Novel Application Method for Mesenchymal Stem Cell Therapy Utilizing Its Attractant-Responsive Accumulation Property
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation and Culture of MSCs
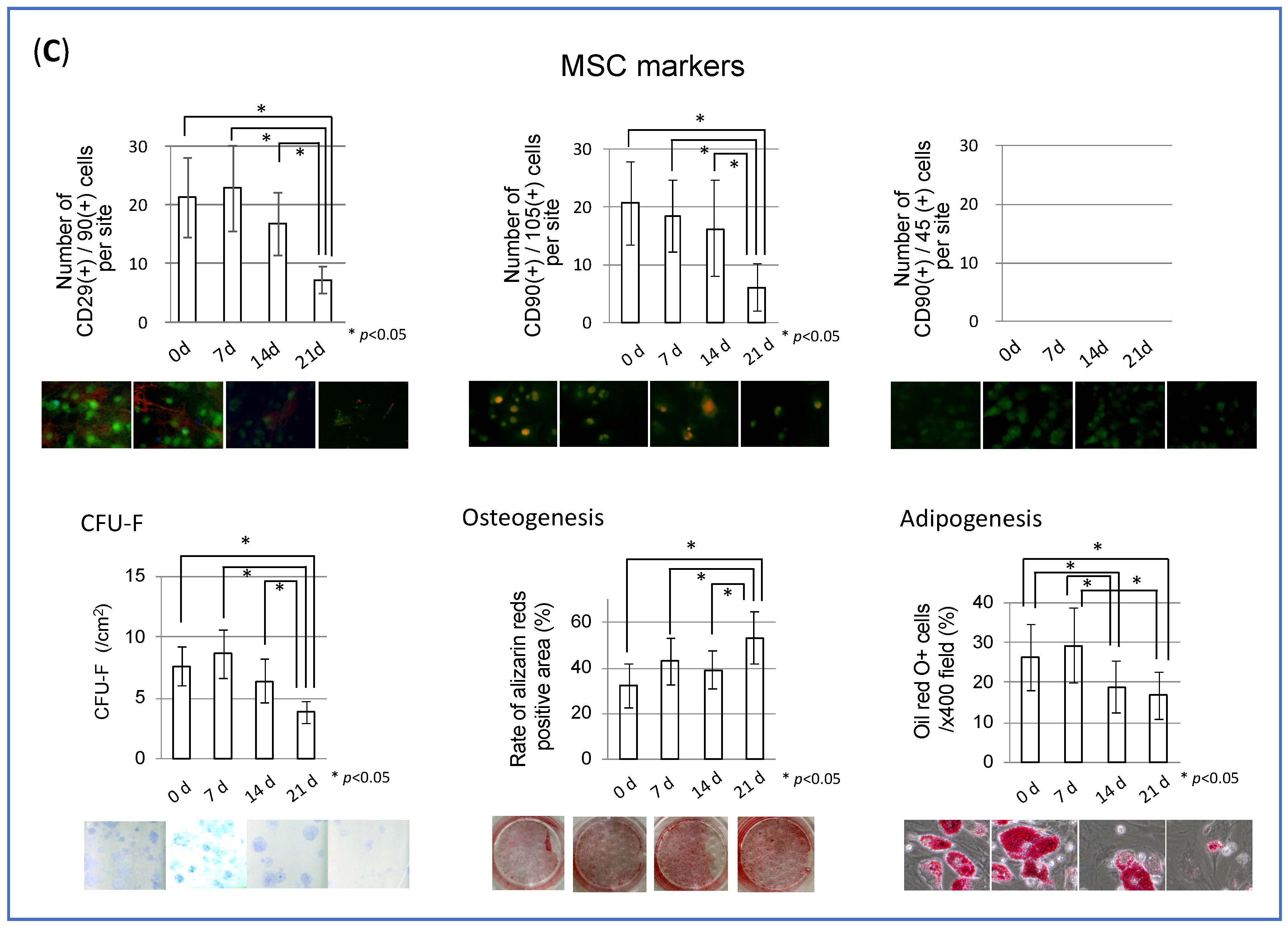
2.3. Osteogenic Differentiation Assay
2.4. Adipogenic Differentiation Assay
2.5. CFU-F Assay
2.6. MSC Injection Via the Tail Vein
2.7. MSC Injection into the Back of Mice
2.8. Cell Cultures
2.9. Collagen Gel Culture
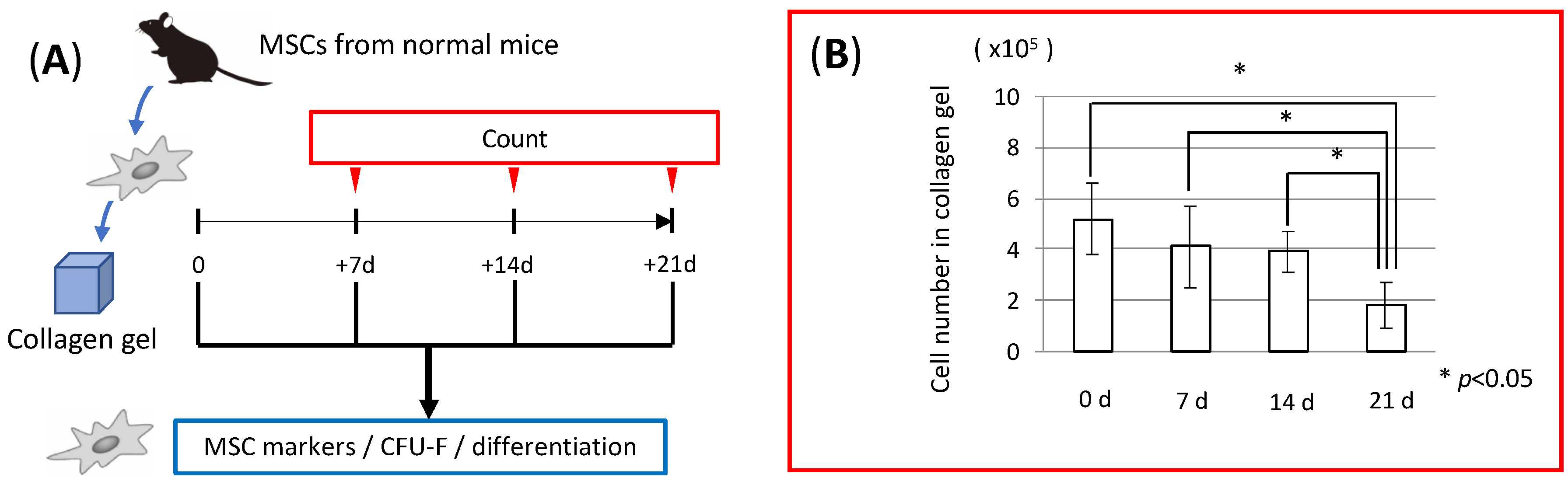
2.10. Counting of MSCs in Collagen
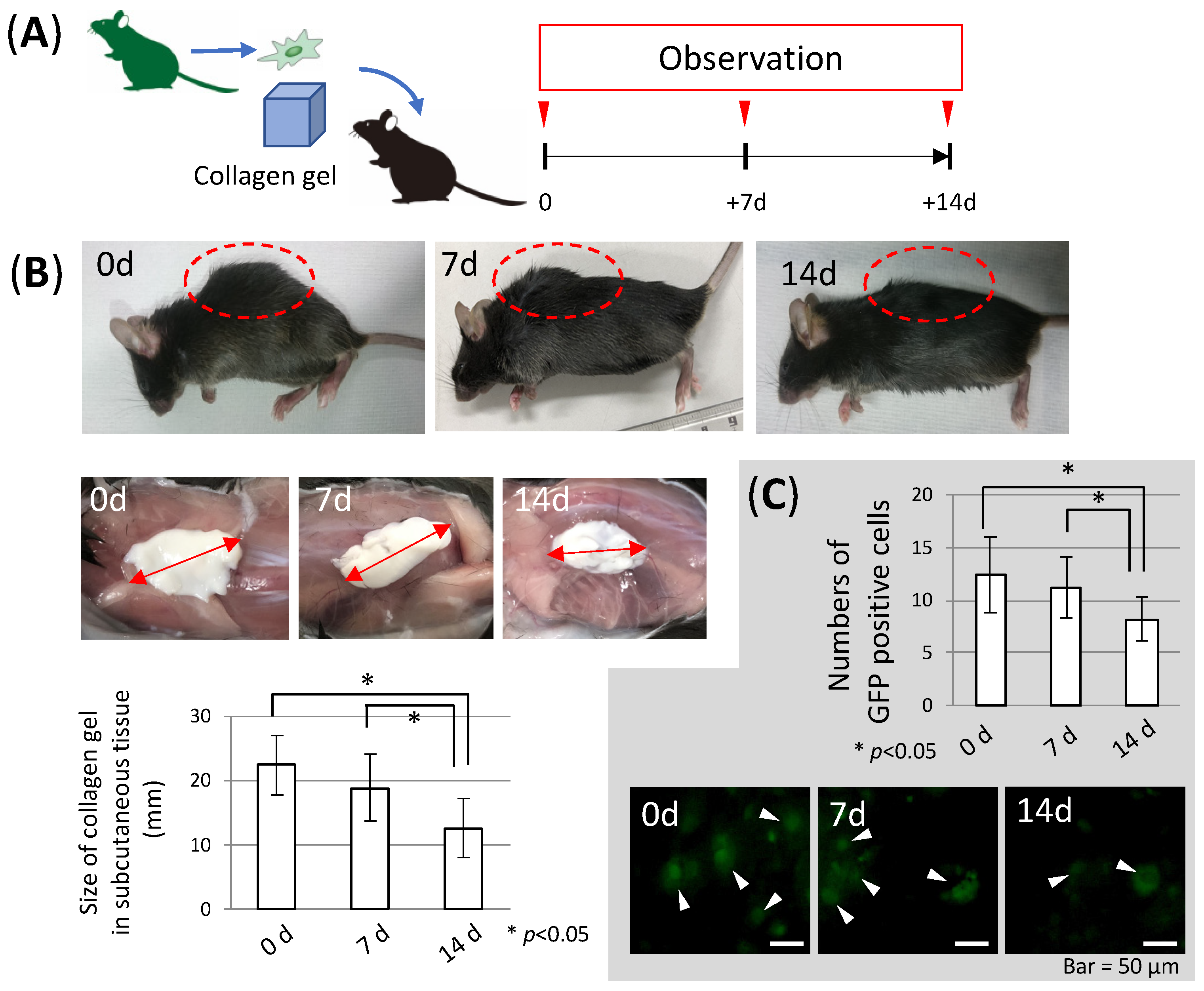
2.11. The Injection of Collagen Gel with MSC
2.12. Counting of Injected MSCs in Collagen Gel
2.13. Counting of Injected MSCs at Tooth Extraction Site and Organs
2.14. Tissue Preparation
2.15. Immunofluorescence Microscopy
2.16. Expression of Adhesion Protein
2.17. Statistical Analysis
3. Results
3.1. MSC Behavior in Collagen Gel Scaffold (In Vitro Study)
3.2. Stability of Collagen Gel in the Body (In Vivo Study)
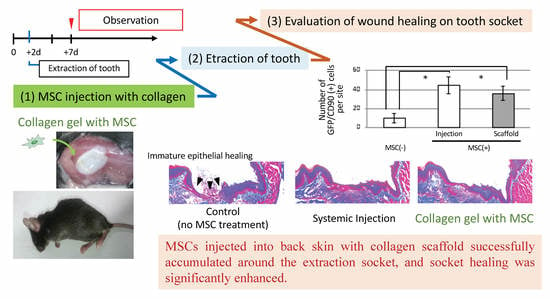
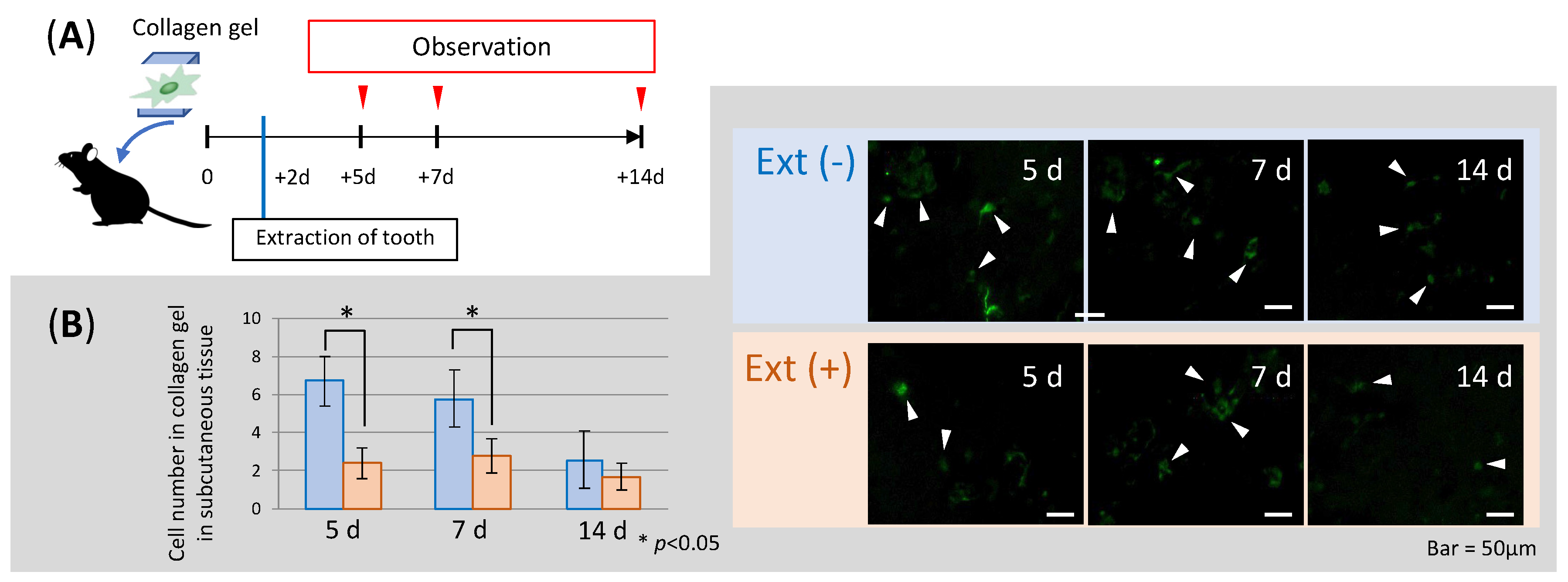
3.3. Changes in Cell Number in Scaffolds before and after Tooth Extraction
3.4. Accumulation of MSCs in Several Organs
3.5. Effect of Delivering MSCs with a Scaffold on Epithelial Healing after Tooth Extraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MEM | alpha minimum essential medium |
| BSA | bovine serum albumin |
| CFU-F | colony forming unit-fibroblast |
| DAPI | 4′,6-diamidino-2-phenylindole |
| Dex | dexamethasone |
| FBS | fetal bovine serum |
| FITC | fluorescein isothiocyanate |
| GFP | green fluorescent protein |
| MSC | mesenchymal stem cell |
| PBS | phosphate-buffered saline |
References
- Uchiyama, A.; Motegi, S.; Sekiguchi, A.; Fujiwara, C.; Perera, B.; Ogino, S.; Yokoyama, Y.; Ishikawa, O. Mesenchymal stem cells-derived MFG-E8 accelerates diabetic cutaneous wound healing. J. Dermatol. Sci. 2017, 86, 187–197. [Google Scholar] [CrossRef]
- Mouiseddine, M.; Mathieu, N.; Stefani, J.; Demarquay, C.; Bertho, J.M. Characterization and histological localization of multipotent mesenchymal stromal cells in the human postnatal thymus. Stem Cells Dev. 2008, 17, 1165–1174. [Google Scholar] [CrossRef]
- Payne, N.L.; Sun, G.; Mcdonald, C.; Layton, D.; Moussa, L.; Emerson-Webber, A.; Veron, N.; Siatskas, C.; Herszfeld, D.; Price, J.; et al. Distinct immunomodulatory and migratory mechanisms underpin the therapeutic potential of human mesenchymal stem cells in autoimmune demyelination. Cell Transplant. 2013, 22, 1409–1425. [Google Scholar] [CrossRef]
- Oh, S.Y.; Lee, S.J.; Jung, Y.; Lee, H.J.; Han, H.J. Arachidonic acid promotes skin wound healing through induction of human MSC migration by MT3-MMP-mediated fibronectin degradation. Cell Death Dis. 2015, 6, e1750. [Google Scholar] [CrossRef]
- Huang, S.; Xu, L.; Zhang, Y.; Sun, Y.; Li, G. Systemic and Local Administration of Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells Promotes Fracture Healing in Rats. Cell Transplant. 2015, 24, 2643–2655. [Google Scholar] [CrossRef]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. J. Immunol. 2007, 179, 1855–1863. [Google Scholar] [CrossRef]
- Ge, J.; Guo, L.; Wang, S.; Zhang, Y.; Cai, T.; Zhao, R.C.H.; Wu, Y. The Size of Mesenchymal Stem Cells is a Significant Cause of Vascular Obstructions and Stroke. Stem Cell Rev. Rep. 2014, 10, 295–303. [Google Scholar] [CrossRef]
- Karp, J.M.; Leng, T.G. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, K.; Liu, X.; Li, D.; Luo, C.; Fu, B.; Cui, S.; Zhu, F.; Zhao, R.C.; Chen, X. Repeated Systemic Administration of Human Adipose-Derived Stem Cells Attenuates Overt Diabetic Nephropathy in Rats. Stem Cells Dev. 2013, 22, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Bazhanov, N.; Ylostalo, J.H.; Bartosh, T.J.; Tiblow, A.; Mohammadipoor, A.; Foskett, A.; Prockop, D.J. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Res. Ther. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, M.; Urzua, C.A.; Montecino, S.; Leal, K.; Conget, P.; Ezquer, F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res. Ther. 2016, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Himeoka, Y.; Kaneko, K. Enzyme oscillation can enhance the thermodynamic efficiency of cellular metabolism: Consequence of anti-phase coupling between reaction flux and affinity. Phys. Biol. 2016, 13, 026002. [Google Scholar] [CrossRef] [PubMed]
- Aali, E.; Mirzamohammadi, S.; Ghaznavi, H.; Madjd, Z.; Larijani, B.; Rayegan, S.; Sharifi, A.M. A comparative study of mesenchymal stem cell transplantation with its paracrine effect on control of hyperglycemia in type 1 diabetic rats. J. Diabetes Metab. Disord. 2014, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, G.; Liu, M.; Zhou, T.; Zhou, H. Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. J. Biosci. Bioeng. 2016, 121, 213–219. [Google Scholar] [CrossRef]
- Burk, J.; Berner, D.; Brehm, W.; Hillmann, A.; Horstmeier, C.; Josten, C.; Paebst, F.; Rossi, G.; Schubert, S.; Ahrberg, A.B. Long-Term Cell Tracking following Local Injection of Mesenchymal Stromal Cells in the Equine Model of Induced Tendon Disease. Cell Transplant. 2016, 25, 2199–2211. [Google Scholar] [CrossRef]
- Liu, Y.S.; Ou, M.E.; Liu, H.; Gu, M.; Lv, L.W.; Fan, C.; Chen, T.; Zhao, X.-H.; Jin, C.Y.; Zhang, X.; et al. The effect of simvastatin on chemotactic capability of SDF-1α and the promotion of bone regeneration. Biomaterials 2014, 35, 4489–4498. [Google Scholar] [CrossRef]
- Matsuura, Y.; Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Kondo, R.; Takahashi, A.; Ueda, N.; Oshiro, W.; Tsukiyama, Y.; Koyano, K. Therapeutic interactions between mesenchymal stem cells for healing medication-related osteonecrosis of the jaw. Stem Cell Res Ther. 2016, 7, 119. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 28 November 2018).
- Duscher, D.; Rennert, R.C.; Januszyk, M.; Anghel, E.; Maan, Z.N.; Whittam, A.J.; Perez, M.G.; Kosaraju, R.; Hu, M.S.; Walmsley, G.G.; et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci. Rep. 2014, 4, 7144. [Google Scholar] [CrossRef]
- Kurata, K.; Heino, T.J.; Higaki, H.; Väänänen, H.K. Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J. Bone Miner. Res. 2006, 21, 616–625. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, M. Ultrastructural localization of laminin-5 (γ2 chain) in the rat peri-implant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Mino, S.; Goto, T.; Kido, M.A.; Terada, Y.; Tanaka, T. Changes in the distribution of laminin-5 during peri-implant epithelium formation after immediate titanium implantation in rats. Biomaterials 2005, 26, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Takamori, Y.; Atsuta, I.; Nakamura, H.; Sawase, T.; Koyan, K.; Hara, Y. Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin. Oral Implants Res. 2017, 28, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Von, D.F.; Kramer, M.; Wermke, M.; Wehner, R.; Röllig, C.; Alakel, N.; Stölzel, F.; Parmentier, S.; Sockel, K.; Krech, M.; et al. Mesenchymal Stromal Cells for Treatment of Acute Steroid-Refractory Graft Versus Host Disease: Clinical Responses and Long-Term Outcome. Stem Cells 2016, 34, 357–366. [Google Scholar]
- Li, X.H.; Gao, C.J.; Da, W.M.; Cao, Y.B.; Wang, Z.H.; Xu, L.X.; Wu, Y.M.; Liu, B.; Liu, Z.Y.; Yan, B.; et al. Reduced Intensity Conditioning, Combined Transplantation of Haploidentical Hematopoietic Stem Cells and Mesenchymal Stem Cells in Patients with Severe Aplastic Anemia. PLoS ONE 2014, 9, e89666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antonio, U.; Lorenzo, M.; Vito, P. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar]
- Kondo, R.; Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Therapeutic interaction of systemically-administered mesenchymal stem cells with peri-implant mucosa. PLoS ONE 2014, 9, e90681. [Google Scholar] [CrossRef]
- Kanazawa, M.; Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Kondo, R.; Matsuura, Y.; Koyano, K. The influence of systemically or locally administered mesenchymal stem cells on tissue repair in a rat oral implantation model. Int. J. Implant Dent. 2018, 4, 2. [Google Scholar] [CrossRef]
- Holder, A.J.; Badiei, N.; Hawkins, K.; Wright, C.; Williams, P.R.; Curtis, D.J. Control of collagen gel mechanical properties through manipulation of gelation conditions near the sol-gel transition. Soft Matter 2018, 14, 574–580. [Google Scholar] [CrossRef]
- Kimura, H.; Ouchi, T.; Shibata, S.; Amemiya, T. Stem cells purified from human induced pluripotent stem cell- derived neural crest-like cells promote peripheral nerve regeneration. Sci. Rep. 2018, 8, 10071. [Google Scholar] [CrossRef]
- Ogawa, M.; Ogawa, S.; Bear, C.E.; Ahmadi, S.; Chin, S.; Li, B. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat. Biotechnol. 2015, 33, 853–861. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, N.; Marsano, A.; Vunjak, N.G.; Zhang, Y.; Lopez, M.J. In vitro mesenchymal trilineage differentiation and extracellular matrix production by adipose and bone marrow derived adult equine multipotent stromal cells on a collagen scaffold. Stem Cell Rev. Rep. 2013, 9, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Shen, L.; Huang, Q.; Mi, J.; Wu, Y.; Yang, M.; Zeng, W.; Li, L.; Chen, W.; Zhu, C. The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials 2013, 34, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Isaji, M.; Hayashi, M.; Tsurufuji, S. Selective inhibition of collagen breakdown by proteinase inhibitors in granulation tissue in rats. J. Biochem. 1981, 89, 1081–1090. [Google Scholar] [PubMed]
- Kühl, T.; Mezger, M.; Hausser, I.; Handgretinger, R.; Bruckner, T.L.; Nyström, A. High local concentrations of intradermal MSCs restore skin integrity and facilitate wound healing in dystrophic epidermolysis bullosa. Mol. Ther. 2015, 23, 1368–1379. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Nagaishi, K.; Ataka, K.; Echizen, E.; Arimura, Y.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic hepatocyte damage in mice by inhibiting infiltration of bone marrow-derived cells. Hepatology 2014, 59, 1816–1829. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.; Xu, Q.; Park, W.; Lee, S.; Furuhashi, A. Neural Progenitor-Like Cells Induced from Human Gingiva-Derived Mesenchymal Stem Cells Regulate Myelination of Schwann Cells in Rat Sciatic Nerve Regeneration. Stem Cells Transl Med 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Peired, A.J.; Sisti, A.; Romagnani, P. Mesenchymal Stem Cell-based therapy for kidney disease: A Review of clinical evidence. Stem Cells Int. 2016, 4798639. [Google Scholar] [CrossRef]
- Elizabeth, B.; Yanzhou, L.; Li, C.; Austin, M.G. Eicosanoids: Emerging contributors in stem cell-mediated wound Healing. Prostaglandins Other Lipid Mediat. 2016, 132, 17–24. [Google Scholar]
- Yu, Y.; Wu, R.X.; Gao, L.N.; Xia, Y.; Tang, H.N.; Chen, F.M. Stromal cell-derived factor-1-directed bone marrow mesenchymal stem cell migration in response to inflammatory and/or hypoxic stimuli. Cell Adh. Migr. 2016, 10, 342–359. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, N.; Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Furuhashi, A.; Narimatsu, I.; Matsuura, Y.; Kondo, R.; Watanabe, Y.; Zhang, X.; et al. Novel Application Method for Mesenchymal Stem Cell Therapy Utilizing Its Attractant-Responsive Accumulation Property. Appl. Sci. 2019, 9, 4908. https://doi.org/10.3390/app9224908
Ueda N, Atsuta I, Ayukawa Y, Yamaza T, Furuhashi A, Narimatsu I, Matsuura Y, Kondo R, Watanabe Y, Zhang X, et al. Novel Application Method for Mesenchymal Stem Cell Therapy Utilizing Its Attractant-Responsive Accumulation Property. Applied Sciences. 2019; 9(22):4908. https://doi.org/10.3390/app9224908
Chicago/Turabian StyleUeda, Nobuyuki, Ikiru Atsuta, Yasunori Ayukawa, Takayoshi Yamaza, Akihiro Furuhashi, Ikue Narimatsu, Yuri Matsuura, Ryosuke Kondo, Yu Watanabe, Xiaoxu Zhang, and et al. 2019. "Novel Application Method for Mesenchymal Stem Cell Therapy Utilizing Its Attractant-Responsive Accumulation Property" Applied Sciences 9, no. 22: 4908. https://doi.org/10.3390/app9224908
APA StyleUeda, N., Atsuta, I., Ayukawa, Y., Yamaza, T., Furuhashi, A., Narimatsu, I., Matsuura, Y., Kondo, R., Watanabe, Y., Zhang, X., & Koyano, K. (2019). Novel Application Method for Mesenchymal Stem Cell Therapy Utilizing Its Attractant-Responsive Accumulation Property. Applied Sciences, 9(22), 4908. https://doi.org/10.3390/app9224908