Conjugation of Urokinase to Water-Soluble Magnetic Nanoparticles for Enhanced Thrombolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
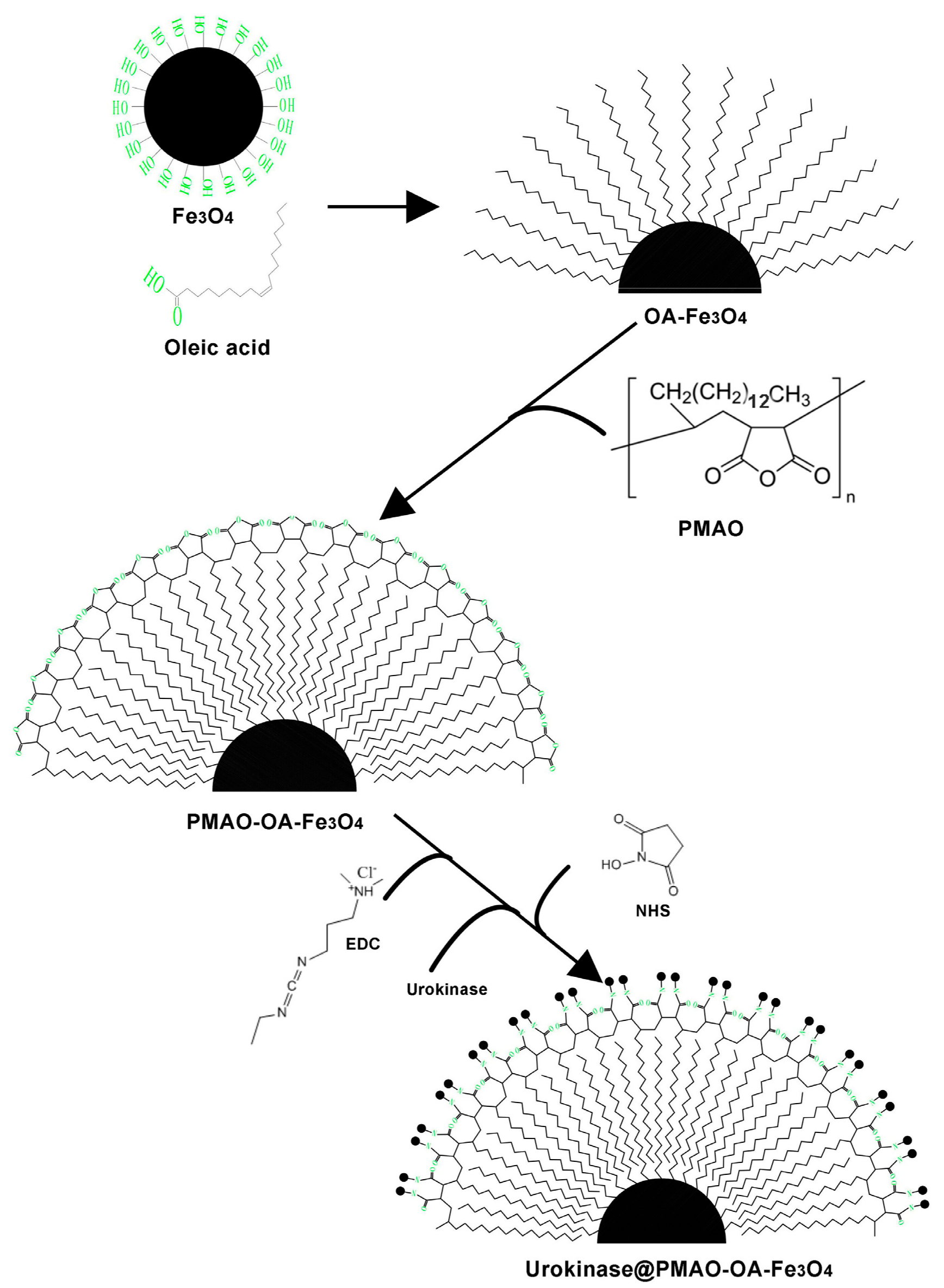
2.2. Synthesis of Urokinase@PMAO-OA-Fe3O4 NPs
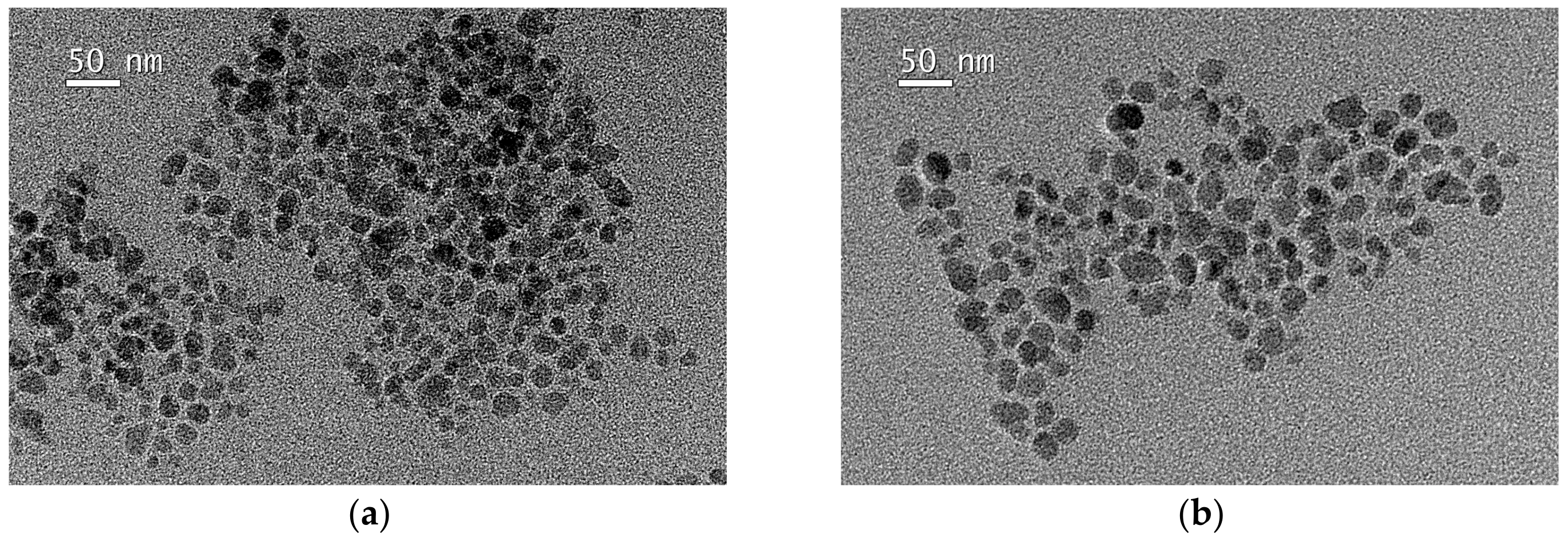
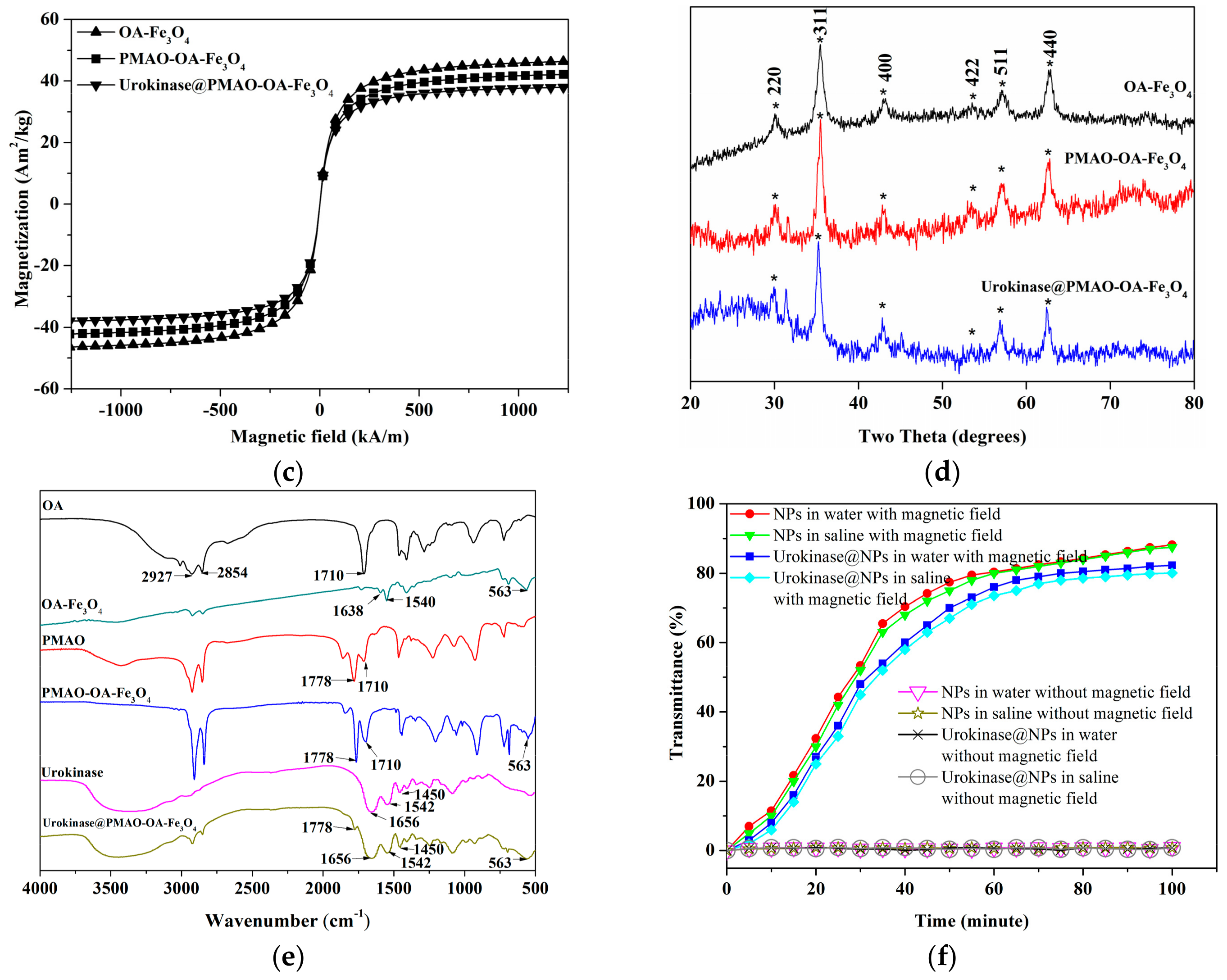
2.3. Characterization of Magnetic Nanoparticles (NPs)
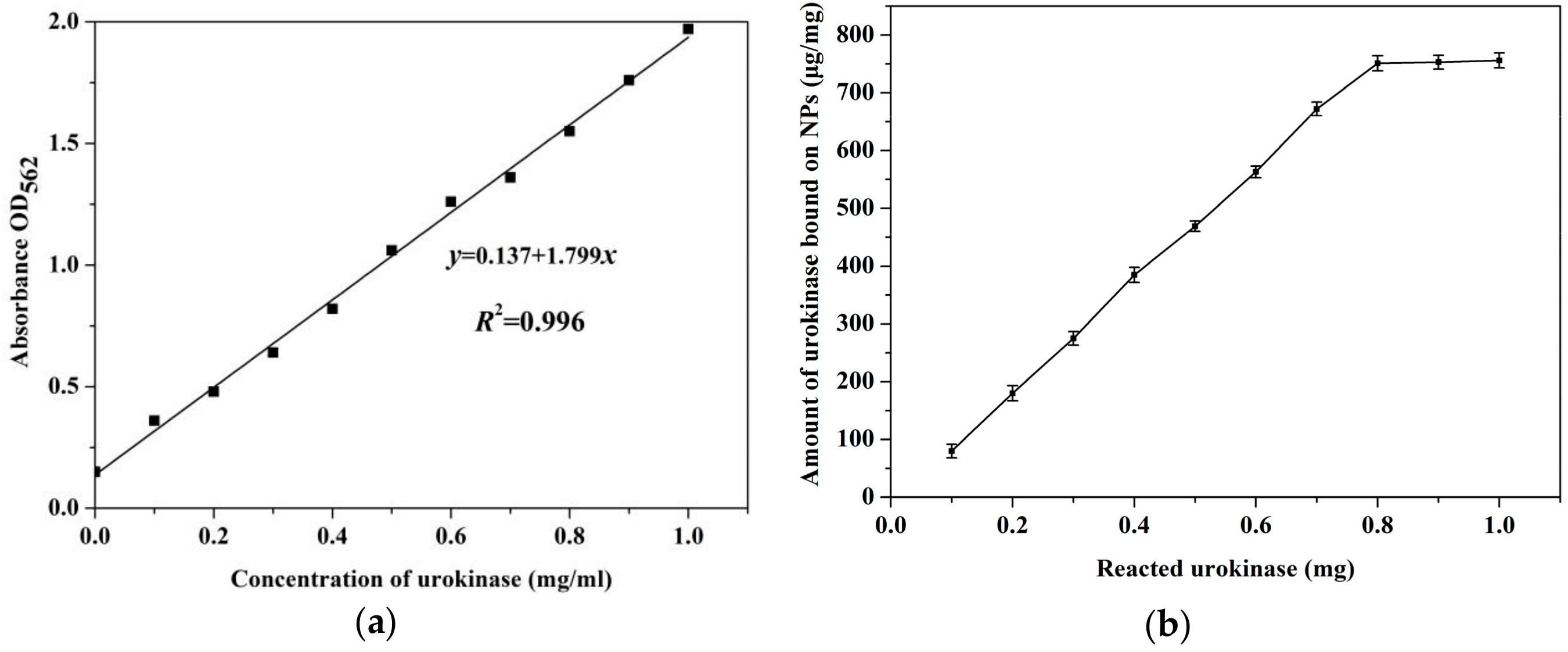
2.4. Evaluation of the Amount of Urokinase Bounding on PMAO-OA-Fe3O4 NPs
2.5. Toxicity Assay of Urokinase@PMAO-OA-Fe3O4 NPs
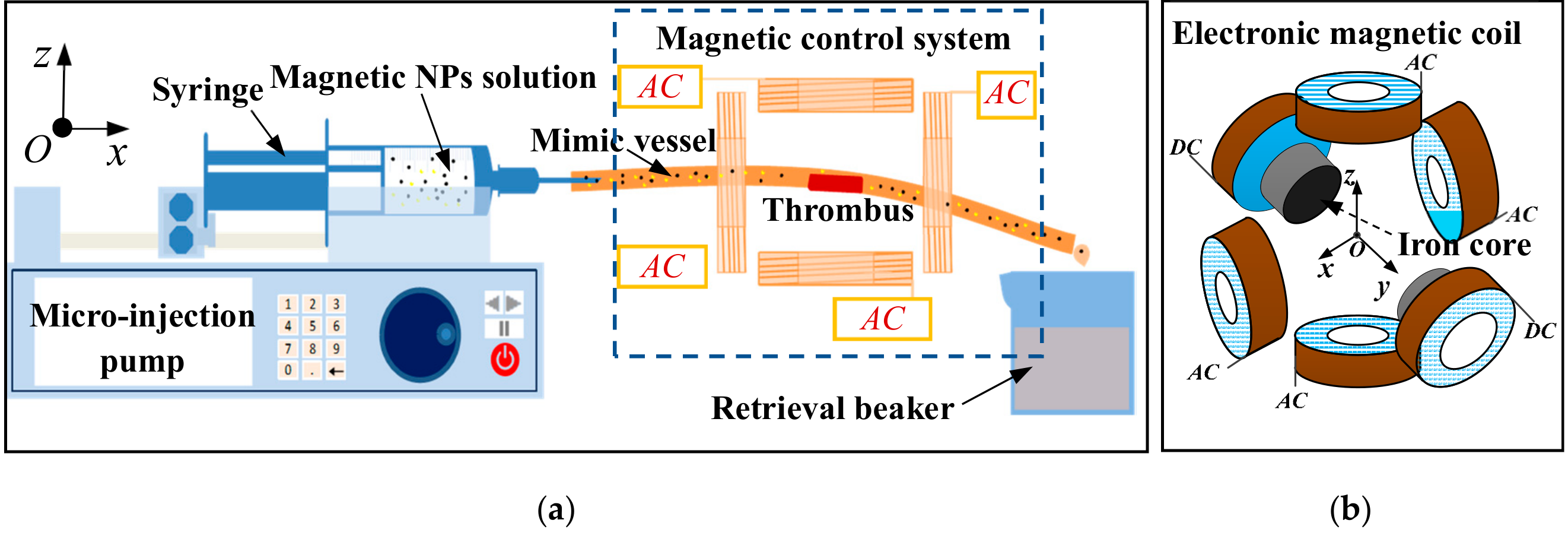
2.6. In Vitro Thrombolysis Experimental System
2.7. Statistical Analysis
3. Results and Discussions of Thrombolysis Experiment In Vitro
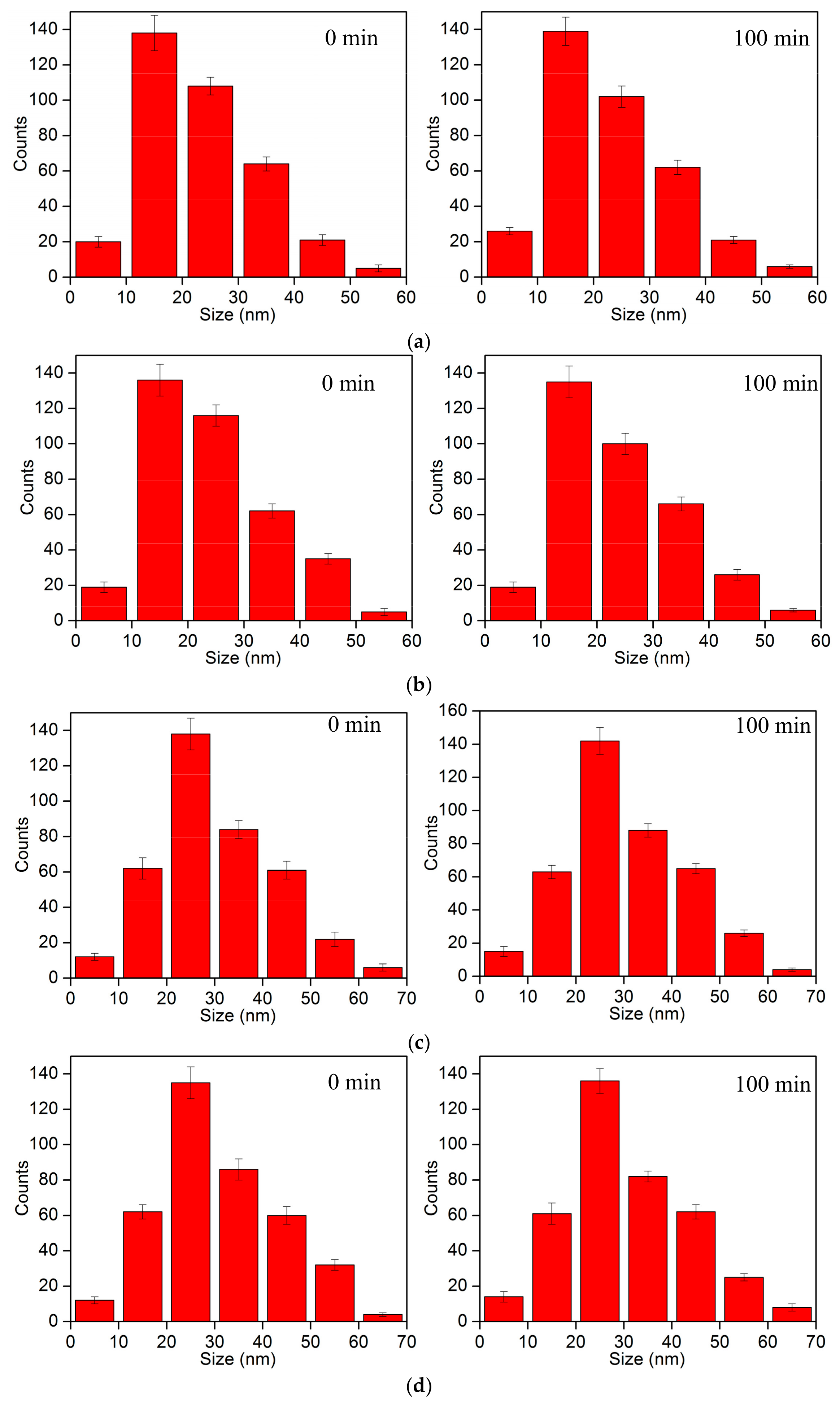
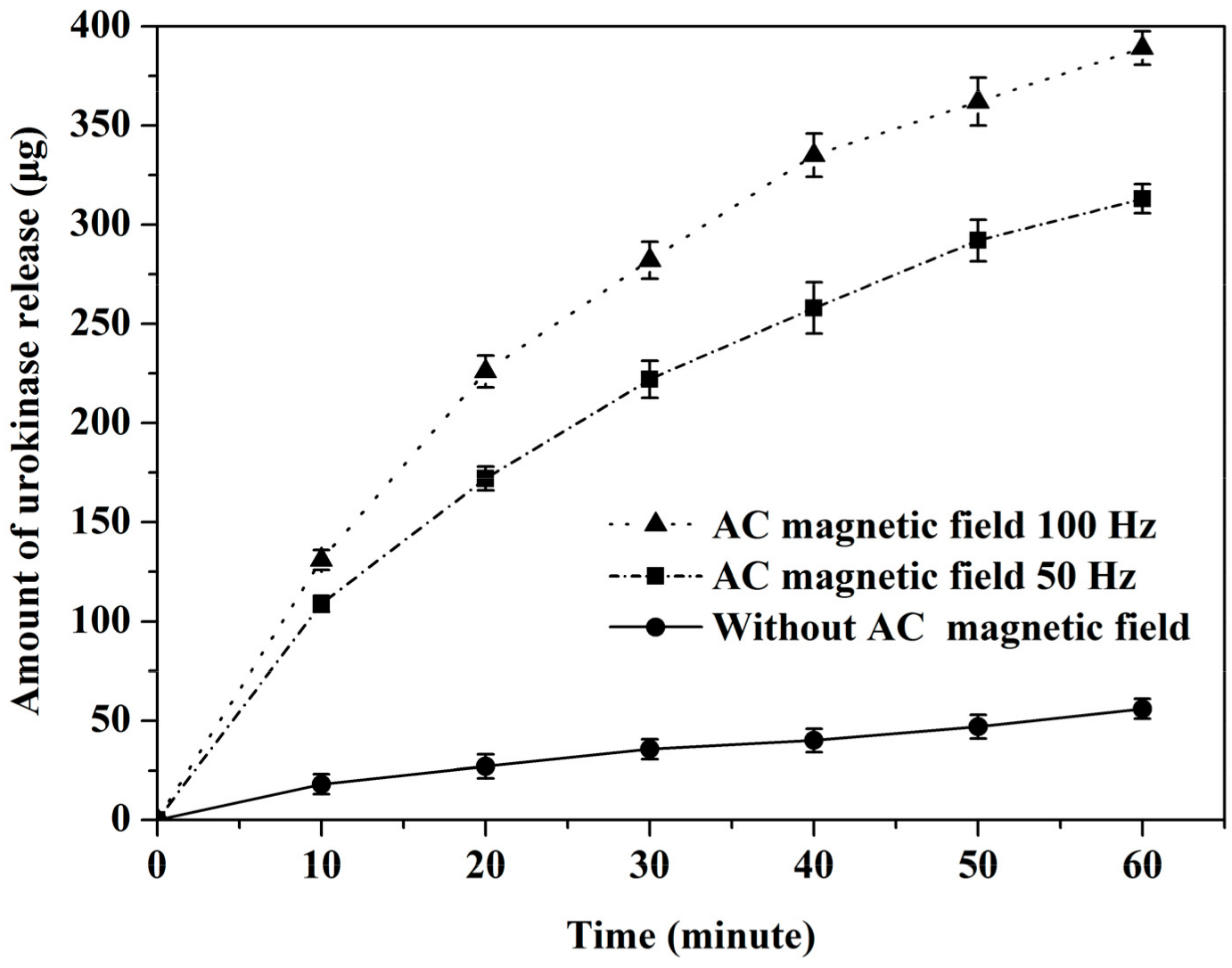
3.1. Drug Release Experiment
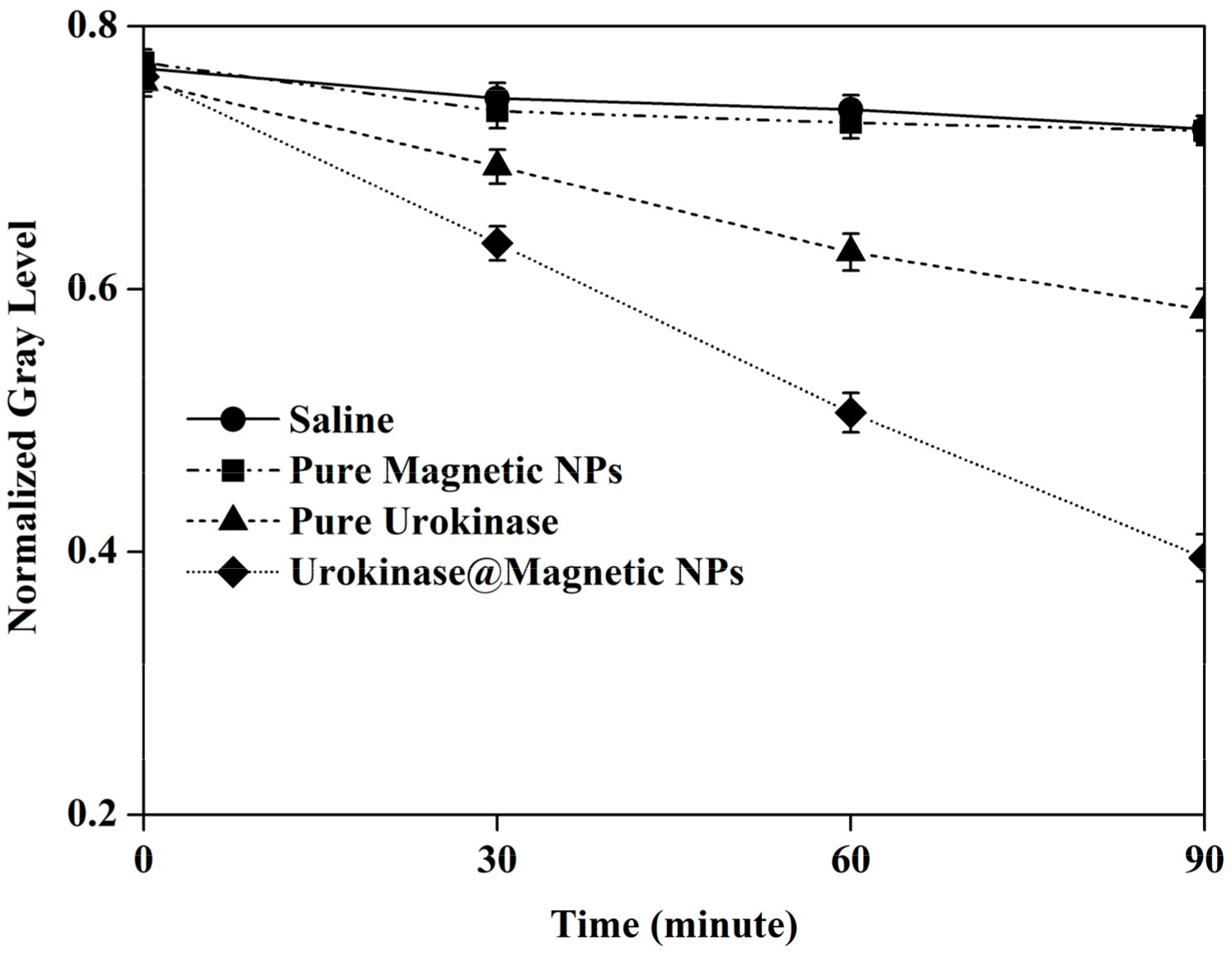
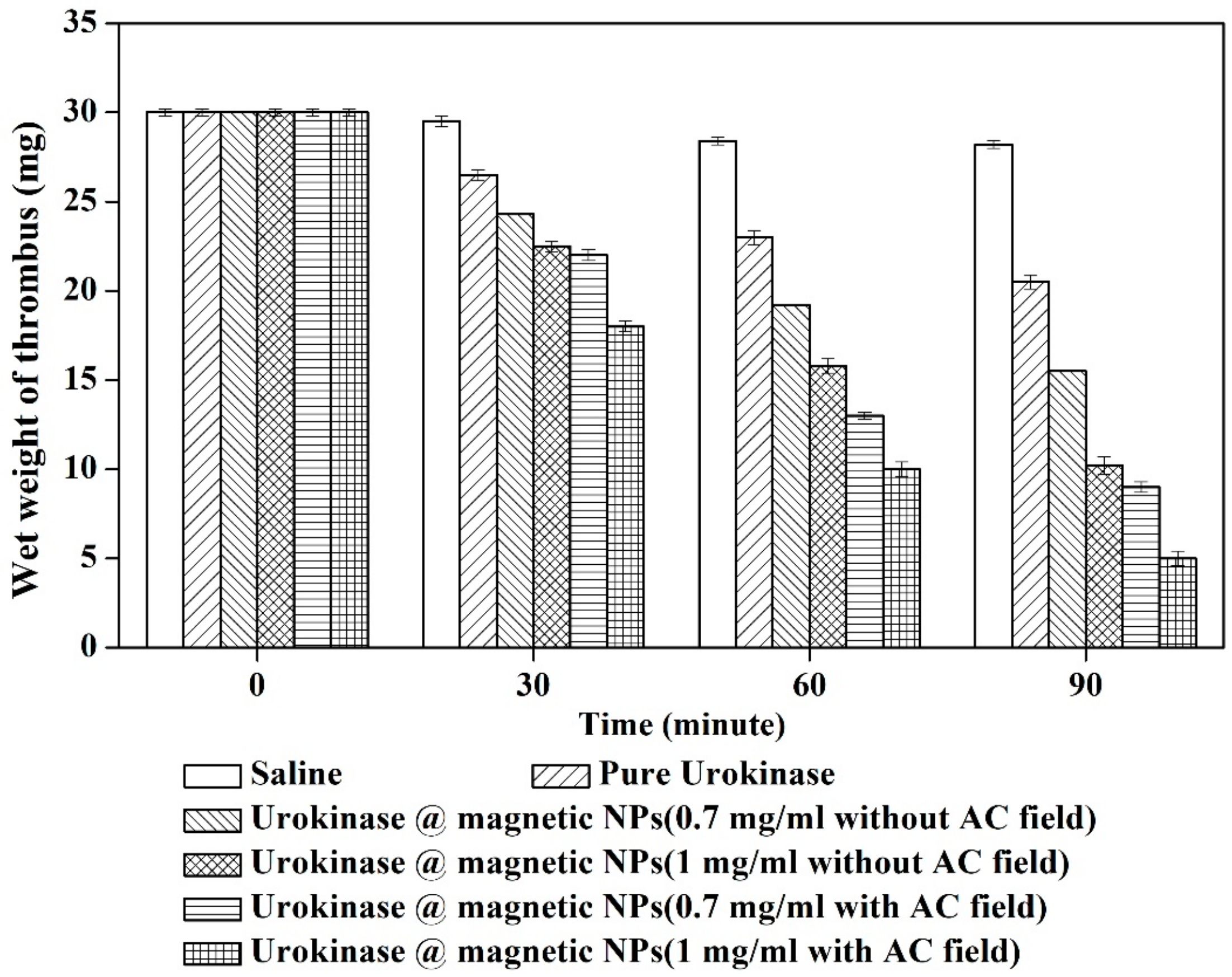
3.2. Thrombolysis Experiment In Vitro
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolach, O.; Sellar, R.S.; Martinod, K.; Cherpokova, D.; McConkey, M.; Chappell, R.J.; Silver, A.J.; Adams, D.; Castellano, C.A.; Schneider, R.K. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 2018, 10, eaan8292. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.; Broderick, C.; Armon, M.P. Thrombolysis for acute deep vein thrombosis. Cochrane Db. Syst. Rev. 2016, 11, CD002783. [Google Scholar] [CrossRef] [PubMed]
- Engelberger, R.P.; Spirk, D.; Willenberg, T.; Alatri, A.; Do, D.; Baumgartner, I.; Kucher, N. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ-Cardiovasc. Inte. 2015, 8, e002027. [Google Scholar]
- Ebben, H.P.; Nederhoed, J.H.; Lely, R.J.; Meijerink, M.R.; Meijs, B.B.V.D.; Wisselink, W.; Yeung, K.K.; Hoksbergen, A.W.J. Low-dose Thrombolysis for Thromboembolic Lower Extremity Arterial Occlusions is Effective Without Major Hemorrhagic Complications. Eur. J. Vasc. Endovasc. 2014, 48, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, W.J.; van Geloven, N.; van der Laan, M.H.; Vis, M.M.; Baan, J.; Koch, K.T.; Peters, R.J.; de Winter, R.J.; Piek, J.J.; Tijssen, J.G. The prognostic value of bleeding academic research consortium (BARC)-defined bleeding complications in ST-segment elevation myocardial infarction: A comparison with the TIMI (Thrombolysis In Myocardial Infarction), GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries), and ISTH (International Society on Thrombosis and Haemostasis) bleeding classifications. J. Am. Coll. Cardiol. 2014, 63, 1866–1875. [Google Scholar] [PubMed]
- Yang, F.Y.; Fang, S.F. Behavioural Assessment of Blood-Brain Barrier Opening Induced by Various Ultrasound Parameters. Proc. Eng. Technol. Innov. 2016, 3, 13–15. [Google Scholar]
- Yang, F.Y.; Chang, W.Y.; Li, J.J.; Wang, H.E.; Chen, J.C.; Chang, C.W. Pharmacokinetic analysis and uptake of 18F-FBPA-Fr sfter ultrasound-induced blood-brain barrier disruption for potential enhancement of boron delivery for neutron capture therapy. J. Nucl. Med. 2014, 55, 616–621. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-dimensional nanomaterials for biomedical applications: emerging trends and future prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Miller, M.A.; Gadde, S.; Pfirschke, C.; Engblom, C.; Sprachman, M.M.; Kohler, R.H.; Yang, K.S.; Laughney, A.M.; Wojtkiewicz, G.; Kamaly, N. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 2015, 7, 314ra183. [Google Scholar] [CrossRef]
- Hejazian, M.; Li, W.; Nguyen, N. Lab on a chip for continuous-flow magnetic cell separation. Lab Chip 2015, 15, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. A. 2016, 104, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.T.; Shah, S.; Pasquale, N.J.; Garbuzenko, O.B.; Minko, T.; Lee, K. Stem cell-based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer. Biomaterials 2015, 81, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Orza, A.; Lu, Q.; Guo, P.; Wang, L.; Yang, L.; Mao, H. Magnetic nanoparticle facilitated drug delivery for cancer therapy with targeted and image-guided approaches. Adv. Funct. Mater. 2016, 26, 3818–3836. [Google Scholar] [CrossRef]
- Ragelle, H.; Danhier, F.; Préat, V.; Langer, R.; Anderson, D.G. Nanoparticle-based drug delivery systems: A commercial and regulatory outlook as the field matures. Expert Opin. Drug Del. 2017, 14, 851–864. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, J.; Su, Y.; Tang, Y.; Liu, J. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials. 2009, 30, 5125–5130. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Vinogradov, V.V. Leach-proof magnetic thrombolytic nanoparticles and coatings of enhanced activity. Sci. Rep. UK 2016, 6, 28119. [Google Scholar] [CrossRef]
- Chang, M.; Lin, Y.H.; Gabayno, J.L.; Li, Q.; Liu, X. Thrombolysis based on magnetically-controlled surface-functionalized Fe3O4 nanoparticle. Bioengineered 2017, 8, 29–35. [Google Scholar] [CrossRef]
- Sanz, B.; Calatayud, M.P.; Torres, T.E.; Fanarraga, M.L.; Ibarra, M.R.; Goya, G.F. Magnetic hyperthermia enhances cell toxicity with respect to exogenous heating. Biomaterials 2017, 114, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Uson, L.; Arruebo, M.; Sebastian, V.; Santamaria, J. Single phase microreactor for the continuous, high-temperature synthesis of<4 Nm superparamagnetic iron oxide nanoparticles. Chem. Eng. J. 2018, 340, 66–72. [Google Scholar]
- Gabayno, J.L.F.; Liu, D.W.; Chang, M.; Lin, Y.H. Controlled manipulation of Fe3O4 nanoparticles in an oscillating magnetic field for fast ablation of microchannel occlusion. Nanoscale 2015, 7, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Prilepskii, A.Y.; Fakhardo, A.F.; Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Shtil, A.A.; Bel’Tyukov, P.P.; Shibeko, A.M.; Koltsova, E.M.; Nechipurenko, D.Y. Urokinase-conjugated magnetite nanoparticles as a promising drug delivery system for targeted thrombolysis: synthesis and preclinical evaluation. ACS Appl. Mater. Inter. 2018, 10, 36764–36775. [Google Scholar] [CrossRef] [PubMed]
- Inada, Y.; Ohwada, K.; Yoshimoto, T.; Kojima, S.; Takahashi, K.; Kodera, Y.; Matsushima, A.; Saito, Y. Fibrinolysis by urokinase endowed with magnetic property. Biochem. Bioph. Res. Co. 1987, 148, 392–396. [Google Scholar] [CrossRef]
- Ren, L.; Wang, X.; Wu, H.; Shang, B.; Wang, J. Conjugation of nattokinase and lumbrukinase with magnetic nanoparticles for the assay of their thrombolytic activities. J. Mol. Catal. B-Enzym. 2010, 62, 190–196. [Google Scholar] [CrossRef]
- Yang, H.W.; Hua, M.Y.; Lin, K.J.; Wey, S.P.; Tsai, R.Y.; Wu, S.Y.; Lu, Y.C.; Liu, H.L.; Wu, T.; Ma, Y.H. Bioconjugation of recombinant tissue plasminogen activator to magnetic nanocarriers for targeted thrombolysis. Int. J. Nanomed. 2012, 7, 5159–5173. [Google Scholar]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Hsieh, S.; Huang, B.Y.; Hsieh, S.L.; Wu, C.C.; Wu, C.H.; Lin, P.Y.; Hunag, Y.S.; Chang, C.W. Green fabrication of agar-conjugated Fe3O4 magnetic nanoparticles. Nanotechnology. 2010, 21, 445601. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.Z.; Ahamed, F.J.; Maitra, A.N.; Prashant, C.K.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomedicine. 2010, 5, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hu, S.; Hsiao, C.; Chen, Y.; Liu, D.; Chen, S. Multifunctional magnetically removable nanogated lids of Fe3O4–capped mesoporous silica nanoparticles for intracellular controlled release and MR imaging. J. Mater. Chem. 2011, 21, 2535–2543. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, X.; Lu, Z.; Yang, W.; Lei, Z.; Chang, M. Conjugation of Urokinase to Water-Soluble Magnetic Nanoparticles for Enhanced Thrombolysis. Appl. Sci. 2019, 9, 4862. https://doi.org/10.3390/app9224862
Li Q, Liu X, Lu Z, Yang W, Lei Z, Chang M. Conjugation of Urokinase to Water-Soluble Magnetic Nanoparticles for Enhanced Thrombolysis. Applied Sciences. 2019; 9(22):4862. https://doi.org/10.3390/app9224862
Chicago/Turabian StyleLi, Qian, Xiaojun Liu, Zhen Lu, Wenjun Yang, Zili Lei, and Ming Chang. 2019. "Conjugation of Urokinase to Water-Soluble Magnetic Nanoparticles for Enhanced Thrombolysis" Applied Sciences 9, no. 22: 4862. https://doi.org/10.3390/app9224862
APA StyleLi, Q., Liu, X., Lu, Z., Yang, W., Lei, Z., & Chang, M. (2019). Conjugation of Urokinase to Water-Soluble Magnetic Nanoparticles for Enhanced Thrombolysis. Applied Sciences, 9(22), 4862. https://doi.org/10.3390/app9224862




