Study on Preparation of Aluminum Ash Coating Based on Plasma Spray
Abstract
:1. Introduction
2. Experiments
2.1. Raw Materials
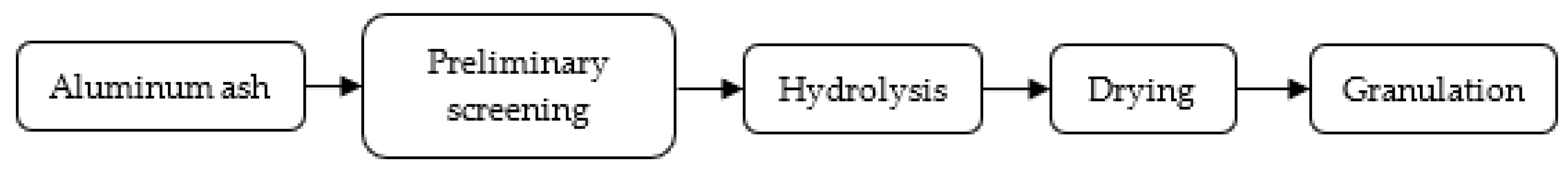
2.2. Preparation of Ultimate Spray Powder
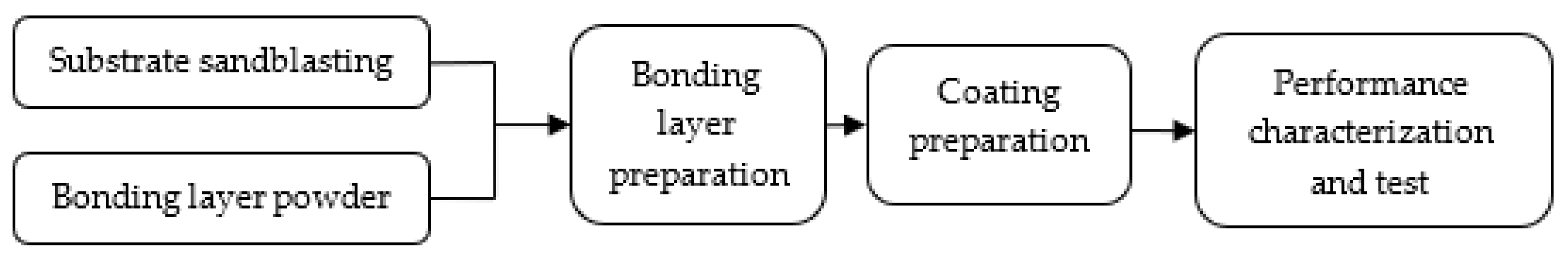
2.3. Preparation of Ultimate Coating
2.4. Characterization and Test
3. Results and Discussion
3.1. Characterization and Evaluation of Ultimate Spray Powder
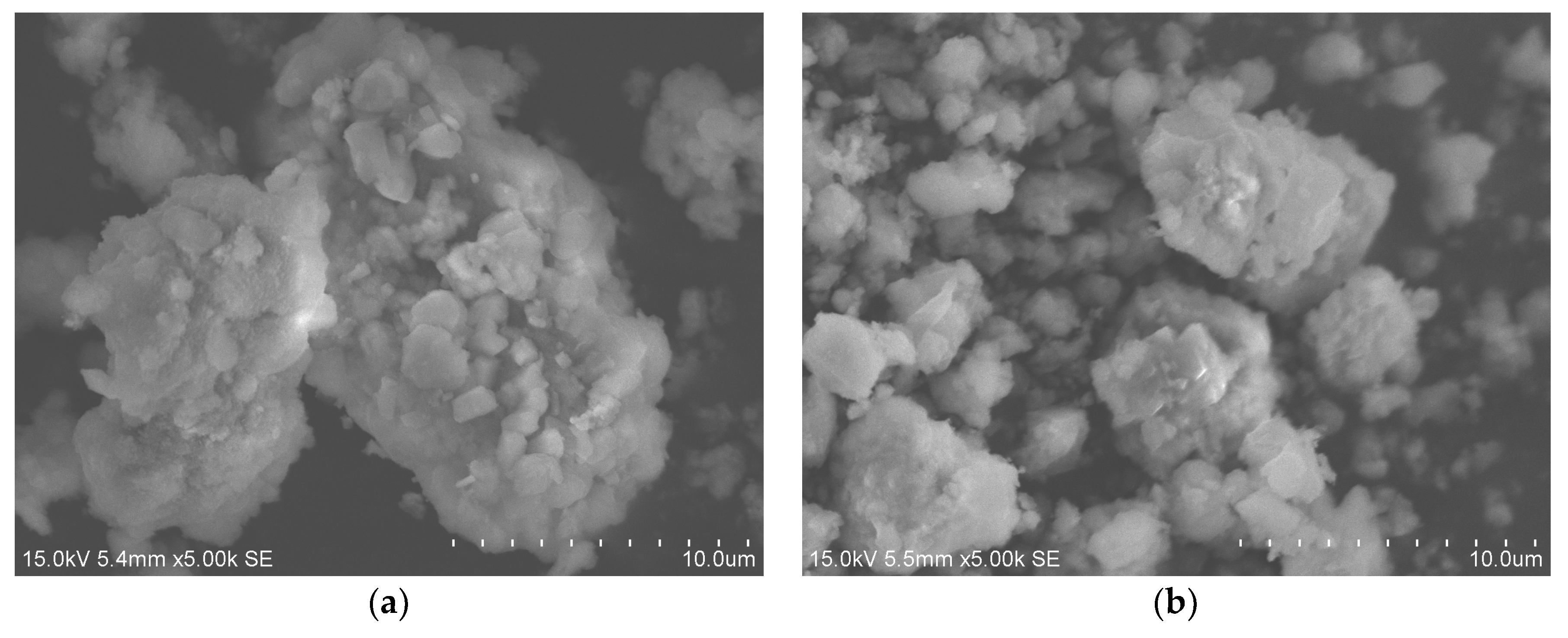
3.1.1. Observation and Analysis of Microstructure
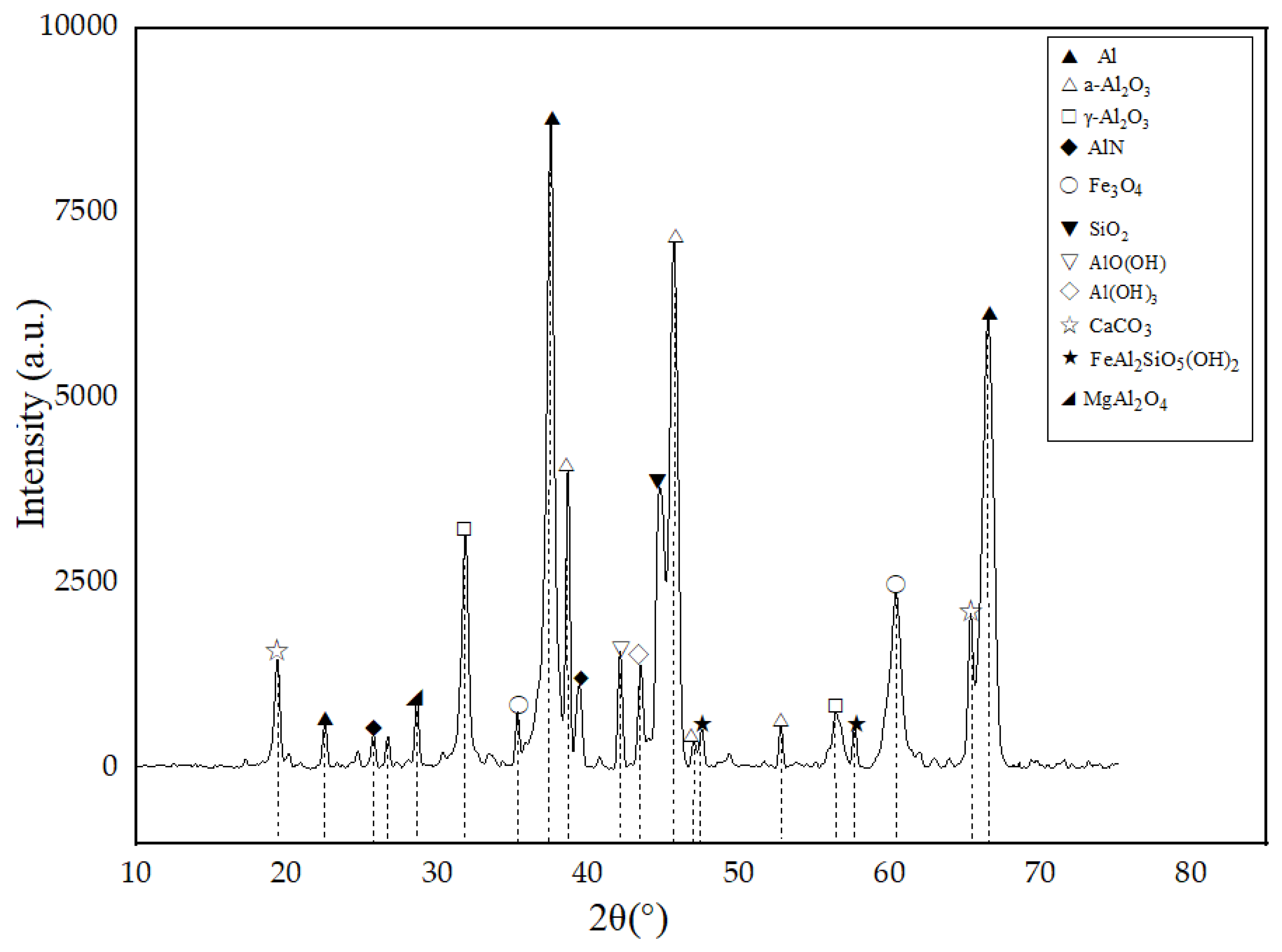
3.1.2. Composition Analysis
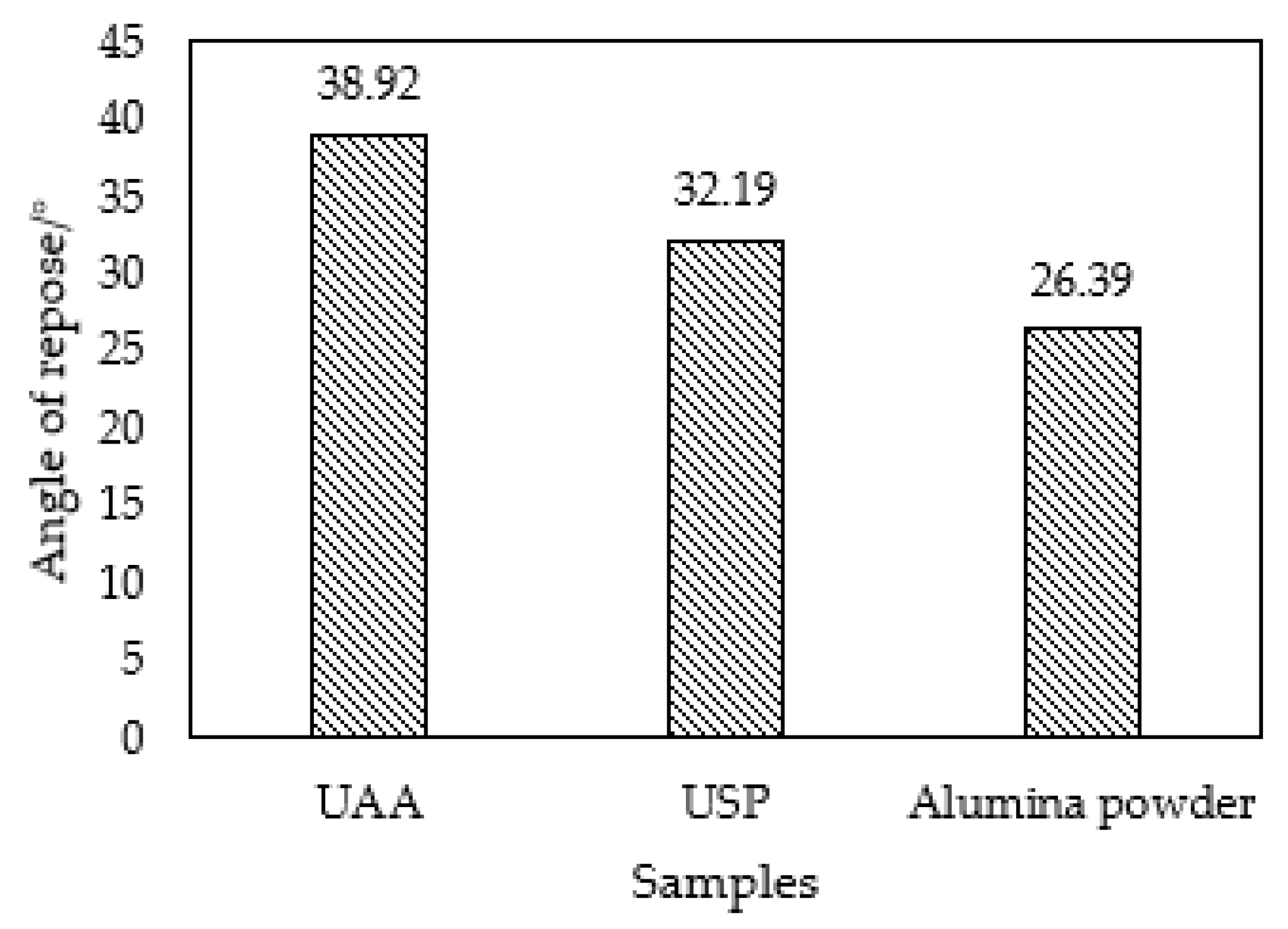
3.1.3. Evaluation of the Flowability
3.2. Effects of Spray Process on Coating Properties
3.2.1. Orthogonal Experiment
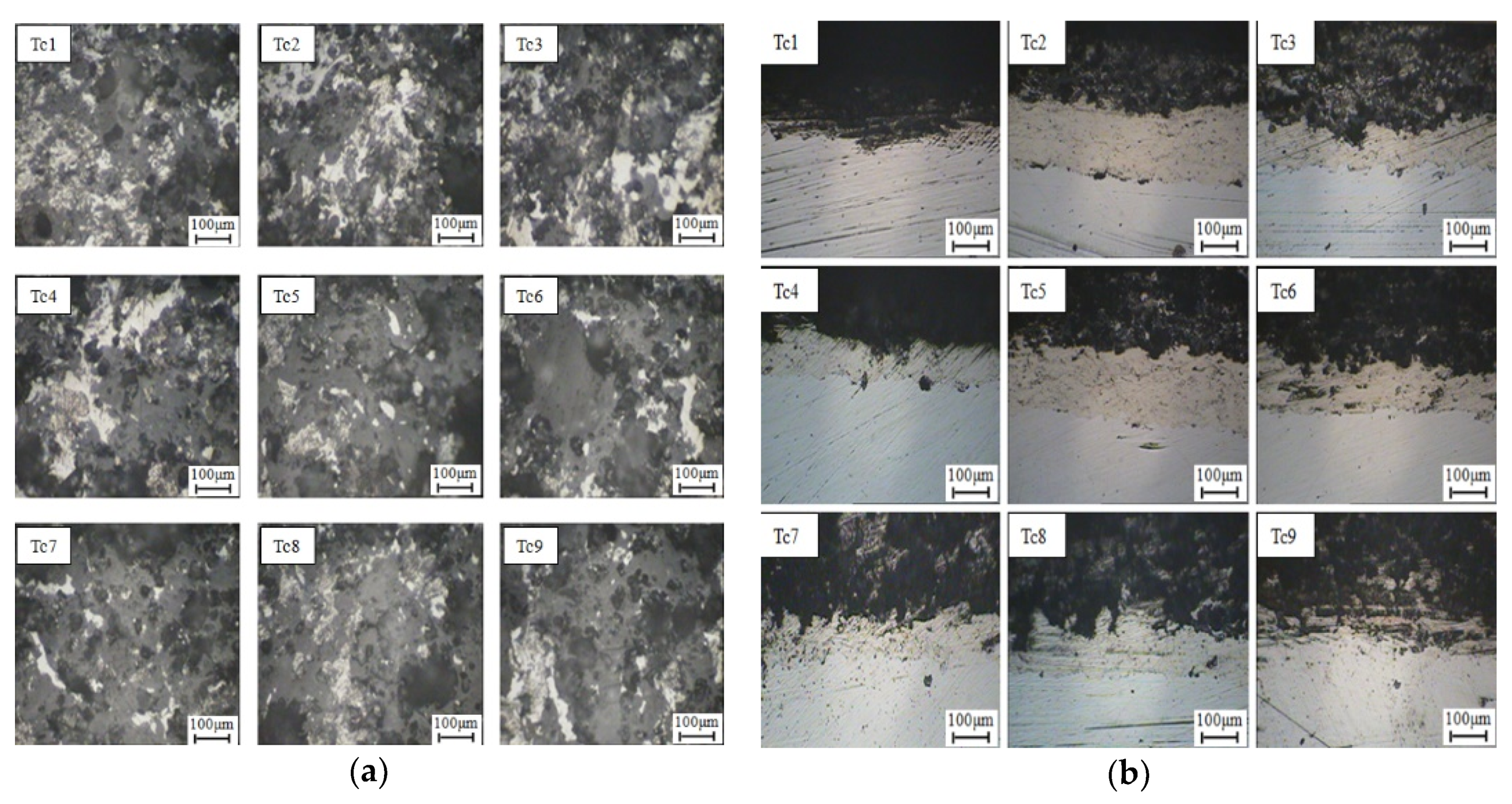
3.2.2. Microstructure of Coating
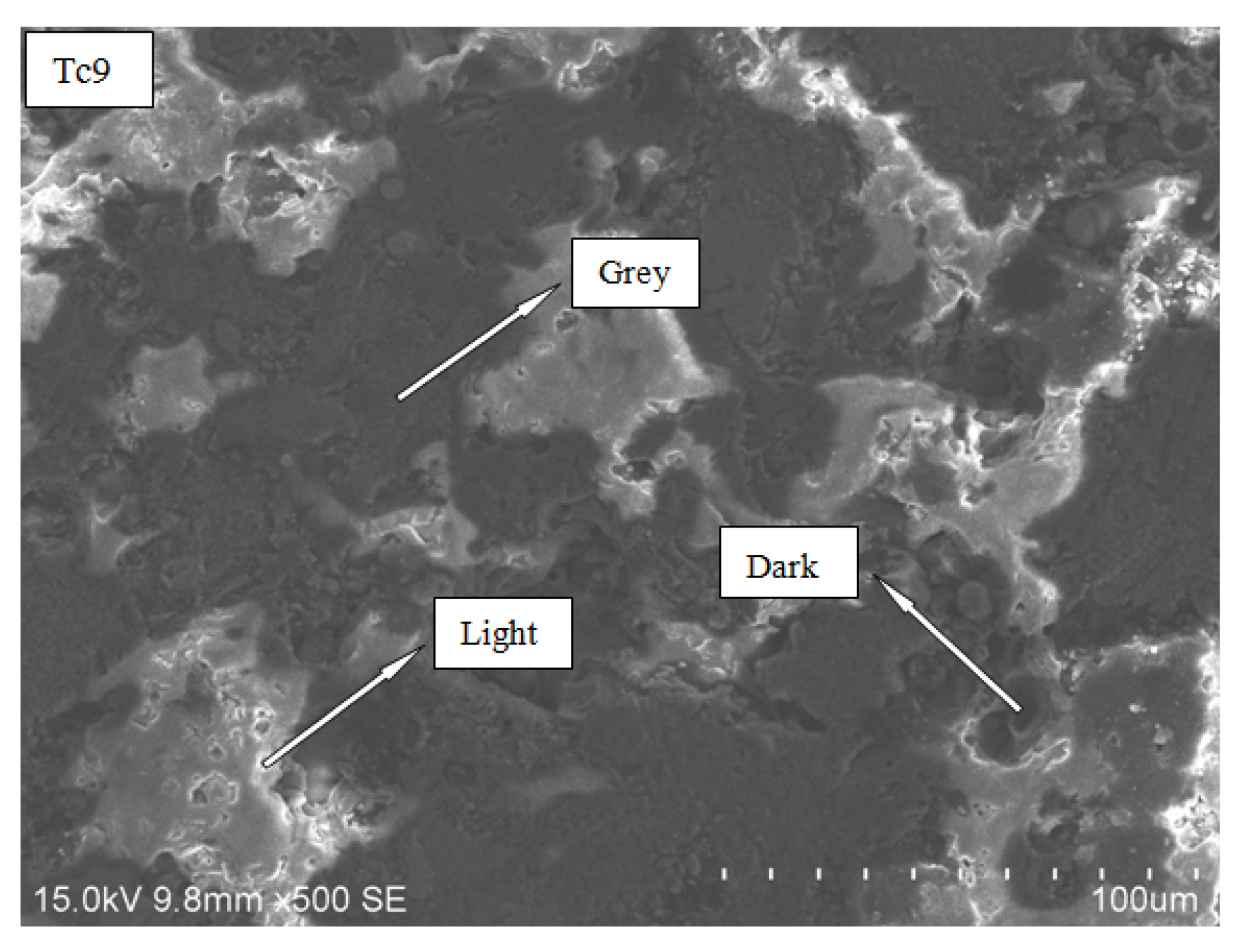
3.2.3. Energy Spectrum Analysis
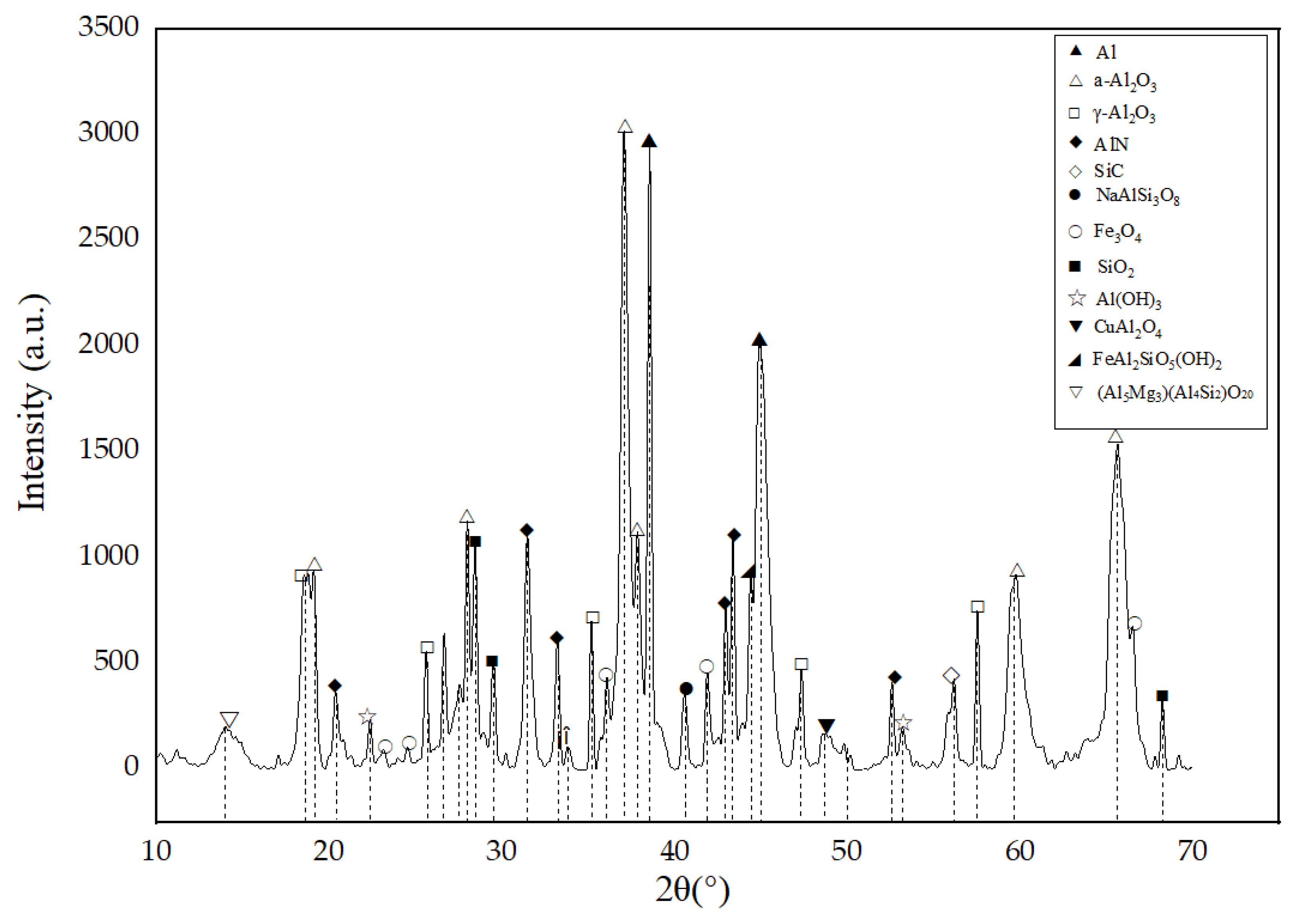
3.2.4. Phase Analysis
4. Conclusions
- (1)
- After hydrolysis and granulation of the UAA, the particle size becomes more uniform, and the impurities are significantly decreased. The angle of repose of prepared USP is 32.19° with great flowability, which meets the requirements of plasma spraying.
- (2)
- The primary and secondary order of the factors affecting the comprehensive performance of the ultimate coating is as follows: spray current > spray voltage > powder flow rate > main air flow. The preferred spraying parameters are as follows: spray current 600 A, spray voltage 55 V, powder flow rate 22 g/min, and main air flow 33 lspm. Also, the feasibility of preparing the coating based on the UAA was verified.
- (3)
- Different regions are formed on the surface of the ultimate coating. Among them, the bright and gray regions comprise more aluminum and oxygen elements, and the hardness is higher; the dark regions have higher carbon elements and lower hardness. Therefore, during the spray process, the formation of dark regions should be avoided in order to increase the hardness of the coating.
- (4)
- During the spray process, owing to the action of high temperature, some sodium salts undergo sublimation, and compounds such as Al(OH)3 and AlO(OH) undergo a chemical decomposition reaction. As a result, the type of the phase of the ultimate coating is reduced, and the characteristic peak of the diffraction pattern is less. However, substances such as Al, AlN, and SiO2 still exist, and their influence on coating properties needs further exploration. Besides, in the future, more spray process parameters can be taken into consideration to modify the orthogonal experiments and obtain a better spray solution.
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Wu, C.Y.; Yu, H.F.; Zhang, H.F. Extraction of aluminum by pressure acid-leaching method from coal fly ash. Trans. Nonferrous Met. Soc. China 2012, 22, 2282–2288. [Google Scholar] [CrossRef]
- Li, X.L.; Ou, Y.J.; Li, C.L.; Zhu, J.K.; Zhi, P.G. Preparation of Alumina from Aluminum Ash by Sintering with Sodium hydroxide. IOP Conf. Ser. Earth Environ. Sci. 2018, 233, 042027. [Google Scholar] [CrossRef]
- Aubert, J.E.; Husson, B.; Vaquier, A. Metallic aluminum in MSWI fly ash: Quantification and influence on the properties of cement-based products. Waste Manag. 2004, 24, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.L.; Qin, B.K. Effect of Reaction Temperature on Silicon-Aluminum Alloy Prepared from Fly Ash. IOP Conf. Ser. Mater. Sci. Eng. 2019, 592, 012045. [Google Scholar] [CrossRef]
- Shusei, K.; Takeshi, H.; Tetsuya, M.; Kazuo, Y.; Motohide, M. Selective synthesis of zeolites A and X from two industrial wastes: Crushed stone powder and aluminum ash. J. Environ. Manag. 2019, 231, 749–756. [Google Scholar]
- Lisa, W.; Kristin, H.; Thomas, L. High cycle fatigue behavior of the severely plastically deformed 6082 aluminum alloy with an anodic and plasma electrolytic oxide coating. Surf. Coat. Technol. 2018, 349, 576–583. [Google Scholar]
- Abyzov, V.A. Refractory Cellular Concrete Based on Phosphate Binder from Waste of Production and Recycling of Aluminum. Procedia Eng. 2017, 206, 783–789. [Google Scholar] [CrossRef]
- Abyzov, V.A. Lightweight Refractory Concrete Based on Aluminum-Magnesium-Phosphate Binder. Procedia Eng. 2016, 150, 1440–1445. [Google Scholar] [CrossRef]
- Zhan, D.P.; Zhang, H.S.; Jiang, Z.H. Effects of AIMnCa and AIMnFe Alloys on Deoxidization of Low Carbon and Low Silicon Aluminum Killed Steels. J. Iron Steel Res. Int. 2008, 15, 15–18. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chou, K.C. Deoxidation of Molten Steel by Aluminum. J. Iron Steel Res. Int. 2015, 22, 905–908. [Google Scholar] [CrossRef]
- Peng, H.L.; Zhong, S.X.; Xiang, J.X.; Lin, Q.T.; Yao, C.; Dong, J.H.; Yin, G.C.; Yao, K.; Zeng, S.Y.; Zhong, J. Characterization and secondary sludge dewatering performance of a novel combined aluminum-ferrous-starch flocculant (CAFS). Chem. Eng. Sci. 2017, 173, 335–345. [Google Scholar] [CrossRef]
- Lin, Q.T.; Peng, H.L.; Zhong, S.X.; Xiang, J.X. Synthesis, characterization, and secondary sludge dewatering performance of a novel combined silicon–aluminum–iron–starch flocculant. J. Hazard. Mater. 2015, 285, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kachalova, G.S. Modern coagulants and flocculants in the cleaning of washing waters of water treatment plants. IOP Conf. Ser. Mater. Sci. Eng. 2018, 451, 012226. [Google Scholar] [CrossRef]
- Tripathy, A.K.; Mahalik, S.; Sarangi, C.K.; Tripathy, B.C.; Sanjay, K.; Bhattacharya, I.N. A pyro-hydrometallurgical process for the recovery of alumina from waste aluminium dross. Miner. Eng. 2019, 137, 181–186. [Google Scholar] [CrossRef]
- Parvati, R.; Shravan, R.; Sushmit, B.; Sylvester, A.G. Synthesis of high temperature (1150 °C) resistant materials after extraction of oxides of Al and Mg from Aluminum dross. Mater. Today Proc. 2019. [Google Scholar] [CrossRef]
- Leiva, C.; Galiano, Y.L.; Arenas, C.; Fariñas, B.A.; Pereira, F. A porous geopolymer based on aluminum-waste with acoustic properties. Waste Manag. 2019, 95, 504–512. [Google Scholar] [CrossRef]
- Dirisu, J.O.; Fayomi, O.S.I.; Oyedepo, S.O.; Jolayemi, K.J.; Moboluwarin, D.M. Critical evaluation of aluminium dross composites and other potential building ceiling materials. Procedia Manuf. 2019, 35, 1205–1210. [Google Scholar] [CrossRef]
- Victor, E.I.; Fernando, E.I.; Carlos, R.M. Ceramic panels versus aluminium in buildings: Energy consumption and environmental impact assessment with a new methodology. Procedia Manuf. 2019, 233, 959–974. [Google Scholar]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. An investigation of oxide coating synthesized on an aluminum alloy by plasma electrolytic oxidation in molten salt. Appl. Sci. 2017, 7, 889. [Google Scholar] [CrossRef]
- Takajo, S.; Hollis, K.J.; Cummins, D.R.; Tegtmeier, E.L.; Dombrowski, D.E.; Vogel, S.C. Texture evolution in U-10Mo nuclear fuel foils during plasma spray coating with Zr. Quantum Beam Sci. 2018, 2, 12. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. Effect of pulse current mode on microstructure, composition and corrosion performance of the coatings produced by plasma electrolytic oxidation on AZ31 Mg alloy. Coatings 2019, 9, 688. [Google Scholar] [CrossRef]
- Kolås, T.; Røyset, A.; Grandcolas, M.; Cate, M.T.; Lacau, A. Cool coatings with high near infrared transmittance for coil coated aluminium. Sol. Energy Mater. Sol. Cells 2019, 196, 94–104. [Google Scholar] [CrossRef]
- John, G.O.; Li, W.G.; Zhao, Y.T.; Li, C.L. Porosity and its significance in plasma-sprayed coatings. Coatings 2019, 9, 460. [Google Scholar]

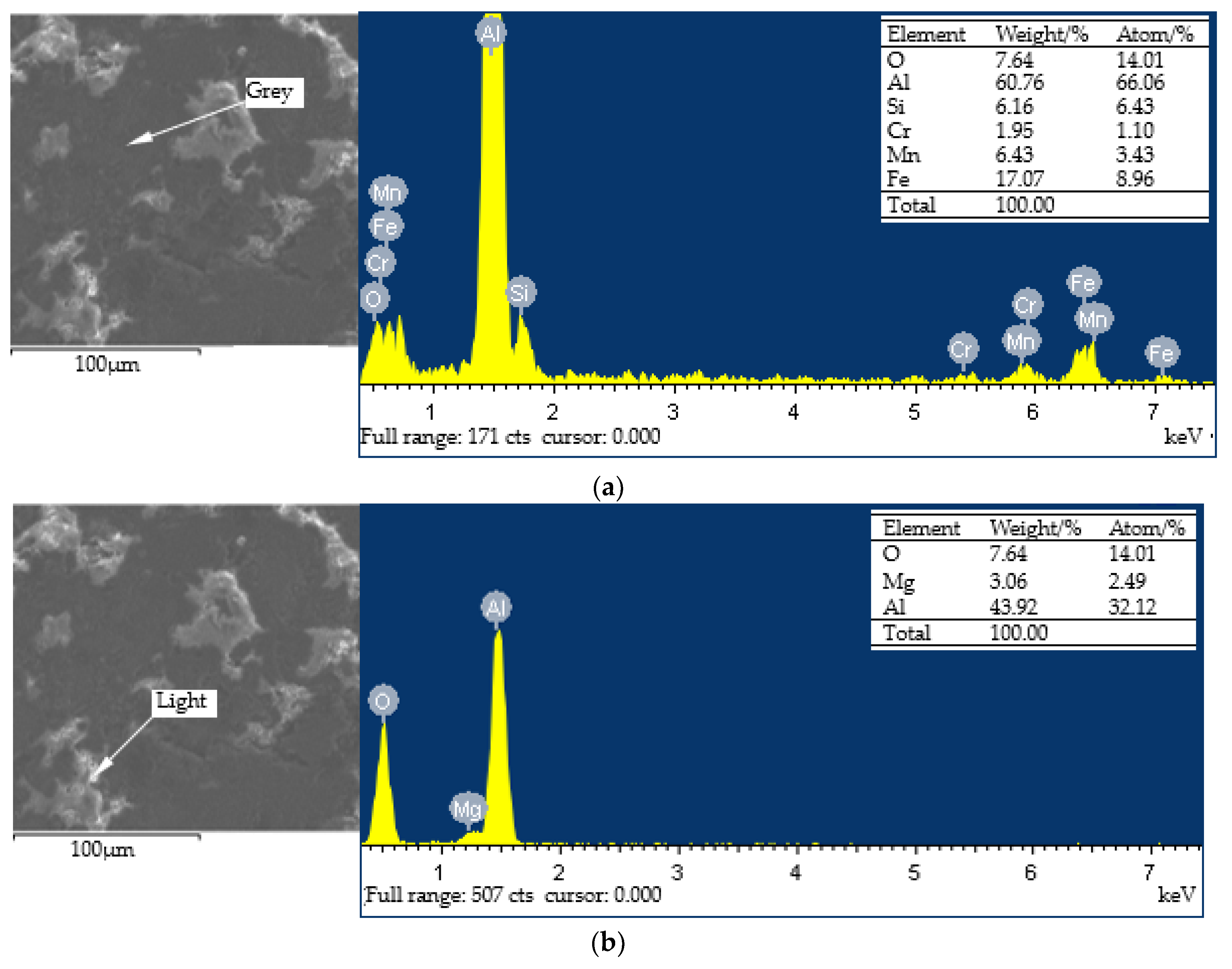
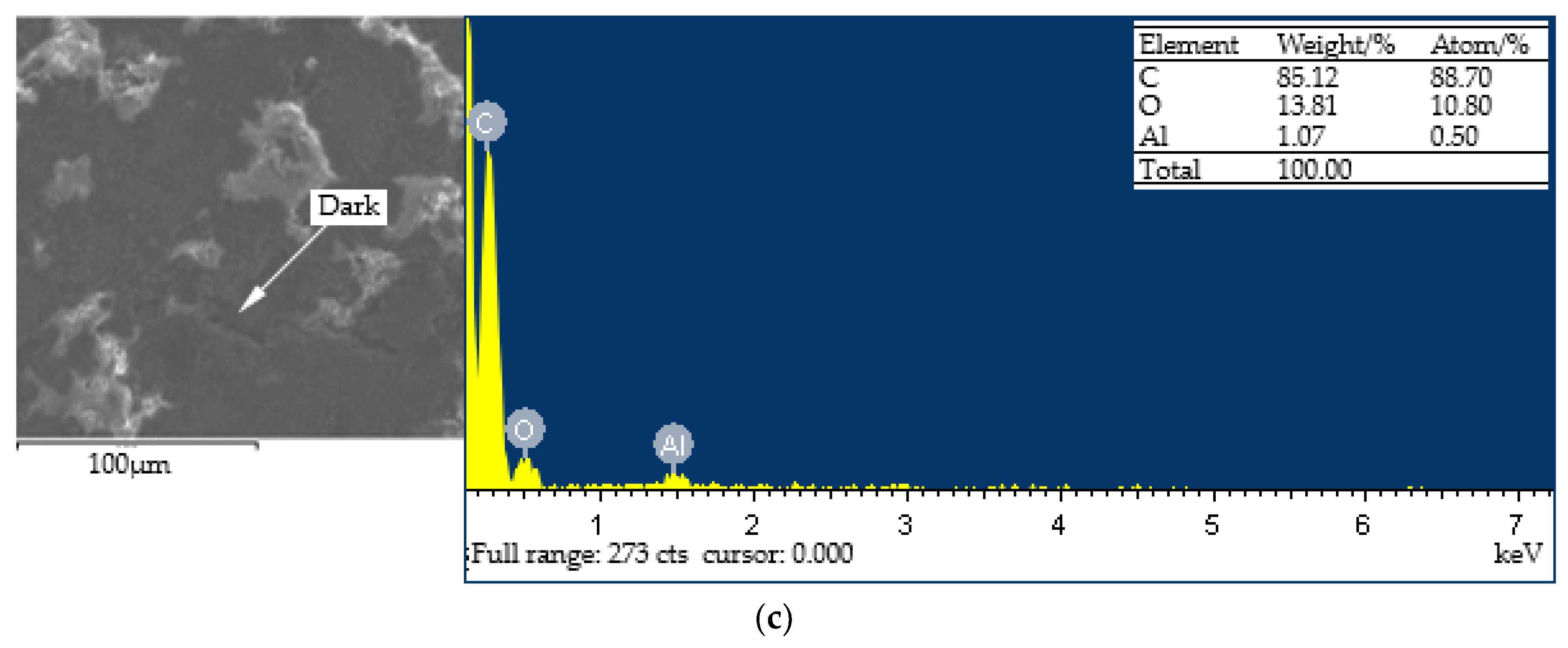
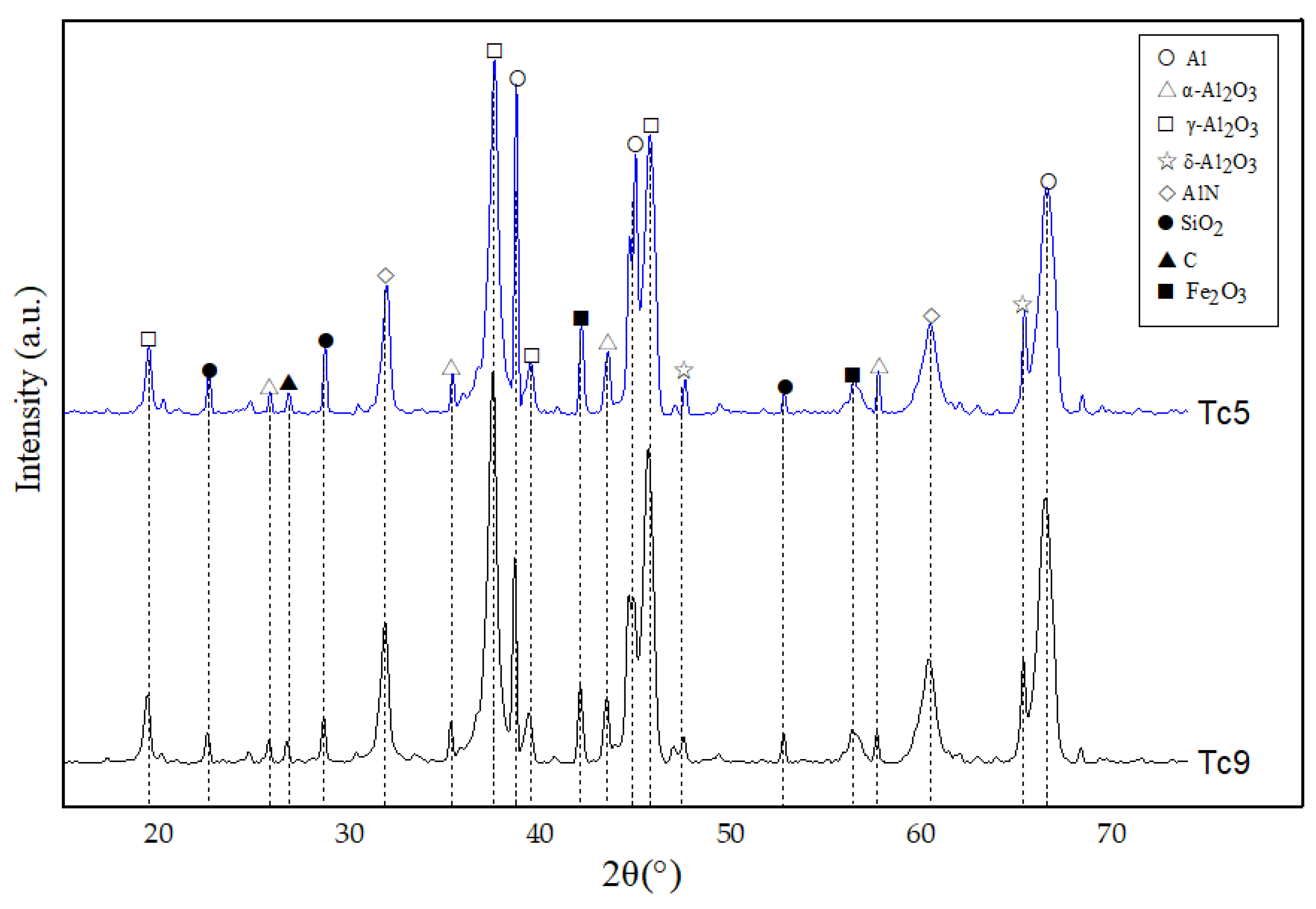
| Element | Al | Fe | Ca | Si | N | Ti | Zn | Cl | Mn | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Wt.% | 54.2 | 14.8 | 7.7 | 3.9 | 3.8 | 3.3 | 2.5 | 2.1 | 1.6 | 6.1 |
| Molecular Formula | Al | Al2O3 | AlN | AlO(OH) | SiO2 | Others |
|---|---|---|---|---|---|---|
| Semi-quantitative (%) | 5 ± 2 | 20 ± 2 | 22 ± 2 | 3 ± 1 | 1 | 49 ± 3 |
| Powder | Spray Current/A | Spray Voltage/V | Main air Flow/slpm | Powder Flow Rate/g·min−1 |
|---|---|---|---|---|
| Al/Ni | 550 | 55 | 33 | 24 |
| Levels | Factors | |||
|---|---|---|---|---|
| A Spray Voltage/V | B Spray Current/A | C Powder Flow Rate/g·min−1 | D Main Air Flow/Slpm | |
| 1 | 50 | 500 | 22 | 30 |
| 2 | 55 | 550 | 24 | 33 |
| 3 | 60 | 600 | 26 | 36 |
| Element | Al | Fe | Ca | Si | N | Ti | Zn | Cl |
|---|---|---|---|---|---|---|---|---|
| Wt.% | 51.8 | 14.7 | 6.0 | 3.7 | 0.8 | 2.7 | 2.2 | 3.5 |
| No. | A | B | C | D | Microhardness/HV | Porosity/% | Abrasion Rate 10−3 g/min | Adhesive Strength/MPa | Comprehensive Score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 93.44 | 0.15 | 32.4 | 8 | −66.15 |
| 2 | 1 | 2 | 2 | 2 | 370.92 | 0.18 | 11.2 | 5 | −45.2 |
| 3 | 1 | 3 | 3 | 3 | 377.18 | 0.17 | 20.02 | 5 | −51.53 |
| 4 | 2 | 1 | 2 | 3 | 382.96 | 0.15 | 20.24 | 3 | −48.41 |
| 5 | 2 | 2 | 3 | 1 | 654.87 | 0.23 | 12.44 | 12 | −39.33 |
| 6 | 2 | 3 | 1 | 2 | 599.57 | 0.16 | 9.71 | 16 | −10.52 |
| 7 | 3 | 1 | 3 | 2 | 607.29 | 0.20 | 20.71 | 6 | −50.44 |
| 8 | 3 | 2 | 1 | 3 | 688.83 | 0.15 | 25.88 | 5 | −38.65 |
| 9 | 3 | 3 | 2 | 1 | 623.71 | 0.16 | 14.15 | 19 | −9.71 |
| k1 | −54.30 | −55.00 | −38.44 | −38.40 | |||||
| k2 | −32.76 | −41.06 | −34.44 | −35.39 | |||||
| k3 | −32.94 | −23.92 | −47.10 | −46.20 | |||||
| R | 21.54 | 31.08 | 12.66 | 10.81 | |||||
| Factors primary to secondary | B > A > C > D | ||||||||
| Preferred scheme | B3A2C2D2 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuaishuai, L.; Jiaqiao, Z.; Hongjun, N.; Xingxing, W.; Yu, Z.; Tao, G. Study on Preparation of Aluminum Ash Coating Based on Plasma Spray. Appl. Sci. 2019, 9, 4980. https://doi.org/10.3390/app9234980
Shuaishuai L, Jiaqiao Z, Hongjun N, Xingxing W, Yu Z, Tao G. Study on Preparation of Aluminum Ash Coating Based on Plasma Spray. Applied Sciences. 2019; 9(23):4980. https://doi.org/10.3390/app9234980
Chicago/Turabian StyleShuaishuai, Lv, Zhang Jiaqiao, Ni Hongjun, Wang Xingxing, Zhu Yu, and Gu Tao. 2019. "Study on Preparation of Aluminum Ash Coating Based on Plasma Spray" Applied Sciences 9, no. 23: 4980. https://doi.org/10.3390/app9234980
APA StyleShuaishuai, L., Jiaqiao, Z., Hongjun, N., Xingxing, W., Yu, Z., & Tao, G. (2019). Study on Preparation of Aluminum Ash Coating Based on Plasma Spray. Applied Sciences, 9(23), 4980. https://doi.org/10.3390/app9234980





