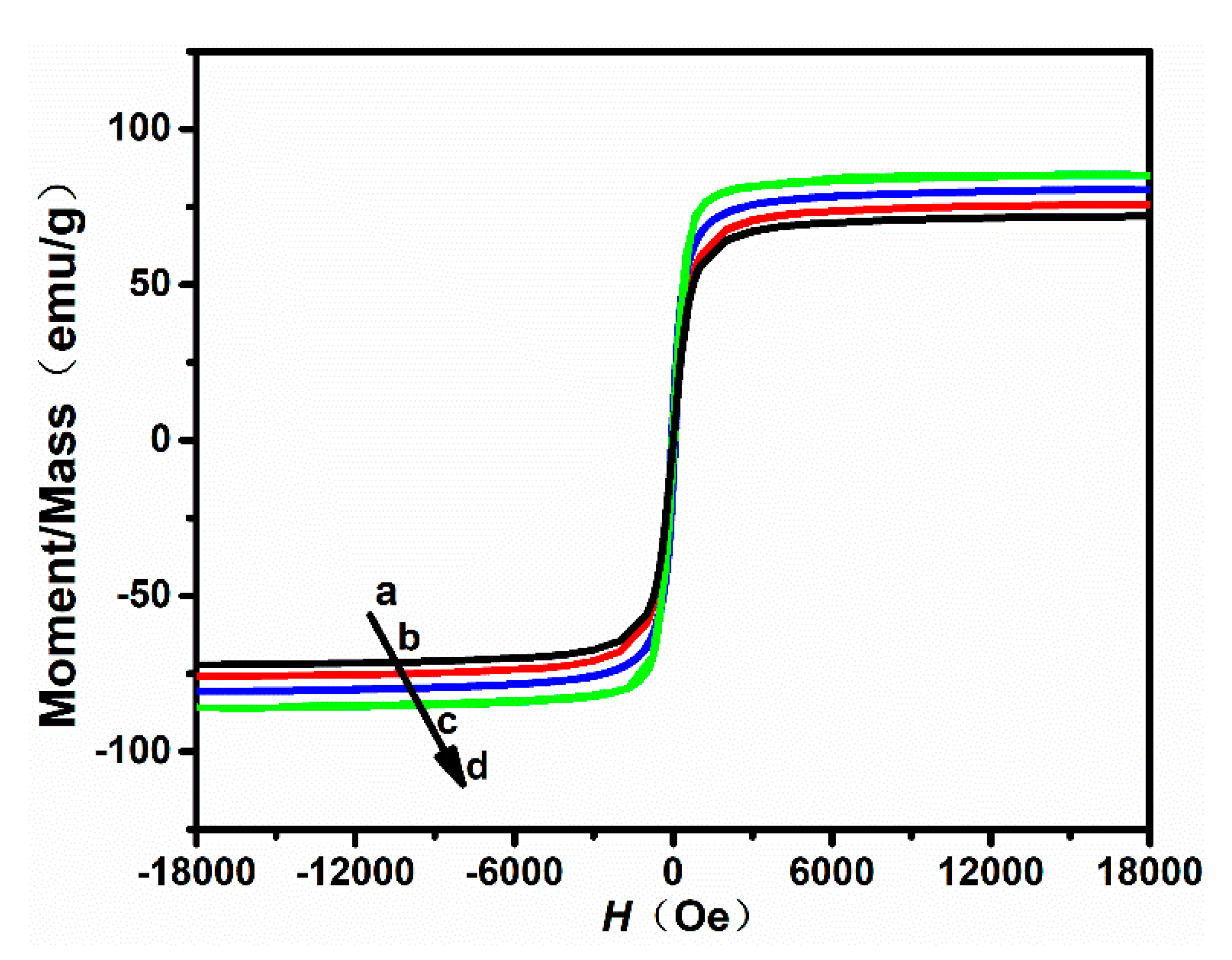
3.2. Magnetic Properties
3.3. Formation Mechanism of Fe3O4 Nanoparticles with Tunable Sizes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed.-Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Indira, T.K.; Lakshmi, P.K. Magnetic nanoparticles–A review. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 1035–1042. [Google Scholar]
- Alexiou, C.; Tietze, R.; Schreiber, E.; Jurgons, R.; Richter, H.; Trahms, L.; Rahn, H.; Odenbach, S.; Lyer, S. Cancer therapy with drug loaded magnetic nanoparticles—Magnetic drug targeting. J. Magn. Magn. Mater. 2011, 323, 1404–1407. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, P.K.; Behera, S.; Lockey, R.F.; Mohapatra, S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomed.-Nanotechnol. Biol. Med. 2010, 6, 64–69. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Kempe, H.; Kempe, M. The use of magnetite nanoparticles for implant-assisted magnetic drug targeting in thrombolytic therapy. Biomaterials 2010, 31, 9499–9510. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Le, H.; Yin, Y. Magnetic field guided colloidal assembly. Mater. Today 2013, 16, 110–116. [Google Scholar] [CrossRef]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, X.Y.; Ma, Y.F.; Huang, Y.; Wang, Y.S.; Chen, Y.S. Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009, 19, 2710–2714. [Google Scholar] [CrossRef]
- Padella, F.; Alvani, C.; La, B.A.; Ennas, G.; Liberatore, R.; Varsano, F. Mechanosynthesis and process characterization of nanostructured manganese ferrite. Mater. Chem. Phys. 2005, 90, 172–177. [Google Scholar] [CrossRef]
- Mathur, P.; Thakur, A.; Singh, M. Processing of high density manganese zinc nanoferrites by co-precipitation method. Z. Phys. Chem.-Int. J. Res. Phys. Chem. 2007, 221, 887–895. [Google Scholar] [CrossRef]
- Rondinone, A.J.; Liu, C.; Zhang, Z.J. Determination of magnetic anisotropy distribution and anisotropy constant of manganese spinel ferrite nanoparticles. J. Phys. Chem. B 2001, 10, 7967–7971. [Google Scholar] [CrossRef]
- Bao, N.Z.; Shen, L.M.; Wang, Y.H.; Padhan, P.; Gupta, A. A facile thermolysis route to monodisperse ferrite nanocrystals. J. Am. Chem. Soc. 2007, 129, 12374–12375. [Google Scholar] [CrossRef] [PubMed]
- Gabal, M.A.; Ata-Allah, S.S. Concerning the cation distribution in MnFe2O4 synthesized through the thermal decomposition of oxalates. J. Phys. Chem. Solids 2004, 65, 995–1003. [Google Scholar] [CrossRef]
- Bai, F.; Wang, D.S.; Huo, Z.Y.; Chen, W.; Liu, L.P.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y.D. A versatile bottom-up assembly approach to colloidal spheres from nanocrystals. Angew. Chem.-Int. Ed. 2007, 46, 6650–6653. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.C.; Yang, J.; Xu, H.Y.; Qian, Y.T. One-step hydrothermal synthesis of ZnFe2O4 nano-octahedrons as a high capacity anode material for Li-ion batteries. Nano Res. 2012, 5, 477–485. [Google Scholar] [CrossRef]
- Guo, P.Z.; Cui, L.J.; Wang, Y.Q.; Lv, M.; Wang, B.Y.; Zhao, X.S. Facile synthesis of ZnFe2O4 nanoparticles with tunable magnetic and sensing properties. Langmuir 2013, 29, 8997–9003. [Google Scholar] [CrossRef]
- Xing, R.M.; Lu, L.; Huang, H.P.; Liu, S.H.; Niu, J.Y. Facile Synthesis of Carboxylic Functionalized MnFe2O4 (M = Mn, Co, Zn) Nanospheres. J. Nanosci. Nanotechnol. 2015, 15, 5175–5179. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Wang, Y.H.; Tan, Q.Q.; Zhong, Z.Y.; Su, F.B. Facile solvothermal synthesis of mesoporous manganese ferrite (MnFe2O4) microspheres as anode materials for lithium-ion batteries. J. Colloid Interface Sci. 2013, 398, 185–192. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.Y.; Zhou, L.B.; Ma, W.; Zhang, D.; Ren, F.L.; Yan, H.L.; Qiu, G.Z.; Liu, X.H. A facile solvothermal synthesis and magnetic properties of MnFe2O4 spheres with tunable sizes. J. Am. Ceram. Soc. 2012, 95, 3569–3576. [Google Scholar] [CrossRef]
- Zheng, H.H.; Zhou, B.F.; Chen, L.; Wang, Y.Q.; Zhang, X.L.; Zhou, S.M. Gel-assisted synthesis of oleate-modified Fe3O4@Ag composite microspheres as magnetic SERS probe for thiram detection. CrystEngComm 2015, 17, 6393–6398. [Google Scholar] [CrossRef]
- Zhang, X.L.; Niu, C.Y.; Wang, Y.Q.; Zhou, S.M.; Liu, J. Gel-limited synthesis of dumbbell-like Fe3O4-Ag composite microspheres and their SERS applications. Nanoscale 2014, 6, 12618–12625. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Wang, S.X.; Li, G.X. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.N.; Liu, T.H.; Wang, D.Q.; Liu, J.; Meng, L.J. Controlled synthesis of water-dispersible and superparamagnetic Fe3O4 nanomaterials by a microwave-assisted solvothermal method: From nanocrystals to nanoclusters. CryStengComm 2017, 19, 5089–5099. [Google Scholar] [CrossRef]
- Yan, A.G.; Liu, X.H.; Qiu, G.Z.; Wu, H.Y.; Yi, R.; Zhang, N.; Xu, J. Solvothermal synthesis and characterization of size-controlled Fe3O4 nanoparticles. J. Alloys Compd. 2008, 458, 487–491. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Ding, J.; Xue, J.M. Synthesis of magnetite nanooctahedra and their magnetic field-induced two-/three-dimensional superstructure. Chem. Mater. 2010, 22, 3183–3191. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Bodnarchuk, M.I.; Lechner, R.T.; Hesser, G.; Schaffler, F.; Heiss, W. Fatty acid salts as stabilizers in size-and shape-controlled nanocrystal synthesis: The case of inverse spinel iron oxide. J. Am. Chem. Soc. 2007, 129, 6352–6353. [Google Scholar] [CrossRef]
- Redl, F.X.; Black, C.T.; Papaefthymiou, G.C.; Sandstrom, R.L.; Yin, M.; Zeng, H.; Murray, C.B.; O’Brien, S.P. Magnetic, electronic, and structural characterization of nonstoichiometric iron oxides at the nanoscale. J. Am. Chem. Soc. 2004, 126, 14583–14599. [Google Scholar] [CrossRef]
- Duan, L.F.; Wang, Y.X.; Wang, L.N.; Zhang, F.F.; Wang, L.M. Mesoporous MFe2O4 (M = Mn, Co, and Ni) for anode materials of lithium-ion batteries: Synthesis and electrochemical properties. Mater. Res. Bull. 2015, 61, 195–200. [Google Scholar] [CrossRef]
- Li, S.C.; Zhang, T.L.; Tang, R.Z.; Qiu, H.; Wang, C.Q.; Zhou, Z.N. Solvothermal synthesis and characterization of monodisperse superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2015, 379, 226–231. [Google Scholar] [CrossRef]
- Xuan, S.H.; Wang, F.; Wang, Y.X.J.; Yu, J.C.; Leung, K.C.F. Facile synthesis of size-controllable monodispersed ferrite nanospheres. J. Mater. Chem. 2010, 20, 5086–5094. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-Assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Larcher, D.; Sudant, G.; Patrice, R.; Tarascon, J.M. Some insights on the use of polyols-based metal alkoxides powders as precursors for tailored metal-oxides particles. Chem. Mater. 2003, 15, 3543–3551. [Google Scholar] [CrossRef]


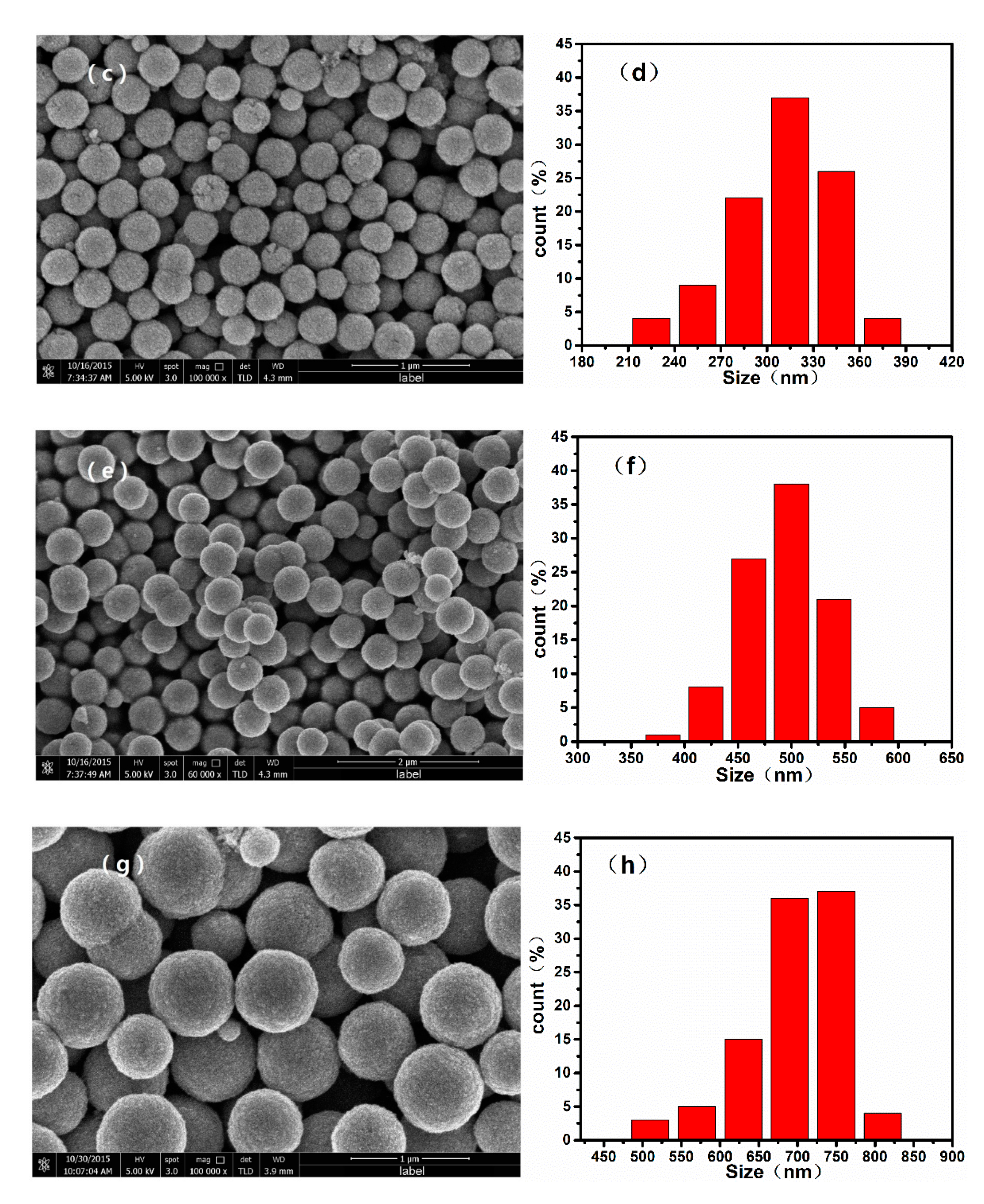
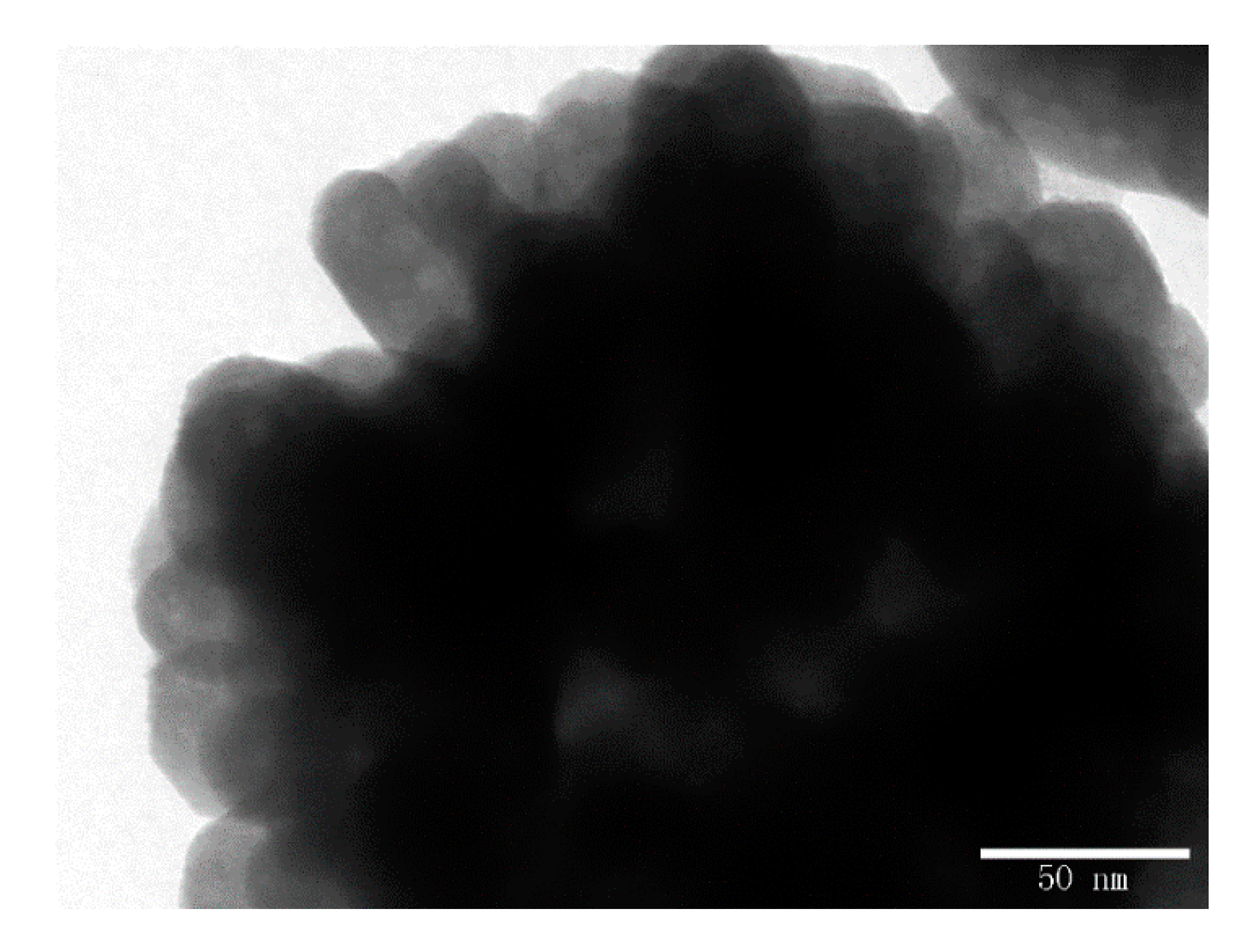

| Sample | EG/DEG | Grain Size (nm) |
|---|---|---|
| a | 40/60 | 23.62 |
| b | 60/40 | 25.25 |
| c | 80/20 | 25.65 |
| d | 100/0 | 27.86 |
| Sample | Saturation Magnetization () emu/g | Remanent Magnetization () emu/g |
|---|---|---|
| a | 72.14 | 3.34 |
| b | 75.94 | 3.97 |
| c | 80.28 | 3.26 |
| d | 85.41 | 4.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, J.; Wang, Z.; Zhou, Z. Solvothermal Synthesis of Size-Controlled Monodispersed Superparamagnetic Iron Oxide Nanoparticles. Appl. Sci. 2019, 9, 5157. https://doi.org/10.3390/app9235157
Chen Y, Zhang J, Wang Z, Zhou Z. Solvothermal Synthesis of Size-Controlled Monodispersed Superparamagnetic Iron Oxide Nanoparticles. Applied Sciences. 2019; 9(23):5157. https://doi.org/10.3390/app9235157
Chicago/Turabian StyleChen, Yongpeng, Jianguo Zhang, Zhixin Wang, and Zunning Zhou. 2019. "Solvothermal Synthesis of Size-Controlled Monodispersed Superparamagnetic Iron Oxide Nanoparticles" Applied Sciences 9, no. 23: 5157. https://doi.org/10.3390/app9235157
APA StyleChen, Y., Zhang, J., Wang, Z., & Zhou, Z. (2019). Solvothermal Synthesis of Size-Controlled Monodispersed Superparamagnetic Iron Oxide Nanoparticles. Applied Sciences, 9(23), 5157. https://doi.org/10.3390/app9235157






