1. Introduction
Valvular heart disease is a major cause of disability and premature mortality worldwide [
1]. Clinically moderate or severe valvular heart disease will double before 2050 from 1.5 million in 2015 to 3.3 million in 2056 [
2] resulting in a sharp increase of heart valve replacements, either surgical or transcatheter. To date, damaged native valves are replaced with mechanical and tissue heart valves. However, neither mechanical nor tissue prostheses are ideal [
3,
4]. Their implantation is associated with reduced quality of life. Mechanical prostheses require life-long anticoagulation therapy and are associated with a substantially high risk of bleedings [
5]. Tissue heart valves are susceptible to calcification and degeneration resulting in the structural failure, requiring repeat surgery [
6,
7]. Numerous approaches have been proposed to solve the existing problem and develop a durable heart valve: tissue-engineering and hybrid materials [
8,
9,
10], novel shape memory designs and magnetically triggered devices [
11], bioinert ceramics [
12], and polymeric materials with enhanced properties [
13,
14,
15]. Tissue-engineered heart valves strive to overcome the limitations of mechanical and bioprosthetic valves, because they are living tissues capable of active remodeling and self-repair [
16]. However, more studies are required to determine the optimal composition, manufacturing technique, and biofunctionalization of the selected scaffolds [
17]. Functional properties of mechanical heart valves may be addressed by additional coatings able to change the roughness and wettability of the product and control the resultant hemocompatibility and biocompatibility [
18,
19]. Promising results have been shown by de Avila et al. who reported zirconia material to possess optimal properties for biofilm formation [
20]. Though the data were presented for dental implants, they seem to be promising for developing artificial heart valve.
Recent advances in polymer synthesis and processing have opened new fronts for developing novel materials with unique morphology, mechanical, and physical properties. The ability to control the material properties has given the light to develop medical devices of given patterns to address compelling needs of various medical fields, including cardiothoracic surgery [
21,
22]. Thus, superior polymeric materials may ensure the development of artificial heart valves able to replicate native valve function and overcome major limitations of commercially available prostheses [
23]. Optimal polymeric heart valves should combine the main advantages of tissue and mechanical heart valve prostheses. Despite that the concept of the polymeric heart valve is not new and dates back to the 1950s, it has been put forward again with technologically advanced polymer synthesis and processing. The variety of polymers was used to design optimal flexible leaflets: polytetrafluoroethylene (PTFE and ePTFE) [
24,
25], silicone [
26], polyurethanes [
27], three-dimensionally cross-linked polyvinyl alcohol [
28], POSS-PCU nanocomposite [
29], copolymers based on styrene and isobutylene (SIBS) [
30], and others. However, almost all of them have several drawbacks limiting them from successful clinical introduction.
Poly (Styrene-
block-IsoButylene-
block-Styrene) (SIBS) is a biostable thermoplastic elastomer with physical properties that overlap silicone rubber and polyurethane [
31]. It was first developed in the Akron University, Ohio, USA in the late 1990s and was commercialized by Boston Scientific Corporation’s (BSC; Natick, MA, USA) in 2002. The development of the Drug Eluting TAXUS Coronary Stent reduced the rate of the bypass procedures with the subsequently improved rate of complications. Spurred by superior TAXUS results, Innovia LLC started working on its application for manufacturing polymeric heart valve able to mimic native valve and fully replicate its function. Original SIBS was reported to be highly hemocompatible material, but its poor physical properties forwarded the research groups towards its reinforcement. Galloher et al. assessed effective fiber reinforcement (Dacron vs. LARS
®) to improve the fatigue resistance of SIBS by minimizing stress concentration in the matrix. LARS
®-reinforced valve demonstrated superior physical properties over Dacron-reinforced valve, but they were still not enough to eradicate the main problem [
32]. Wang et al. (2010) tested dimyristoyl phosphatidylcholine (DMPC) precoating of SIBS [
33]. Despite good hemocompatibility, inferior functional properties limited its use for manufacturing polymeric heart valve potentially suitable for the clinical practice. Although several researches have been done on SIBS, no study to date has performed comprehensive assessment of its properties as a potential material for manufacturing heart valve leaflets and has compared with Gore-tex
TM, a benchmark material routinely used in the clinical practice.
Thus, our research is aimed at assessing physical properties, hemocompatibility and biocompatibility of SIBS to set reference range for selecting optimal nanofillers or functional fragments able to reinforce its structure, improve hemocompatibility and its application potential for manufacturing polymeric heart valve leaflets.
In order to achieve the set aim, we synthesized SIBS with Mn of 33,000 and compared its properties with Gore-texTM, a polymer that is routinely used for manufacturing medical devices for cardiothoracic surgery. Surface topography of both materials was described with scanning electron microscopy and atomic force microscopy. Uniaxial tension allowed describing the mechanical properties. The water contact angle was used to evaluate hydrophilicity/hydrophobicity of the study samples. Hemocompatibility was evaluated using cell lines (Ea.hy 926), donor blood, and in vivo.
2. Materials and Methods
2.1. SIBS Synthesis
Stabilized styrene (Sigma-Aldrich, St. Louis, MO, USA, >99%) was treated with 10% KOH aqueous solution, repeatedly washed with water, dried first with CaCl
2 and then with CaH
2, and, finally, distilled two times over CaH
2 under reduced pressure. Methylene chloride and hexane (both from Ekos-1, chemically pure grade) were treated with sulphuric acid, washed with aqueous sodium bicarbonate, dried over CaCl
2, and distilled twice from CaH
2 under an inert atmosphere Titanium tetrachloride (Sigma-Aldrich, St. Louis, MO, USA, 99.9%) was distilled before use. Isobutylene (Sigma Aldrich, St. Louis, MO, USA; 99%) was dried in the gaseous state by passing through the column packed with drierite and condensed into the Schlenk tube. 2,6-Di(
tert-butyl)pyridine (Sigma-Aldrich, St. Louis, MO, USA; 97%) and dicumyl alcohol (Sigma Aldrich, St. Louis, MO, USA; 97%) were used as received. Dicumyl chloride was synthesized by passing gaseous HCl through solution of dicumyl alcohol in CH
2Cl
2 at 0 °C [
34]. The purity of dicumyl chloride was conformed by
1H NMR spectroscopy to be >99%.
Triblock copolymer of isobutylene and styrene (SIBS) was synthesized by the living cationic polymerization according to the method reported by Mishra et al. [
34]. Polymerization was performed under inert argon atmosphere in a three-necked flask. The reactor was consequently charged with dicumyl chloride (0.095 mmol), 25 mL of solvents (hexane-methylene chloride mixture 60:40
v/
v), 2,6-di(tert-butyl)pyridine (0.20 mmol), and isobutylene (24 mmol) at −60 °C. Then, the reaction mixture was cooled to −80 °C and 1.9 mmol of titanium tetrachloride was added to initiate the polymerization. After 25 min since the beginning of the polymerization, 9.2 mmol of a preliminarily cooled 2.0 M solution of styrene in a hexane-methylene chloride mixture 60:40
v/
v was added into the reactor. The reaction was quenched by 2.0 mL of prechilled methanol 50 min after the initiation. The product was precipitated twice into a tenfold excess of cooled ethanol. The cooled sample was separated by centrifugation, washed with a small amount of ethanol, and dried in a vacuum at 55–60 °C/2 mmHg to constant weight.
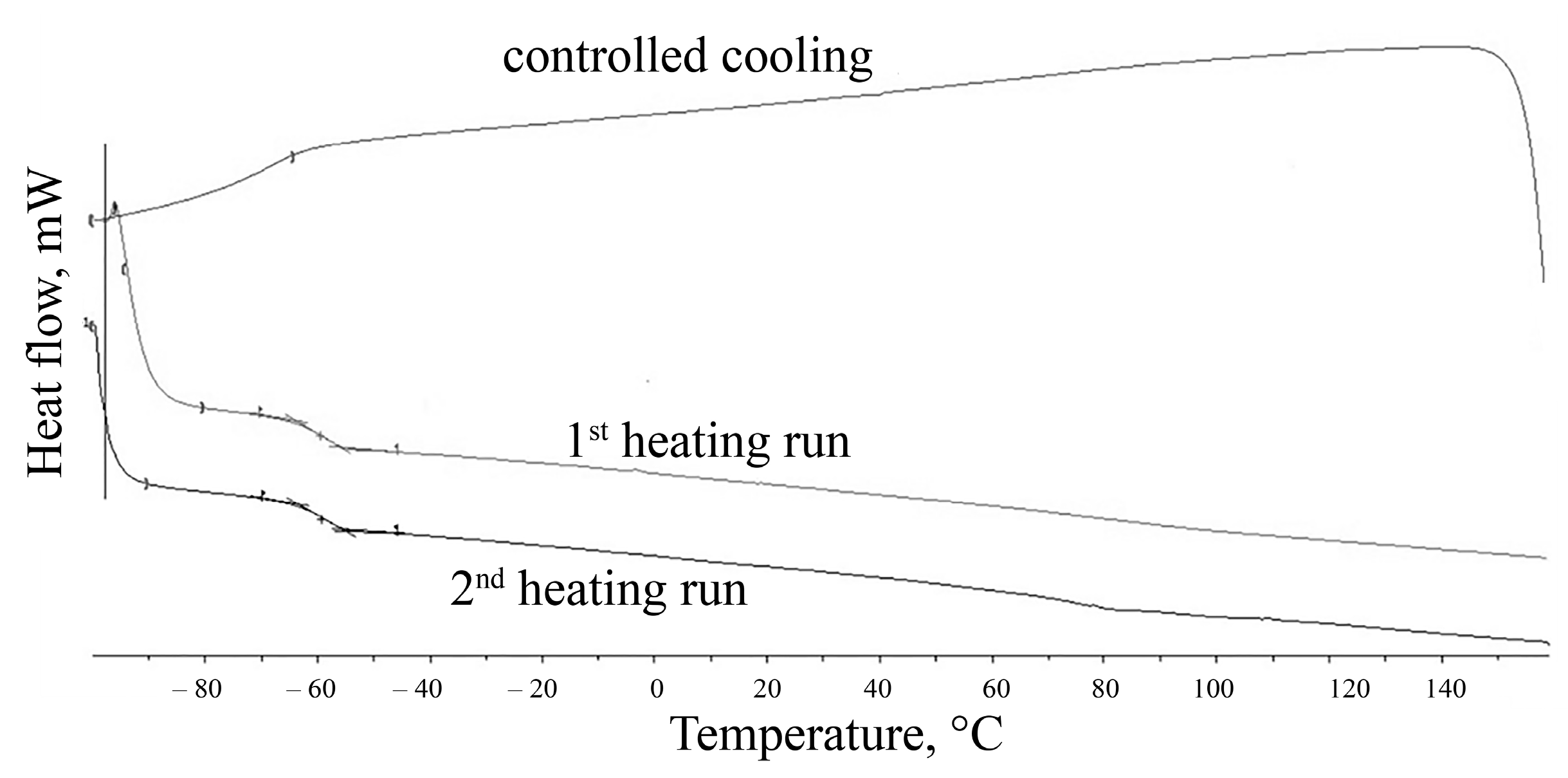
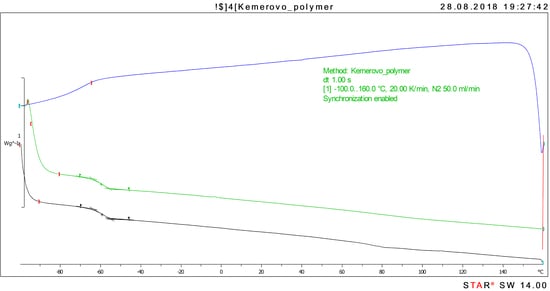
The structure of newly synthesized SIBS was identified with the differential scanning calorimetry (DSC). The phase and relaxation transitions of the polymer were studied by differential scanning calorimetry on DSC 3+ (Mettler Toledo, Greifensee, Switzerland) with a liquid nitrogen cooling system in the range of −100–160 °C with a heating rate of 20 deg/min and a cooling rate of 20 deg/min and with a nitrogen flow of 50 mL/min. The first and second heating cycles were examined in thermograms, the glass transition temperature Tg was evaluated by analyzing the inflection point.
Number-average molecular weight and polydispersity of SIBS were determined by gel permeation chromatography (GPC). Measurements were performed using an Ultimate 3000 (Thermo Fisher Scientific Dionex, Sunnyvale, CA, USA) device with a PLgel MIXED-C column (7.5 × 300 mm, particle size 5 μm, Agilent Technologies, Santa Clara, CA, USA) column and one precolumn (PLgel 5 μm Guard, Agilent Technologies, Santa Clara, CA, USA) thermostated at 30 °C. GPS traces were achieved using a refractometer or a UV-detector (λ = 255 nm). Tetrahydrofuran was used as a mobile phase with a flow rate of 1.0 mL min−1. The molecular weights and polydispersity were calculated based on polystyrene standards (EasiCal, Agilent Technologies, Santa Clara, CA, USA) with Mw/Mn ≤ 1.05 and using Chromeleon 7.0 program (Thermo Fisher Scientific Dionex, Sunnyvale, CA, USA).
2.2. SIBS and Gore-TexTM Samples
The SIBS films were solvent cast. 1,6 g of SIBS pellets were dissolved in 8 mL of chloroform (Sigma-Aldrich, St. Louis, MO, USA, ≥99%). The polymer solution was cast into a petri dish with the surface area of 21 cm. Slow evaporation was processed at room temperature for 1 h and at 35 °C for 3 h. The resultant films were then vacuum dried for 24 h at room temperature and pressure of <0.133 mbar using FREEZONE 2.5 (Labconco, Kansas City, MO, USA). The thickness of the obtained films was 240 μm.
A commercial thin-walled Gore-tex™ (Gore & Associates, Inc., Newark, DE, USA) stretch vascular graft (5 mm in diameter and 10 cm in length) was used as the reference sample to set the range of physical and hemocompatible properties that are required for a cardiovascular implant. Gore-tex™ is expanded PTFE with a microporous structure and a high strength-to-weight ratio, biocompatibility, and high thermal resistance. It is manufactured by means of a heating, stretching, and extruding process [
35].
2.3. Mechanical Properties
SIBS and Gore-texTM samples were tested in uniaxial tension using a universal testing machine (Zwick/Roell, Zwick GmbH & Co. KG, Ulm, Germany) equipped with a 50 N load cell according to the editorial rules of the ISO 37: 2017. Given the absence of any reinforcing fillers, fibers, and particles, SIBS referred to the isotropy group of materials and did not require any specific considerations of its orientation mode. Ten SIBS samples were tested for one tensile direction. In contrast, Gore-texTM had remarkable anisotropy, and was cut in two mutually perpendicular directions, longitudinal and circumferential. Ten Gore-texTM samples were tested for each tensile directions. Both sets of samples were cut to dumbbells with the narrow portion of 2 mm and of 10 mm length using type 1A blade according to the ISO 37: 2017. Samples were then mounted in Vulkollan grips in the “Zwick/Roell” (Zwick GmbH & Co. KG, Ulm, Germany). Samples were subjected to one loading cycle of applied strain at a constant rate of 10 mm/min until rupture. The tensile strength was measured with the maximum tensile stress (MPa), taking into account the cross-section area of the samples. Elastic deformation was assessed with the relative elongation adjusted to the elongation at break (%) and Young’s modulus (MPa). The last was determined in the range of small deformations corresponding to the range of physiological loading.
2.4. Assessment of Surface Properties
Surface structure of samples was assessed using scanning electron microscopy (SEM) and atomic force microscopy (AFM). Tapping mode atomic force microscopy was performed under standard laboratory conditions. Samples were cut into 5 mm × 5 mm sizes, fixed on a flat metal tab and studied using a MultiMode 8 microscope (Bruker, Billerica, MA, USA) equipped with SNL-10 probes (Bruker, Billerica, MA, USA).
Three SIBS and three Gore-texTM samples were cut into 5 mm × 5 mm sizes. Samples were then mounted on the tabs using conductive tape and gold/palladium (Au/Pd) coating sputtered with an EM ACE200 (Leica Mikrosysteme GmbH, Wetzlar, Germany). Scanning electron microscopy was performed using a S-3400N microscope (Hitachi, Tokyo, Japan). Secondary electron images were obtained at accelerating voltage of 5 kV and magnification of 5000.
The water contact angle was estimated using the sessile drop method. A drop of distilled water, approximately 15 μL, was dispensed on the flat platform and stabilized in during the same time for each measurement at room temperature. The obtained images were processed using the ImageJ Contact Angle plugin. The analysis was repeated eight times for each group of samples. An average contact angle with standard deviation (S.D.) was reported.
2.5. In Vitro Cytotoxicity and Cell Viability Assessment
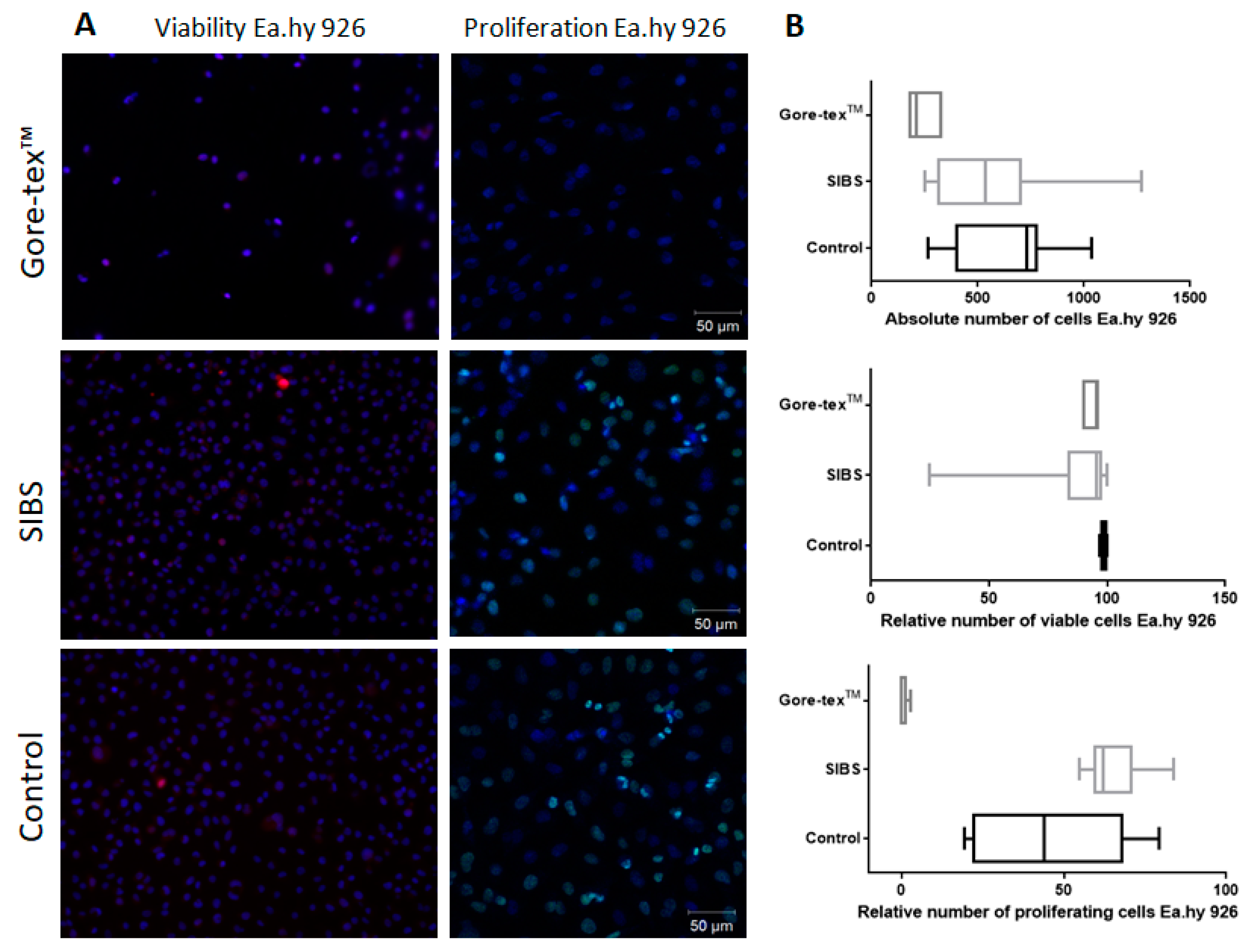
The experiment was performed using the Ea.hy 926 cell line, a hybridoma made from human umbilical vein endothelial cells (HUVEC) and a human lung carcinoma cell line. Cell culture plastic was used as a control in the experiments. Samples of sterile matrices (n = 5 each group, sterilized with ethylene oxide) were seeded with cells at a density of 2.0 × 105 cells/cm2 and cultured for 5 days in DME/F12 medium (Sigma Aldrich, St. Louis, MO, USA) containing 1% HEPES buffer (Hyclone, Logan, UT, USA), 10% fetal bovine serum (Sigma Aldrich, USA), 1% L-glutamine, 100 units/mL penicillin, 0.1 µg/mL streptomycin, 0.1 µg/mL amphotericin B, and hypoxanthine–aminopterin–thymidine (HAT; Sigma Aldrich, St. Louis, MO, USA) at 37 °C and 5% CO2. The medium was changed every alternative once in 2 days. The absolute number of cells per 1 mm2 surface and the relative amount of dead cells were assessed using a fluorescence microscope (Axio Observer Z1, Carl Zeiss, Oberkochen, Germany) after adding Hoechst 33342 nuclear fluorescent dyes (Molecular Probes, Eugene, OR, USA) in an amount of 2 μg/mL of ethidium bromide (EtBr; AppliChem, Darmstadt, Germany) at a concentration of 0.03 mg/mL. Cells were calculated in five different fields at a magnification of ×200 followed by a further counting under 1 mm2 of surface. The relative amount of dead cells was measured using the ratio of the absolute number of dead cells under 1 mm2 of surface to the absolute number of cells under 1 mm2 of surface. Cell proliferation was evaluated with sterile matrices (n = 5 each group) using Click-iT Plus EdU Imaging Kits (Molecular Probes, Eugene, OR, USA). Fluorescence microscopy was performed on an LSM 700 (Carl Zeiss) laser scanning microscope. The relative amount of proliferating cells was measured using the ratio of the absolute number of proliferating cells under 1 mm2 of surface to the absolute number of cells under 1 mm2 of surface.
2.6. In Vitro Calcification Assessment
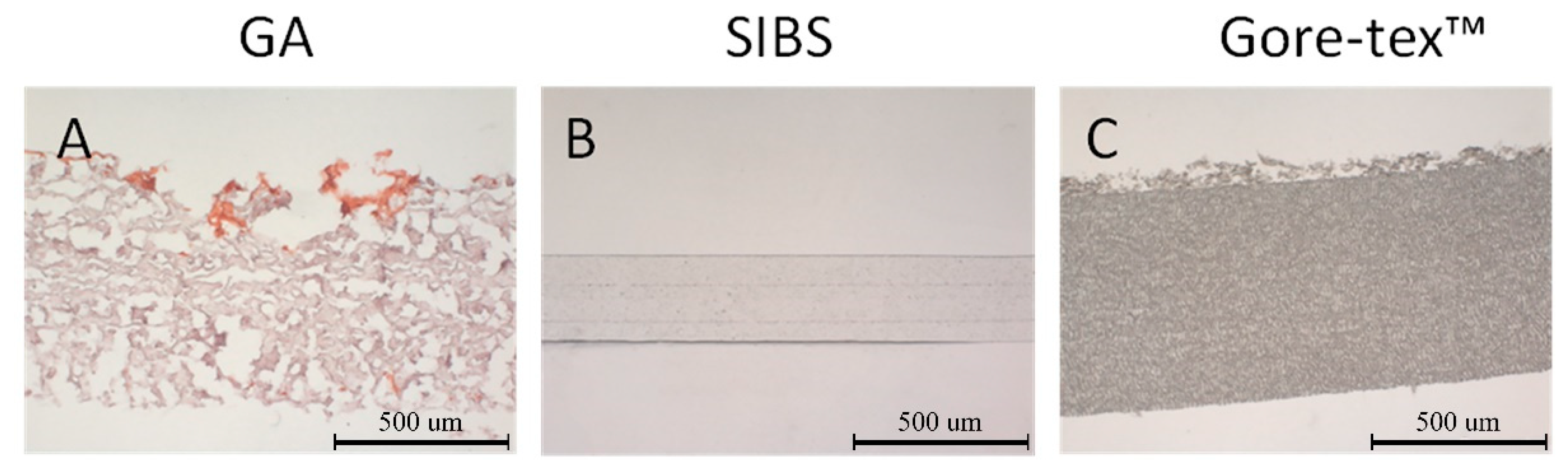
Samples with width of 5 mm × 5 mm (n = 5 each group) were placed in 2 mL solution based on DMEM medium (Sigma Aldrich, St. Louis, MO, USA) and serum albumin (FBS, Sigma Aldrich, St. Louis, MO, USA) containing CaCl2 and Na2HPO4 and kept in a CO2 incubator at 37 °C and 5% CO2. The degree of calcification was assessed 3 and 6 weeks after the incubation. Glutaraldehyde GA treated bovine xenopericardium (NeoCor, Russian Federation) was chosen as the positive control. Cryosections of the biomaterial and polymer samples were stained with alizarin red S (Reakhim, Moscow, Russian Federation) and evaluated with a light microscope (AXIO Imager A1 microscope, Carl Zeiss, Oberkochen, Germany).
2.7. In Vivo Hemocompatibility Assessment
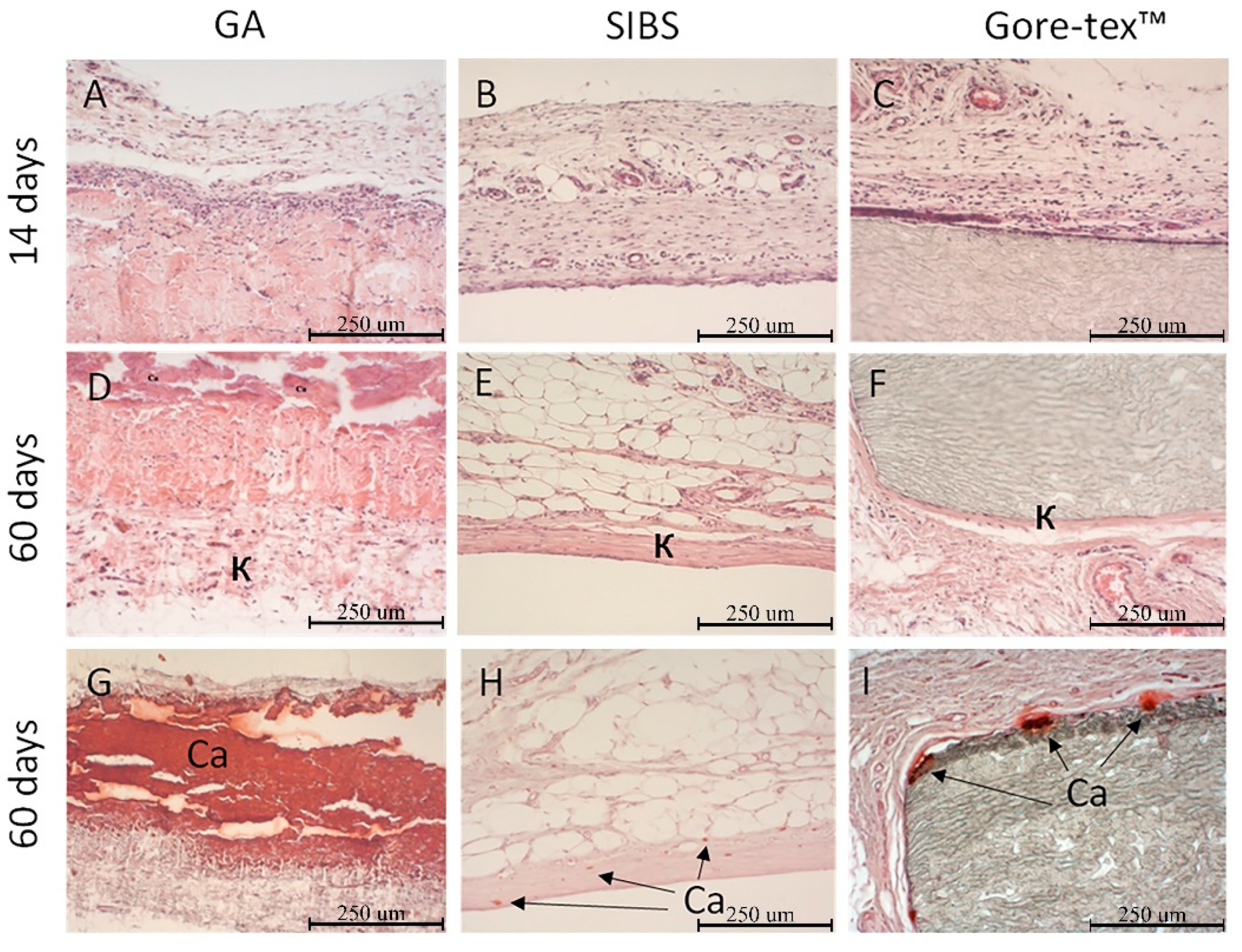
In vivo inflammatory response and calcification were evaluated with the study samples of 5 mm × 5 mm in size implanted subcutaneously into male Wistar rats each weighting 55–70 g for two weeks (tissue response, n = 5) and two months (tissue response, n = 5; calcification, n = 5). The study design was approved by the Local Ethics Committee of the Research Institute. The implants and surrounding tissues were excised once the experiment was completed and fixed in 4% formalin solution (MiniMed, Bryansk City, Russian Federation), then embedded in paraffin (Biovitrum, Saint-Petersburg, Russian Federation). Sections were stained with hematoxylin and eosin (Biovitrum, Saint-Petersburg, Russian Federation), according to Van Gieson (Biovitrum, Saint-Petersburg, Russian Federation) and alizarin red S (Reakhim, Moscow, Russian Federation). Samples were viewed with a light microscope (AXIO Imager A1, Carl Zeiss, Oberkochen, Germany). Inflammatory response was measured according to the editorial rules of the ISO 10993-6: 2016. The number of neovascularization foci was calculated in five different fields for each animal at a magnification of ×400. Fibrous capsule thickness (the mean ten equally distributed measurements of perpendicular distance starting at the capsule-implant interface and moving outward), fatty infiltrate, and the number of lymph nodes were calculated to evaluate tissue response. The rest samples were subjected to hydrolysis in perchloric acid solution followed by heating to assess the amount of calcium (in case of the Gore-texTM samples, calcifications formed during the experiment were present in the solution). The calcium content was measured with the inductively coupled plasma optical emission spectrometry using an iCAP 6500 DUO spectrometer (Thermo Fisher Scientific, Sunnyvale, CA, USA). GA-treated bovine xenopericardium (NeoCor, Kemerovo, Russian Federation) was the positive control.
2.8. In Vitro Hemocompatibility Assessment
The testing was performed in accordance with the editorial rules of the ISO 10993-4:2017. Fresh human donor blood samples were collected in 3.8% sodium citrate solution (dilution, nine parts of freshly drawn blood to one part sodium citrate solution). The citrated blood was centrifuged at 1000 rpm at 25 °C room for 10 min to obtain platelet-rich plasma (PRP). Platelet-poor plasma (PPP) was obtained by centrifuging the citrated blood at 4000 rpm at room temperature. The polymer matrices from Gore-texTM (Gore-Tex, Gore & Associates, Inc., Newark, DE, USA) and polyethylene (LDPE) ERM-EC590 (Merck, Kenilworth, NJ, USA) were controls.
2.9. Degree of Hemolysis
Samples of 25 cm
2 in size (
n = 5 each material) were placed in the beakers, then 10 mL of physiological saline was added, and the beakers were placed in a thermostat at 37 °C for 120 min. 200 μL of citrated blood was added, mixed, and then kept for 60 min. After incubation, the solution was taken from the cups into tubes, followed by centrifugation at 2800 rpm for 10 min to precipitate red blood cells (RBCs). Saline and distilled water were used as negative and positive controls to assess the degree of hemolysis. The optical density of the solutions under study was measured at a wavelength of 545 nm using a GENESYS 6 spectrophotometer (Thermo Fisher Scientific, Sunnyvale, CA, USA) [
36,
37].
2.10. Platelet Aggregation
Intact platelet-rich plasma was used as the positive control in assessing the impact of polymers on platelet aggregation. Spontaneous platelet activation was measured using a semi-automatic 4-channel platelet aggregation analyzer ARAST 4004 (LABiTec, Ahrensburg, Germany). To initiate spontaneous aggregation in citrate blood, Ca
+2 ions were reduced; the prepared CaCl
2 solution with a molar concentration of 0.25 M was used as a reagent. The ratio of the sample and reagent was 250 μL PRP + 25 μL CaCl
2. The contact time of the samples with PRP was 3 min [
37,
38].
2.11. Platelet Adhesion
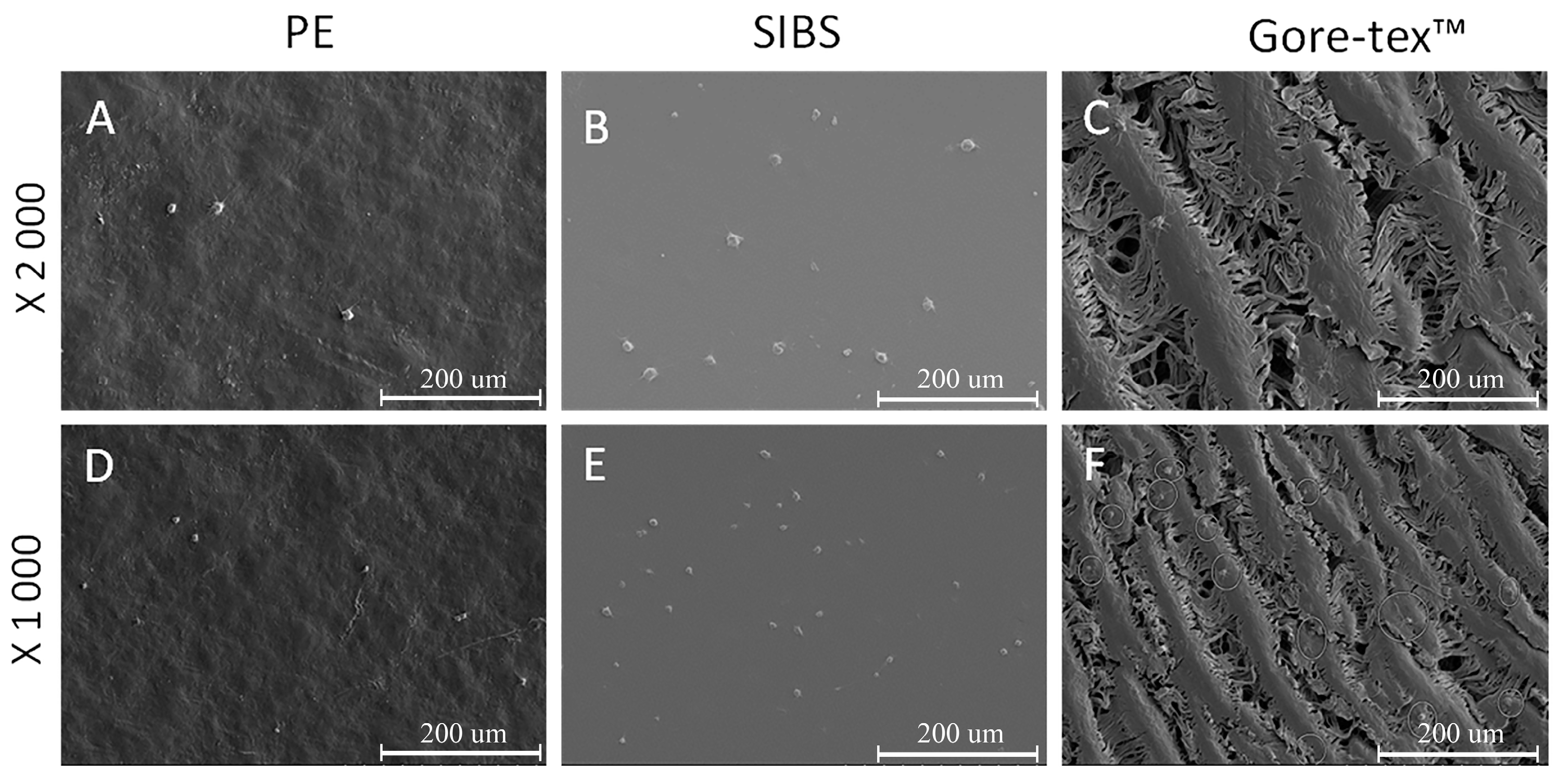
Polymer matrices sized 1 cm
2 were incubated with 500 μL of PRP at 37 °C for 2 h, then gently rinsed with phosphate-buffered saline (PBS) to remove non-adhered plasma components. Samples were then fixed in a 2% glutaraldehyde solution prepared in 0.1 M phosphate buffer for 6 h. Samples were dehydrated with ascending grades of alcohol (50%, 75%, 95%, and 100%). Platelet adhesion was measured using the scanning electron microscopy. Adhesive properties of the surface were evaluated in eight randomly selected fields of view using the deformation index [
36,
37,
38].
2.12. Statistical Analysis
The statistical analysis was performed using commercially available software package STATISTICA 6.0 (StatSoft, Inc., Tulsa, OK, USA). The non-parametric Mann–Whitney U test was used to assess statistically significant differences between two independent groups. A p level of <0.05 was considered statistically significant.
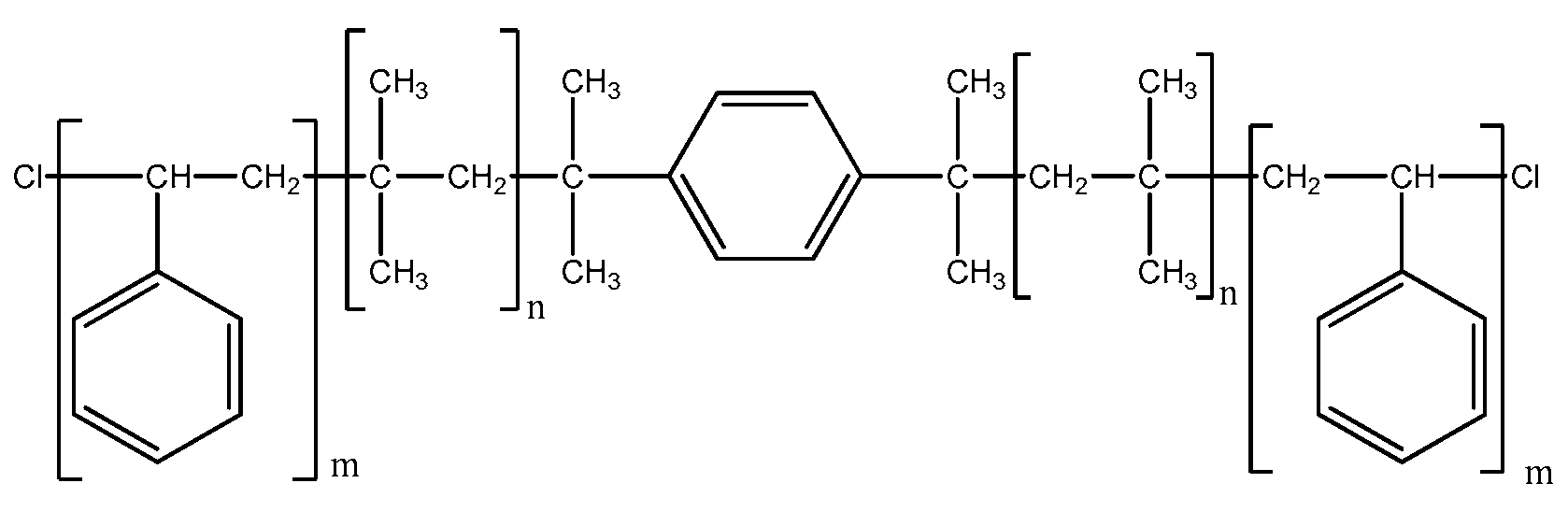
4. Discussion
The development of polymeric heart leaflets requires the use of polymers with superior hemocompatible properties as well as those yielding the best physical and mechanical properties. Molecular and supramolecular architectures are of key importance and drive the choice of the proper polymer. All these requirements encourage the researchers to use co-polymeric materials and composite materials, particularly in biomedicine, due to their unique properties and the ability to combine structural fragments of various functional groups [
13,
39]. A particular attention should be paid to styrene and isobutylene block copolymers as promising materials since they are thermoplastic, elastic, possess high strength, and are resistant to hydrolytic and oxidative effects due to the carbon chain, biological inertness of the backbone and side chains (
Figure 9) [
40].
There are three research groups developing flexible polymeric heart valves and leaflets from original or modified SIBS (i.e., with reinforced structure, added molecules, or following phospholipid modification) [
30,
33,
41]. However, the existing commercial SIBS has some specific disadvantages, including viscoelastic creeping and a tendency to thrombi formation [
31,
42]. In order to meet the demands of cardiovascular surgery, it should possess unique properties [
43]. By combining structural fragments of various types, we may obtain polymers with given patterns materials, i.e., hemocompatibility, biostability, increased strength, and optimal elasticity as compared with homopolymers [
44]. We used the cationic polymerization method to synthesize SIBS with the preferred properties. This is the only method ensuring a simple introduction of additional units into the structure of the polymer molecule that can drastically improve the properties of the resultant material [
45]. First, we analyzed the molecular weight characteristics of the polymer produced under the given conditions to assess the optimal parameters for the synthesis of a new molecule. Then, we synthesized the polymer with the number average molecular weight (M
n) of 33,000 and polydispersity (M
w/M
n) of 1.3 (
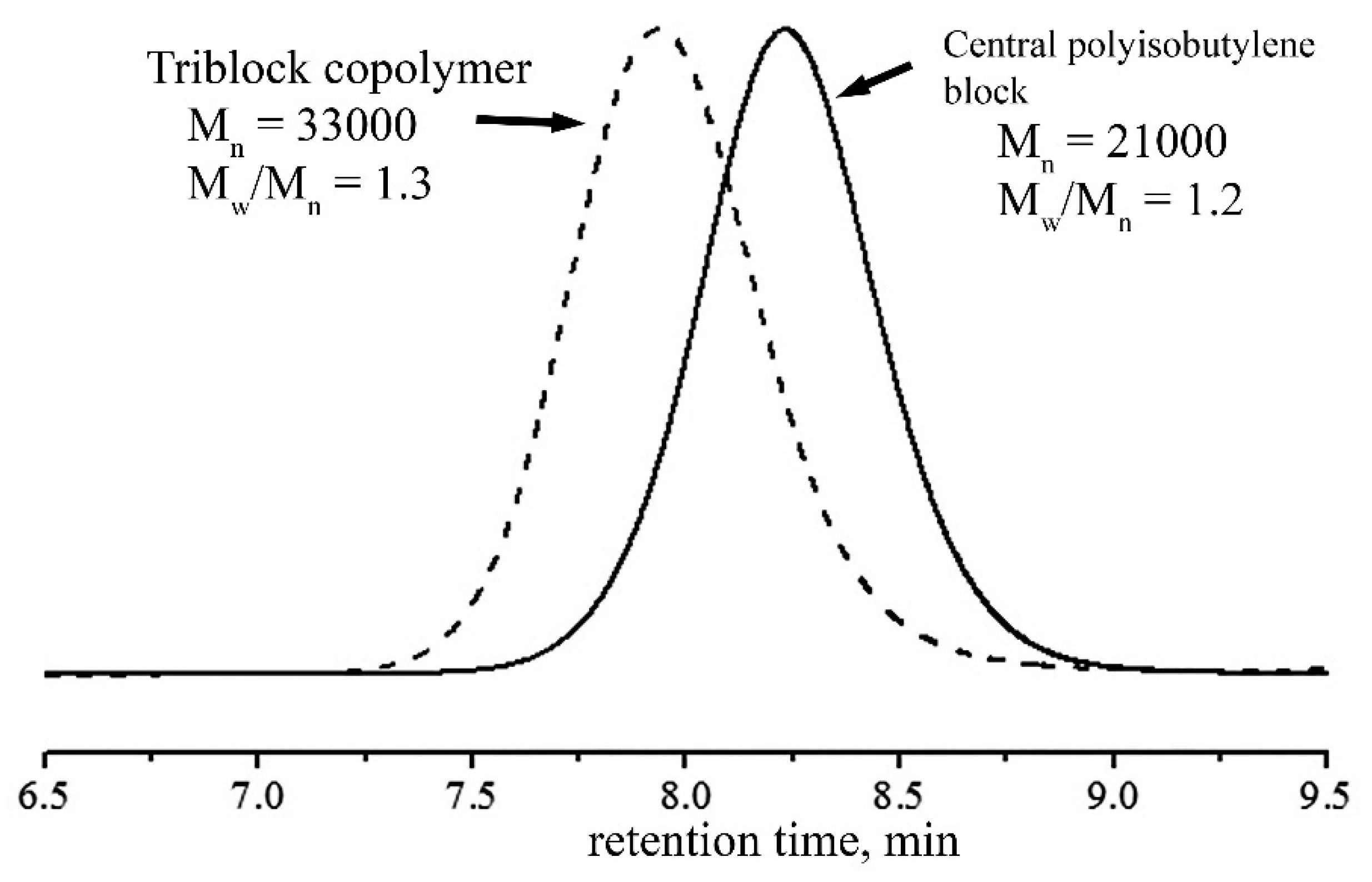
Figure 1). Gore-tex
TM was chosen as a control because it is currently applied in the real clinical practice when compared with other polymers undergoing experimental testing.
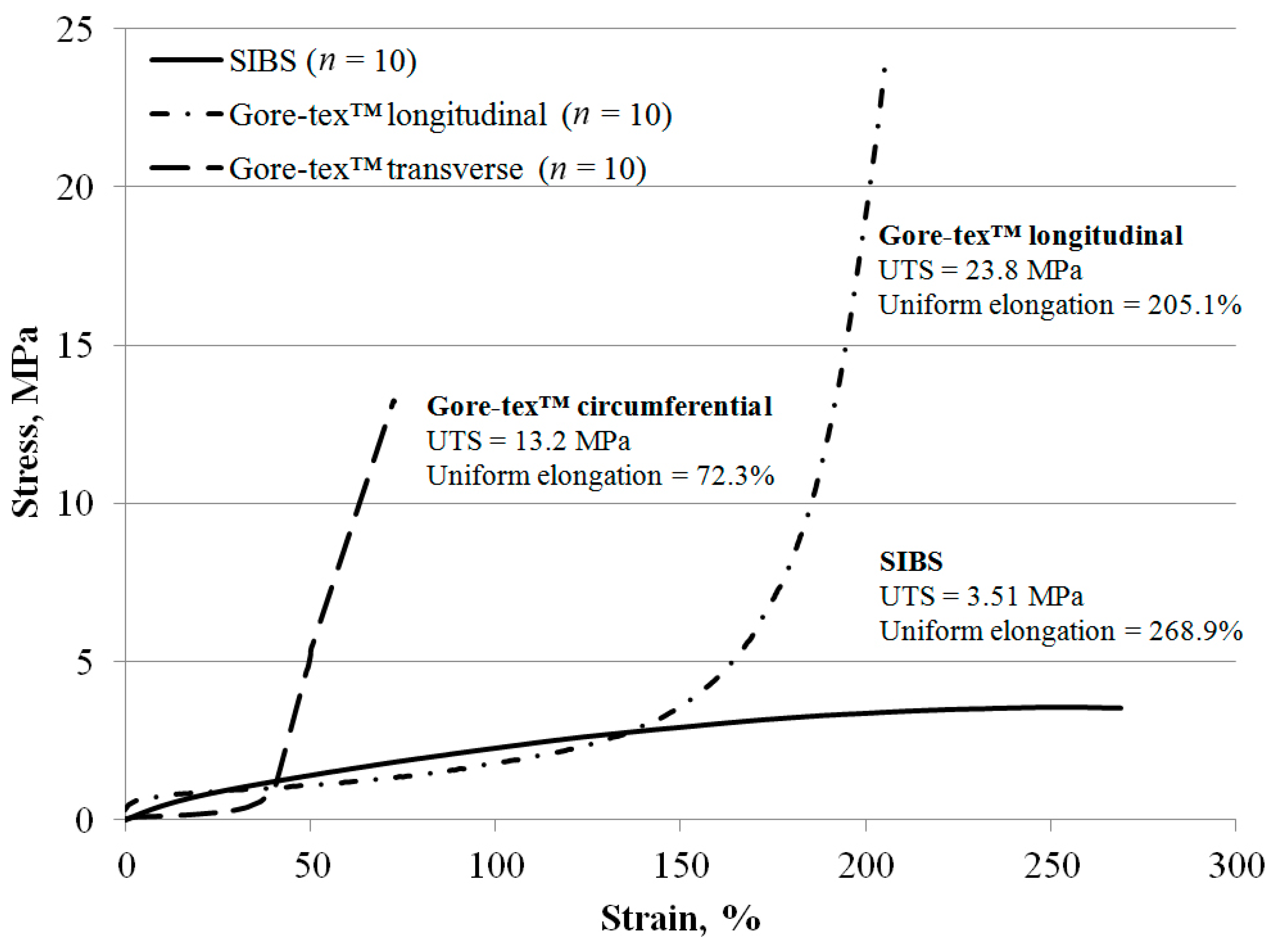
The mechanical properties of each block copolymer may vary depending on its molecular weight, the percentage of the constituent blocks, and the sequence of the polymer chain [
46]. The strength of SIBS was lower than that of Gore-tex™. Despite the superior tensile strength of SIBS that exceeds the tensile strength of the native tissues (heart and vessels), it is worth considering that synthetic materials are not capable of regeneration. As a result, the accumulated fatigue stress can lead to the destruction of the material structure. It means that an optimal material should possess a significant margin of safety [
36]. Due to this, the majority of researchers used SIBS polymers with M
n of 60,000 or even more [
36,
46] with the ultimate tensile strength (MPa) of 10–30 and the ultimate elongation of 300–1100% [
31]. However, a separate study covering this issue is required to enable the choice of the optimal molecular weight. The elasticity modulus in the physiological range of loading is considered as one of the key properties of the mechanical behavior of the material. It determines the suitability of the selected material to be used for developing artificial heart valves. More rigid polymers have a lower tendency to accumulate fatigue stress, but high rigidity of the leaflets may lead to significant hemodynamic alterations [
37]. In our experiment SIBS demonstrated moderate rigidity between the biological material [
38] and Gore-tex
TM. The elasticity of the leaflets ensures minimal energy losses during deformation of the heart valve under the pulsatile blood flow. Therefore, elastic and deformation properties should be considered as one of the main parameters in the decision-making process. Rigidity and elasticity can vary depending on the mass fraction of styrene in the polymer. An increase in rigidity and decrease in elongation is associated with increased styrene rate, and vice versa [
46]. SIBS synthesized in this study demonstrated promising results in terms of rigidity. However, its structure may be altered in case elasticity may require the further optimization.
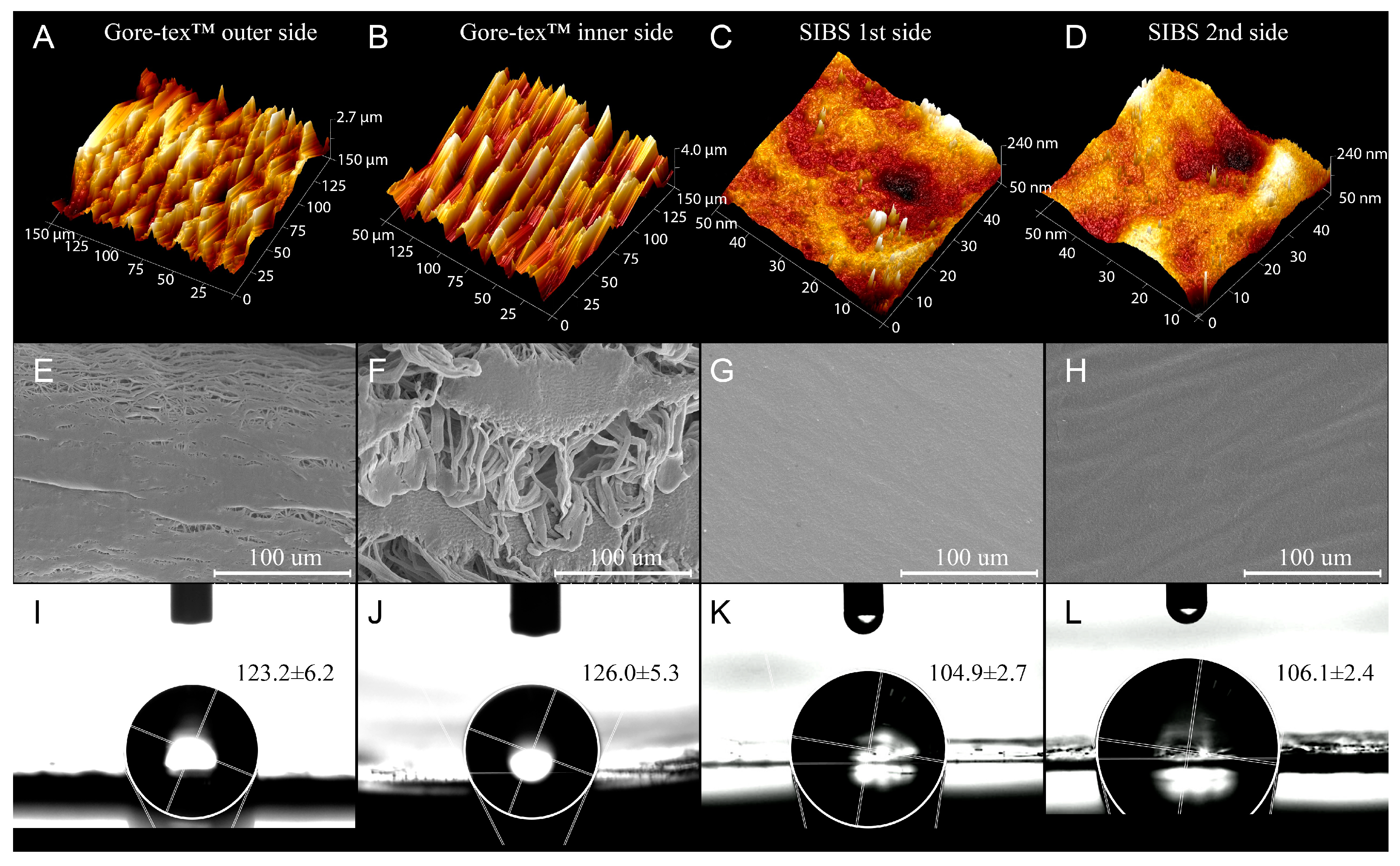
Hydrophobic surfaces are less hemocompatible, since they have a greater tendency to irreversible adsorption of proteins, including the resultant conformational changes, unlike hydrophilic surfaces, which minimally affect the conformation of proteins [
40]. Hydrophilicity/hydrophobicity determines the subsequent adhesion of blood cells and cell proliferation [
47]. However, there is some evidence on using of hydrophobic biomaterials in cardiovascular implants, particularly Gore-tex
TM in artificial heart valve leaflets, in clinical practice [
48]. Our experimental data demonstrated the superiority of SIBS over Gore-tex
TM suggesting the former beneficial potential for the application in the clinical practice. Our results are also consistent with the recent studies [
49]. Since both types of blocks are hydrophobic, hydrophilicity of the SIBS surface may be increased by adding hydrophilic groups (for example, hydroxyl) or by surface modification [
49]. At the same time, the need for such modification may be confirmed by the results of hemocompatibility tests, since the process of contacting with blood is known to be a multifactorial one. The surface properties of the implanted material may also affect its hemocompatibility. Smoother surfaces with regular structures are more biocompatible due to their ability to reduce the severity of the inflammatory process and the adhesion of blood cells [
50,
51]. SIBS demonstrated rather good biostability since there are no gas inclusions accelerating its biodegradation. The more pronounced porosity of the Gore-tex™ sample and its previously reported biocompatibility as compared to the SIBS sample suggest the superior potential of the last according to the surface relief. We might conclude that cast molding with chloroform as a solvent for manufacturing SIBS heart valve prostheses is comparable to THF casting and melting, the well-described methods in the literature [
52].
High hemocompatibility of SIBS and Gore-tex™ was confirmed by cytotoxicity testing using HUVEC cell line. The preceding experiments with the L929 cell line (mouse fibroblasts) [
53] also showed high cell viability on the Kaneka SIBS material. However, we used the HUVEC cell line as it suited more to the objectives of this study. The cell proliferative potential index can complement the functional activity of cells in short-term experiments, which are not able to demonstrate the full range of changes in cell viability [
54]. The ability of cell adhesion and proliferation is critical for tissue-engineered heart valves, a novel trend that has recently emerged. Taking into account the obtained results, we concluded that SIBS has superior potential for the further use in the development of tissue-engineered structures unlike Gore-tex
TM. Moreover, SIBS has higher capacity for spontaneous endothelization. Recent literature data suggest that the presence of the methyl groups in the polymer compound structure does not contribute to the proliferation of cells adhered on the material surface [
55].
Dense fibrous (connective tissue) capsule formation around the SIBS and Gore-tex™ samples indicates the completion of the inflammatory process following the 60-day implantation of the biostable materials. However, the fibrous capsule around the SIBS sample was thicker than that for the Gore-tex™ sample. The obtained results were consistent with the results of other experiments [
52]. Therefore, the molecular weight of the polymer and the structural quantitative composition of the block copolymer did not affect the biocompatibility of the polymer in the short-term experiments. Connective tissue is formed around the artificial heart valve leaflets creating a protective barrier, separating foreign material from the body environment. Moreover, it ensures a tight connection with the aortic root to prevent paravalvular regurgitation and secure the device fixation. Foreign body giant cells visualized in the Gore-tex™ and GA-treated xenopericardium samples may secrete reactive oxygen species and other chemical agents, contributing to oxidative damage and destruction of the implanted devices [
56]. There were no such cells found in the SIBS sample 60 days after the implantation. The biocompatibility of SIBS most likely is related to a thin (10 nm) layer of pure polyisobutylene covering the polymer surface contacting with biological tissues, while the polystyrene phases are buried within the material [
57]. High biocompatibility of SIBS and Gore-tex
TM has recently been confirmed in the experimental and clinical studies [
48,
58].
Calcification is the principal cause leading to stenosis of native and tissue aortic valves [
6]. Polymers, unlike biological tissue, do not contain phosphorus-rich cellular garbage and destroyed collagen provoking the mineralization process. Therefore, they are more resistant to calcification. Our results demonstrated the absence of calcium crystals provoking calcification in the studied polymer samples. The results of the quantitative analysis of calcium content in the in vivo model reported higher calcium content in GA-treated xenopericardium (the positive control) as compared to the polymer materials. Our finding was consistent with the literature data [
59]. Gore-tex™ demonstrated the presence of calcific deposits at the interface between the fibrous capsule and the polymer, while SIBS had isolated calcium crystals within the connective tissue capsule [
60]. The obtained results suggest superiority of SIBS to Gore-tex™ in terms of minimal calcification rate, stating the benefits of the former for manufacturing synthetic heart valve prosthesis.
The evaluation of the level of RBC hemolysis induced by SIBS demonstrated the absence of toxic effects on RBCs. Gore-tex™, being the control sample, did not cause the destruction of RBC membrane. The level of RBC hemolysis after direct contact with polyethylene was 1.82%, suggesting its thrombogenicity. The obtained data are consistent with the results of other studies on the impact of SIBS and Gore-tex
TM on RBC hemolysis. The level of RBC hemolysis after contact with Gore-tex
TM was also significantly lower than those for the positive control with 100% hemolysis [
41,
61]. The cationic polymerization method used to synthesize the block copolymer ensures the safety of the produced medical materials as it provides a high degree of monomer conversion. Moreover, none of the solvents and catalytic residues were identified in the resultant material after isolation procedure. Platelet adhesion is the initial stage of blood clot formation. Importantly, adhesion alone is not enough to trigger the thrombogenic cascade. Activated platelets are able to release substances into the blood that can lead to irreversible aggregation. The adhesion of platelets with a regular round shape that have not yet been deformed, does not carry a thrombogenic hazard, since the adsorption of platelets type I is reversible and they can be easily returned to the bloodstream [
62]. Our results on the assessment of adhesion properties showed that platelets adhere to all polymers, regardless of their type. The mechanism of platelet adhesion on the surface of the studied materials can be determined through the potential difference of polymers and platelets, and platelet adhesion can follow the adhesion of protein components of the blood, in this case, the hydrophobicity of polymers plays a crucial role [
4]. The presence of methyl groups (-CH3) and the styrene benzene ring in the SIBS structure may provoke both protein adhesion and platelet adhesion [
56]. Measurement of the aggregation activity of SIBS and Gore-tex™ polymers showed statistically significant advantage over polyethylene. Polyethylene, being the positive control in this experiment, caused a significant increase in the level of aggregation as compared to SIBS and Gore-tex™. Apparently, polyethylene releases substances that irritate blood platelet and lead to the activation of the last followed by the release of aggregation inducers.
Our results confirm that no residual monomer components and catalysts were found in the resultant material. Hemolysis, adhesion, and platelet aggregation demonstrated high hemocompatibility of SIBS as compared to Gore-tex™. However, its mechanical properties require further improvement and may be addressed through the introduction of nanofillers or a new molecule in the chemical structure.